Abstract
Sjögren's syndrome (SS) is an autoimmune inflammatory disease that primarily affects the lacrimal and salivary glands causing dry eyes and mouth. Antibodies to Ro60 are observed frequently in patients with SS; however, the role of these antibodies in SS initiation and progression remains unclear. The sequence Ro60 273–289 (Ro274) is a known B cell epitope of Ro60 and antibodies to this epitope have been observed in a subset of SS patients and in animals immunized with Ro60 protein. Animals immunized with Ro274 linear peptide develop a Sjögren's-like illness. We hypothesized that passive transfer of anti-Ro274-specific immunoglobulin (Ig)G would induce a Sjögren's-like phenotype. To evaluate this hypothesis, we adoptively transferred affinity-purified Ro274 antibodies into naive BALB/c animals, then evaluated salivary gland histology, function and IgG localization 4 days post-transfer. At this time-point, there was no demonstrable mononuclear cell infiltration and salivary glands were histologically normal, but we observed a functional deficit in stimulated salivary flow of animals receiving Ro274 antibodies compared to animals receiving control IgG. Cellular fractionation and enzyme-linked immunosorbent assay revealed Ro274-specific antibodies in the nucleus and cytoplasmic fractions of isolated parotid salivary gland cells that was confirmed by immunohistochemistry. These data support the hypothesis that antibodies to Ro274 deposit in salivary glands can enter intact salivary gland cells and are involved in the dysregulation of salivary flow in SS.
Keywords: antigens/peptides/epitopes, autoantibodies, autoimmunity, rodent, systemic lupus erythematosus
Introduction
Sjögren's syndrome (SS) is a chronic, progressive autoimmune disease that predominately affects the exocrine glands. Ro/SS-A (Ro60) and La/SS-B (La) are ubiquitous nuclear antigens specifically targeted in systemic lupus erythematosus (SLE) and SS and are used as classification criteria. Previous work by our laboratory in BALB/c mice and rabbits immunized with Ro60 demonstrates the production of antibodies to B cell epitopes Ro274 (aa273–289), Ro413 (aa413–428) and Ro480 (aa480–494) 1,2. Of these peptides, the Ro274 amino acid sequence is conserved 100% between mouse and human 2. Immunization of naive BALB/c mice with a Ro274 linear peptide induced salivary gland infiltrates, impaired stimulated salivary flow and led to the production of high titre antibodies to Ro274 that spread subsequently to other epitopes of Ro60 and La, indicating that this peptide has the pathogenic potential to induce an SS-like illness in mice 3. Interestingly, the mice in this model, very similar to patients with primary SS (pSS), are devoid of antibodies against dsDNA and lack the haematological and renal features observed frequently in SLE. Based on these data, we hypothesized that antibodies to Ro274 could, therefore, be directly culpable in the pathology of human SS.
Although much is being learned about the pathogenesis of pSS, the role in which epitope-specific Ro60 antibodies directly impact the pathogenesis of SS currently remains unclear. Several investigators have shown that antibody-mediated diseases, including pemphigus vulgaris, autoimmune myocarditis, vasculitis and experimental autoimmune glomerulonephritis, can be transferred into naive mice following passive transfer of sera or affinity-purified antibodies from affected mice or humans 4–7. With regard specifically to pSS, Robinson et al. demonstrated that passive transfer of purified immunoglobulin (Ig)G from Ro60- and La-positive pSS patients into non-obese diabetic (NOD)/Igμnull mice functionally decreased salivary flow 8. To answer the question as to whether antibodies binding Ro274 are associated pathologically with induction and progression of SS-like disease, we transferred adoptively Ro274-specific or control IgG antibodies into naive BALB/c mice and evaluated histological and functional parameters 4 days post-transfer. With this work, we demonstrate that Ro274-specific antibodies deposit in salivary glands, enter into salivary gland cells and lead to a functional reduction in salivary flow. Thus, antibodies against Ro274 can induce features of SS-like disease.
Materials and methods
Mice
BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, MA, USA) and used between 6 and 10 weeks of age. Animals were housed in the Laboratory Animal Resource Center of the Oklahoma Medical Research Foundation (OMRF) with a 12-h light/dark cycle. Mice were fed standard γ-irradiated mouse chow (Picolab Rodent Diet 20; LabDiet, St Louis, MO, USA) and water ad libitum. All experimental designs were approved by the OMRF Institutional Animal Care and Use Committee according to established guidelines.
Ro274 peptide immunization and antibody titres by enzyme-linked immunosorbent assay (ELISA)
To obtain Ro274-specific antibodies, a cohort of five female BALB/c mice (6 weeks of age) were immunized as described previously by Scofield et al. 3,9 Briefly, mice were immunized intraperitoneally (i.p.) with either 50 μg of Ro273–289 (LQEMPLTALLRNLGKMT) peptide tagged with a terminal cysteine emulsified 1:1 in complete Freund's adjuvant (CFA) or saline in CFA. Subcutaneous boosting was performed with Ro274 peptide or saline in incomplete Freund's adjuvant (IFA) on days 14, 35, 51 and 63. Animals were bled from the retro-orbital sinus on days 21, 42, 58, 70, 84, 105, 126, 147, 168 and 262 and the sera were collected and stored at −20°C until Ro274 ELISA and affinity purification.
Individual mouse sera were screened at 1:100 in duplicate by a direct antigen ELISA for antibodies to Ro274 multiple antigenic peptide, as described previously, using a 1:5000 dilution of anti-mouse IgG–alkaline–phosphatase conjugate (Sigma-Aldrich Inc, St Louis, MO, USA) as the detection antibody 1,9–11. Individual samples were considered positive when optical density was greater than 2 standard deviations above the average optical density (OD) of all pre-immune serum samples.
Protein G purification and passive transfer of total IgG and Ro274-specific IgG antibodies
To purify total normal IgG, multiple sera samples from Freund's-alone immunized mice were pooled and clarified by centrifugation at 12 000 g. The supernatant was then removed, diluted 1:10 with phosphate-buffered saline (PBS) and passed over a protein-G affinity column pre-equilibrated with PBS. The column was washed with PBS until the flow-through had zero absorption at 280 nm. Total bound IgG was eluted with 0·1 M glycine at pH 2·8 and neutralized with 1 M Tris. Collected fractions having positive OD280 measurements were pooled and concentrated by buffer exchange using Centricon filters (Millipore, Inc., Billerica, MA, USA). Total IgG concentration was determined (1·4 OD280 = 1 mg/ml of IgG) and the purified samples were stored at 4°C until passive transfer into naive mice.
Ro274-specific antibodies were affinity-purified from clarified, PBS-diluted sera of Ro274 peptide-immunized BALB/c mice using a Ro274 multiple antigenic peptide (MAP)-coupled cyanogen bromide (CNBr)-activated sepharose column 10,12. Bound anti-Ro274 was eluted with 500 μl of 3 M sodium thiocyanate. Collected fractions having positive OD280 measurements were pooled and concentrated by buffer exchange dialysis overnight in PBS, after which samples were quantitated and stored as described above.
Six-week-old naive BALB/c mice (n = 5/group for anti-274 and 5/group total IgG) were transferred i.p. with 100 μg of total IgG or anti-Ro274 IgG antibodies suspended in PBS on day 0.
Saliva collection
Saliva from individual mice was collected on day 0 prior to and day 4 after transfer, as described previously with modifications 2,9. Briefly, animals were fasted overnight prior to saliva collection. Each animal was anaesthetized with an i.p. injection of 2·5% 2,2,2-tribromoethanol (Avertin; Sigma Chemical Co., St Louis, MO, USA) at 0·10 ml/g body weight followed by an i.p. injection of 50 μg of pilocarpine/100 g of body weight to stimulate saliva flow. Saliva was obtained from the oral cavity over a 10-min period, placed into a sterile 1·5 ml tube and the volume in microlitres was determined using a micropipette.
Histological evaluation of salivary glands
On day 4, salivary gland tissues were harvested from individual animals following saliva collection. A portion of the tissue was cryopreserved in 50:50 optimal cutting temperature : tissue freezing medium (Triangle Biomedical, Durham, NC, USA) for immunofluorescence staining, with the remainder fixed in 10% neutral-buffered formalin and paraffin-embedded for histological analysis. Paraffin-embedded samples were sectioned at 5 μm and stained with haematoxylin and eosin (H&E). Histological evaluations were performed on coded slides by an unbiased veterinary pathologist (T.R.S.) who examined eight sections of salivary gland tissue per animal.
Immunohistochemical staining for IgG deposition and localization in salivary glands
Cryopreserved salivary glands were sectioned at 5 μm and a representative slide from each animal was stained for IgG deposition using Alexa 546 donkey anti-mouse Fab2′ (Invitrogen-Molecular Probes, Carlsbad, CA, USA). Black-and-white images were captured with the Zeiss Axioplan 2i microscope equipped with an MR camera (Zeiss, Inc., Thornwood, NY, USA) using light microscopy at low (×20) and high (×40) power.
To determine IgG localization within the cells and tissues of the salivary glands, 10 μm deparaffinized sections were blocked with 4% immunohistochemistry (IHC)-grade bovine serum albumin (BSA) and stained with donkey anti-mouse IgG-Alexa 647 and wheatgerm agglutinin (WGA) for 1 h at room temperature (RT). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Serial images of salivary gland sections were acquired using a Zeiss LSM 510 confocal microscope (Zeiss).
Detection of anti-Ro60 antibodies in nuclear and cytosolic fractions of murine salivary gland
Salivary gland tissues were collected from BALB/c mice immunized with either Ro274 peptide or saline on days 1, 17, 25, 38, 45, 60 and 78. Salivary glands were homogenized in Tris-sucrose buffer [0·02 M Tris-HCl (pH 7·4) and 0·25 M sucrose] and were then centrifuged at 800 g for 15 min at 4°C. The supernatant was removed and centrifuged at 8000 g for 20 min to remove mitochondria and at 100 000 g for 1 h to remove organelle and microsome fractions, then retained as the cytosolic fraction for antibody testing by ELISA. The pellet containing the cell nuclei was washed twice with 5 ml PBS, centrifuged at 1000 g for 15 min and the remaining supernatant composing the nuclear fraction was collected. Nuclear and cytoplasmic fractions were analysed for anti-Ro274 antibodies by direct anti-Ro274 MAP peptide ELISA as described above. All samples were evaluated in duplicate and averaged.
Results
Immunization with Ro274 peptide produces prolonged high titre antibodies to Ro60 and Ro274 peptide
Immunization of BALB/c mice with Ro274 peptide rapidly induced high titre (ODs >1·0) production of anti-Ro and anti-Ro274 as early as day 16 that persisted throughout the immunization protocol (Fig. 1). These sera were used for purification of Ro274 antibodies for adoptive transfer. The pre-immune sera from Ro274-immunized mice and control animals immunized with Freund's adjuvant alone had no significant reactivity to Ro274-MAP or 60 kD Ro, as expected.
Figure 1.

Immunization with 60 kD Ro274–290 peptide promotes formation of anti-Ro274 antibodies in BALB/c mice. (a) Sera from individual BALB/c mice (n = 5/group) immunized with Ro273–289 peptide (▪) or saline (▴) were tested in duplicate by enzyme-linked immunosorbent assay (ELISA) for antibodies to a Ro273–289 MAP peptide, as described previously. Individual points on the graph represent data obtained from a single mouse. (b) The mean and standard deviation of anti-Ro274 antibody in sera of Ro274-immunized (black bar) compared to saline-immunized (grey bar) mice (P < 0·0001; Student's t-test).
Ro274-specific IgG antibodies deposit in salivary glands of naive BALB/c mice
In order to determine whether anti-Ro274 transferred passively into naive BALB/c mice deposited within the salivary glands 4 days post-transfer, we performed IHC on cryopreserved salivary gland sections. Significantly, five of five (100%) animals receiving anti-Ro274 demonstrated high levels of IgG deposition in the interstitium and serous acinar cells of the parotid salivary tissues, as represented in Fig. 2b (left), whereas those animals receiving total IgG purified from controls animals demonstrated only weak, punctate staining (Fig. 2b, right).
Figure 2.

Immunoglobulin (IgG) antibodies are detected in salivary glands of naive BALB/c mice following Ro274 IgG adoptive transfer. (a) Haematoxylin and eosin (H&E) paraffin sections of salivary tissue from mice 4 days after receiving adoptively transferred anti-Ro274 antibodies at ×20 and ×40 total magnification (top left and bottom left, respectively), and mice receiving control IgG at ×20 and ×40 total magnification (top right and bottom right, respectively). White bar represents 100 μm. (b) Ig deposition in salivary gland cryosections 4 days post-anti-Ro274 (left) and control (right) IgG transfer to naive BALB/c mice assessed by immunofluorescence staining at ×40 total magnification. White bar represents 100 μm.
Histological evaluation of salivary glands in mice receiving adoptively transferred anti-Ro274 IgG do not demonstrate abnormal pathology
To determine whether adoptive transfer of Ro274 IgG led to structural effects on the salivary glands, histological analysis was performed on salivary gland tissue 4 days post-transfer. All the animals receiving anti-Ro274 IgG (five of five) or total IgG (five of five) had normal salivary morphology, with no immune cell infiltration 4 days post-transfer (Fig. 2a, all panels).
Anti-Ro274 is detectable in cytosolic and nuclear fractions of salivary gland cells of Ro274 peptide-immunized mice
In order to determine whether Ro274 antibodies could enter cells and gain access to the nuclear and cytoplasmic compartments, we studied Ro274-immunized animals. Nuclear and cytoplasmic fractions of salivary gland cells were examined for the presence of anti-Ro274 antibodies from days 1 to 78, comparing the levels of antibodies found in the salivary glands to serum levels (Fig. 3a). As observed by ELISA, anti-Ro274 titres were increased significantly in serum samples collected on days 17 and 38 (day 17 OD450 = 1·057 and day 38 OD450 = 1·095) after boosting with Ro274 peptide in IFA on days 14 and 35. Responses peaked at day 38 (day 38 OD450 = 1·095), then declined consistently from days 60 and 78 (day 60 OD450 = 0·531 and day 78 OD450 = 0·272). Levels of cytosolic anti-Ro274 were increased significantly from day 17 to day 60 (day 17 OD450 = 0·190 versus day 60 OD450 = 1·294), then began to decline by day 78 (day 78 OD450 = 0·877). Nuclear concentrations of anti-Ro274 remained low until days 60 and 78 (day 45 OD450 = 0·005 versus day 60 OD450 = 0·101 and day 78 OD450 = 0·449), at which time they increased more than threefold. Levels of anti-Ro274 in Freund's-only immunized mice remained low in serum, nuclear and cytoplasmic compartments following repeated immunization (Fig. 3b), demonstrating the specificity of the response toward the immunogen. Significantly, the nuclear : cytoplasmic (N : C) ratio (Fig. 3c) increased from day 1 (day 1 OD450 = 0·014), peaking at day 78 (day 78 OD450 = 0·512).
Figure 3.

Anti-Ro274 antibodies are detectable in the serum and in the nucleus and cytoplasm of salivary gland acinar cells by enzyme-linked immunosorbent assay (ELISA). (a) Anti-Ro274 titres were determined for cytoplasmic and nuclear extracts of salivary gland acinar cells and serum by ELISA on days 1, 17, 38, 60 and 78 in mice immunized with Ro274–290 peptide. (b) Anti-Ro274 titres in cytoplasmic and nuclear extracts of salivary gland acinar cells and serum were determined for mice immunized with saline at days 17 and 86. (c) The nuclear : cytoplasmic anti-274 antibody ratio was assessed for both Ro274 peptide and saline-immunized mice.
Co-staining of salivary gland sections with donkey anti-mouse IgG-Alexa 647 and WGA to detect IgG antibodies and plasma membranes, respectively, confirmed that IgG could be detected within the nucleus (Fig. 4b) and cytoplasm (Fig. 4c) of salivary gland cells as well as being detected in the interstitium (Fig. 4c,d), particularly around vessels (Fig. 4d). No background staining for IgG was observed in the negative control sample stained only with DAPI and WGA, as expected (Fig. 4a). These data suggest that anti-Ro274 antibodies are probably being endocytosed from the serum into the cytoplasmic space and the nucleus of the acinar cells in the salivary gland, where they would be able to interact directly with Ro60 protein.
Figure 4.
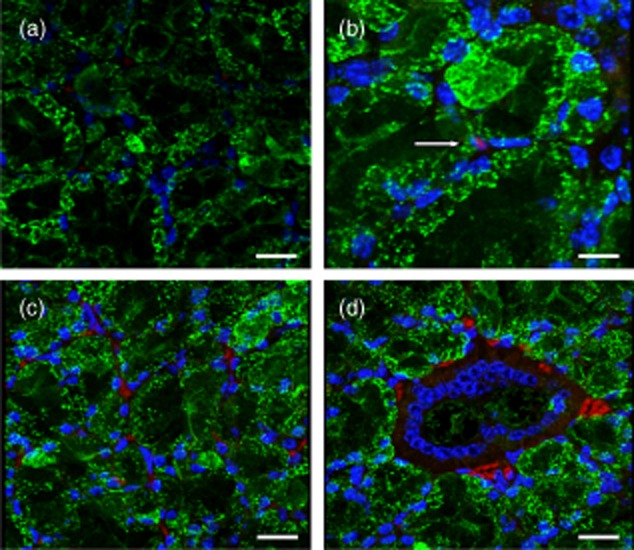
Immunoglobulin (IgG) antibodies are visible in the nucleus, cytoplasm and interstitium in salivary gland tissue of anti-Ro274 recipients. IgG antibodies were detected using donkey anti-mouse IgG-Alexa 647 (red). Cell membranes were delineated using wheat germ agglutinin (green) and cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). (a) Negative section of salivary gland (×400 total magnification). White bar represents 20 μm. (b) Intranuclear IgG staining (white arrow) observed in acinar cells (×630 total magnification). White bar represents 10 μm. (c,d) Interstitial and cytoplasmic IgG staining can be seen throughout the salivary gland tissue (×400 total magnification). White bar represents 20 μm.
Salivary flow is reduced in mice receiving adoptively transferred antibodies to Ro274
One of the primary signs of SS is decreased salivary flow, with the resulting symptom of dry mouth, although the specific order of initiating events leading to salivary dysfunction remains unknown. In order to test the hypothesis that Ro274 IgG antibodies are a significant factor in the mechanism(s) leading to decreased salivary flow in SS, we assessed timed, induced salivary flow in naive BALB/c mice receiving column-purified antibodies to Ro274 or control IgG both prior to and 4 days following the transfer. Median salivary flow (Fig. 5) was significantly lower in mice receiving purified anti-Ro274 IgG (7 μl; range: 2–65 μl; P = 0·0317, Mann–Whitney U-test; Fig. 5) compared to those mice receiving control IgG (34 μl; range: 2–49 μl; Fig. 5). Salivary flow was not significantly different between BALB/c mice assigned to any group prior to immunization (P = 1·0; Mann–Whitney U-test, data not shown). Importantly, the heterogeneity in salivary flow we observed among animals with a genetically identical background was consistent with the dynamic range that we described in our previous work 3.
Figure 5.

Median salivary flow is decreased in naive BALB/c mice receiving adoptively transferred anti-Ro274 antibodies. Median, minimum, maximum, 75th and 25th percentile salivary flow was assessed in naive BALB/c mice 4 days following adoptive transfer of control (left) immunoglobulin (IgG) antibodies or anti-Ro274 (right) antibodies (n = 5/group). Salivary flow is decreased significantly in naive BALB/c mice receiving anti-Ro274 antibodies compared to those receiving control IgG (*P = 0·0317, Mann–Whitney U-test).
These data are consistent with the hypothesis that antibodies with specificity to 60 kD Ro epitopes, particularly Ro274, are likely to be involved directly in the mechanisms leading to inhibition of salivary flow and glandular dysfunction.
Discussion
The role of antibodies binding Ro60 in the pathogenesis of SS and in the initiation of sialoadenitis has yet to be elucidated fully. In this study, we sought to determine if the adoptive transfer of affinity-purified antibodies to Ro274, a disease-promoting epitope of the Ro60 autoantigen, was sufficient to initiate pathogenesis and inhibit salivary flow in naive BALB/c mice. To accomplish this, we purified anti-Ro274 IgG from the sera of Ro274 peptide-immunized BALB/c mice with salivary gland pathology and reduced salivary flow, and transferred adoptively the IgG antibody fraction to naive mice. Timed, stimulated salivary flow was assessed, and salivary and lacrimal glands were analysed for cellular infiltration and IgG deposition. We demonstrated that adoptive transfer of anti-Ro274 alone into naive BALB/c mice leads mainly to IgG antibody deposition in salivary glands and decreased stimulated salivary flow. Significantly, the pattern of IgG staining we observed in the acini and secretory ducts, particularly of the parotid and submandibular salivary glands, correlated strongly with the localization of Ro60 in the acinar nucleoli and nucleoplasm observed in SS patients 12. Furthermore, we demonstrated that antibodies against Ro274 were not only taken up into the cytoplasm of salivary acinar cells but could also be detected in the nucleus, where these antibodies could interact with Ro60 protein. Finally, we were able to demonstrate that pilocarpine-induced salivary flow in naive mice receiving anti-Ro274 antibodies was decreased compared to mice receiving only control IgG. These data support the hypothesis that inhibition of salivary function in SS is initiated by soluble factors, including IgG antibodies, that are directed against epitopes of Ro60.
We have observed a subgroup of patients who met the American European Consensus Group (AECG) for primary pSS classification criteria who have antibodies to Ro60/SSA, exhibit decreased tear and saliva production and have a normal labial biopsy (unpublished data). Importantly, these patients also appear to lack antibodies to muscarinic receptor. Although direct pathological effects have been linked to anti-Ro antibodies in SLE, including skin disease, renal manifestations and complete congenital heart block 2,13, such a correlation has not been described in pSS. This would suggest strongly that antibodies to Ro60 may be involved mechanistically in the exocrine dysfunction observed in pSS.
There are data in the literature suggesting that antibodies to Ro60 can interact directly with calcium channels, particularly of the L-type, that are functional in exocrine tissues. Furthermore, chronic association of Ro60 autoantibodies with these channels can lead ultimately to down-regulation and impairment of channel function. These data lend credence to the hypothesis that these antibodies have a significant role in the pathological progression of Sjögren's-type diseases in exocrine tissues.
Given these observations, it is likely that inflammatory leucocytic infiltration of the salivary glands in SS may be a secondary consequence of an antibody-initiated process. Nonetheless, many SS patients with severely dry mouth have salivary glands that are histologically normal, appearing outside areas of lymphocytic infiltrations; that is, the salivary glands are not destroyed but simply not functioning. Furthermore, disease manifestations in experimental SS can occur independently and often associate with adaptive immune processes that strongly promote disease development, as described by Delaleu and colleagues 14,15.
Other animal models have demonstrated support for this hypothesis. Robinson et al. 8 showed that adoptive transfer of IgG from the sera of SS patients or diseased NOD mice into naive mice was capable of impairing salivary flow, indicating that antibodies played a direct role in the exterpation of salivary function. Furthermore, data obtained in the NOD.B10-H2b mouse showed that salivary flow can remain wholly unaffected even when glands are drastically infiltrated with leucocytes. Significantly, this hypothesis was confirmed when salivary flow was restored in NOD/Igμnull mice that had received IgG antibodies from SS patients or NOD mice after a 1-week washout period, validating the concept that salivary dysfunction was mediated by a soluble factor that could be removed from the system, namely IgG 8. Nguyen et al. 16 demonstrated this further by adoptive transfer of purified autoaantibodies with specificity to M3R, La/SS-B, rat M3 receptor and a parotid secretory protein, in which anti-M3R were capable of inducing salivary flow inhibition to NOD-scid mice. They determined that over-stimulation of the receptor led to receptor down-regulation and subsequently prevented translocation of aquaporin to the apical membrane of acinar cells 16.
Because many autoantigens are intracellular, entry of autoantibodies inside subcellular compartments may be a mechanism of damage to tissues. Alarcon-Segovia et al. 17 first reported the penetration of anti-nuclear ribonucleoprotein (anti-RNP) and anti-DNA into live peripheral blood cells and the entrance was thought to be mediated by Fc receptor 18. Mouse monoclonal anti-dsDNA antibodies have been shown to penetrate cells in vivo and in vitro into culture cells 19,20. A variety of autoimmune antibodies with different antigen specificities have been demonstrated to penetrate live cells. Indeed, IgG immune complexes have been demonstrated to interact with plasma membrane Fc receptors and become endocytosed 21. Entry of human IgG into human intestine is thought to be mediated through the FcRn receptor, independently of clathrin- and caveolin-endocytosis, with macropinocytosis partly involved in the process 22.
Antibodies can also gain entry into cells independently of the Fc receptor. Mouse anti-Sm and anti-La monoclonal antibodies have also been shown to enter different kinds of live cell types from various animal species 23, independently of the Fc receptor. Koscec et al. 24 showed that autoantibodies to ribosomal P proteins gain entry into live human hepatoma cells. This entry is thought to be probably mediated by the binding of the antibody to the human ribosomal phosphoprotein P0 antigen present on the cell surface 25, as Fab fragments of anti-P also gained entry into the cell in a similar time–course to intact IgG. Koscec et al. also found 24, by confocal analysis, that most of internalized anti-P localized within a vesicular compartment and a smaller proportion of autoantibodies localized in the cytoplasm. Other investigators have also shown Fc receptor independent (Fab-mediated) entry of autoantibodies into live cells 26–28.
In this study, we have determined that salivary flow inhibition is not due necessarily to the parasympathetic interference mediated by autoantibodies with anti-MR3 activity alone. We have shown that adoptive transfer of antibodies with specificity to Ro274 can induce functional inhibition of salivary flow. Although the mechanism leading to functional loss of salivary flow is not understood fully, a number of hypotheses could account for how the anti-Ro274 antibodies could be mediating a reduction in salivary flow. Cross-reactivity between the Ro274 epitope and another similar epitope may occur on the membrane of inflammatory or epithelial cells. In an earlier study we found significant neutropenia in 72 SLE patients with anti-Ro60 autoantibodies. These autoantibodies were found to bind to a 64 kD protein D1 on neutrophils, and this is thought to bring about neutropenia in SLE patients 29. In addition, anti-Ro274 could bind Ro60 in apoptotic blebs leading to antigen–antibody complexes that fix complement and induce an inflammatory environment in the salivary glands. Salivary and lacrimal epithelial cells are known to up-regulate the expression of major histocompatibility complex (MHC) class II molecules, which could be presenting Ro peptides obtained during apoptotic breakdown within acinar cell compartments, and to promote epitope spreading 30. Induction of inflammatory molecules such as tumour necrosis factor (TNF)-α and interferon (IFN)-γ can induce the up-regulation of Ro60 and its translocation to the cell membrane, making it an available target for autoantibody binding 31. Furthermore, these effects can lead to the trafficking of immune leucocytes to the glands, leading to further downstream effects.
Salivary flow depends upon the administered levels of pilocarpine. In this study, we have used control mice of similar age and sex in the salivary flow experiment that we carried out. However, the saliva collection methodology is not as simple as stated in all the published papers. There is a large variability in saliva flow between animals, such that we needed to use non-parametric statistics to analyse the data obtained.
Finally, we have demonstrated with this work that antibodies binding Ro274 are capable of transferring functional disease to naive mice, confirming that antibodies to self-antigens can induce autoimmune sialoadenitis independently of cellular infiltration in our mouse model. The mechanism by which these antibodies trigger the pathological cascade inducing a functional block in salivary flow in naive mice is currently under study.
Acknowledgments
This work was supported by grant funding from the National Institutes of Health-National Institute for Dental and Craniofacial Research (R01-DE17564-01). The authors would like to thank Drs Christopher Lessard and Kathy Sivils for providing clinical data from the OMRF-SSRC, Corey Coles and Ali Khalili for their technical assistance and Mollie Rangnow for her administrative assistance.
Disclosure
RHS has been a consultant concerning Sjogren's syndrome to Lily and UCB. The other authors declare they have nothing to disclose.
Author contributions
J. S. M., B. K., A. D., R. J. and R. H. S. participated in the conception and/or design of the study. J. S. M., B. K., A. D., L. B., S. A., Y. D., S. H., O. Y. and D. O. participated in the collection and/or preparation of saliva and tissue. S. H., J. S. M. and O. Y. performed the immunohistochemistry and fluorescence microsopy experiments. L. B. performed the cytoplasmic and nuclear antibody study. J. S. M., B. K. and R. H. S. prepared the manuscript.
References
- Scofield RH, Henry WE, Kurien BT, James JA, Harley JB. Immunization with short peptides from the sequence of the systemic lupus erythematosus-associated 60-kDa Ro autoantigen results in anti-Ro ribonucleoprotein autoimmunity. J Immunol. 1996;156:4059–4066. [PubMed] [Google Scholar]
- Scofield RH, Kaufman KM, Baber U, James JA, Harley JB, Kurien BT. Immunization of mice with human 60-kd Ro peptides results in epitope spreading if the peptides are highly homologous between human and mouse. Arthritis Rheum. 1999;42:1017–1024. doi: 10.1002/1529-0131(199905)42:5<1017::AID-ANR22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Scofield RH, Asfa S, Obeso D, Jonsson R, Kurien BT. Immunization with short peptides from the 60-kDa Ro antigen recapitulates the serological and pathological findings as well as the salivary gland dysfunction of Sjogren's syndrome. J Immunol. 2005;175:8409–8414. doi: 10.4049/jimmunol.175.12.8409. [DOI] [PubMed] [Google Scholar]
- Baiu DC, Barger B, Sandor M, Fabry Z, Hart MN. Autoantibodies to vascular smooth muscle are pathogenic for vasculitis. Am J Pathol. 2005;166:1851–1860. doi: 10.1016/S0002-9440(10)62494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J, Albouainain A, Duda MA, Evans DJ, Pusey CD. Strain susceptibility to active induction and passive transfer of experimental autoimmune glomerulonephritis in the rat. Nephrol Dial Transplant. 2006;21:3398–3408. doi: 10.1093/ndt/gfl523. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Tsunoda K, Hata T, Ishii K, Yamada T, Amagai M. Synergistic pathogenic effects of combined mouse monoclonal anti-desmoglein 3 IgG antibodies on pemphigus vulgaris blister formation. J Invest Dermatol. 2006;126:2621–2630. doi: 10.1038/sj.jid.5700450. [DOI] [PubMed] [Google Scholar]
- Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–8240. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- Robinson CP, Brayer J, Yamachika S, et al. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren's syndrome. Proc Natl Acad Sci USA. 1998;95:7538–7543. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurien BT, Dsouza A, Igoe A, et al. Immunization with 60 kD Ro peptide produces different stages of preclinical autoimmunity in a Sjögren's syndrome model among multiple strains of inbred mice. Clin Exp Immunol. 2013;173:67–75. doi: 10.1111/cei.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield RH, Kurien BT, Zhang F, et al. Protein–protein interaction of the Ro-ribonucleoprotein particle using multiple antigenic peptides. Mol Immunol. 1999;36:1093–1106. doi: 10.1016/s0161-5890(99)00095-4. [DOI] [PubMed] [Google Scholar]
- Kurien BT, Jackson K, Scofield RH. Immunoblotting of multiple antigenic peptides. Electrophoresis. 1998;19:1659–1661. doi: 10.1002/elps.1150191023. [DOI] [PubMed] [Google Scholar]
- de Wilde PC, Kater L, Bodeutsch C, et al. Aberrant expression pattern of the SS-B/La antigen in the labial salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 1996;39:783–791. doi: 10.1002/art.1780390510. [DOI] [PubMed] [Google Scholar]
- Salomonsson S, Sonesson SE, Ottosson L, et al. Ro/SSA autoantibodies directly bind cardiomyocytes, disturb calcium homeostasis, and mediate congenital heart block. J Exp Med. 2005;201:11–17. doi: 10.1084/jem.20041859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaleu N, Immervoll H, Cornelius J, Jonsson R. Biomarker profiles in serum and saliva of experimental Sjogren's syndrome: associations with specific autoimmune manifestations. Arthritis Res Ther. 2008;10:R22. doi: 10.1186/ar2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaleu N, Madureira AC, Immervoll H, Jonsson R. Inhibition of experimental Sjogren's syndrome through immunization with HSP60 and its peptide amino acids 437–460. Arthritis Rheum. 2008;58:2318–2328. doi: 10.1002/art.23656. [DOI] [PubMed] [Google Scholar]
- Nguyen KH, Brayer J, Cha S, et al. Humphreys-Beher, evidence for antimuscarinic acetylcholine receptor antibody-mediated secretory dysfunction in NOD mice. Arthritis Rheum. 2000;43:2297–2306. doi: 10.1002/1529-0131(200010)43:10<2297::AID-ANR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Alarcon-Segovia D, Ruiz-Arguelles A, Fishbein E. Antibody to nuclear ribonucleoprotein penetrates live human mononuclear cells through Fc receptors. Nature. 1978;271:67–69. doi: 10.1038/271067a0. [DOI] [PubMed] [Google Scholar]
- Alarcon-Segovia D, Llorente L. Antibody penetration into living cells. IV. Different effects of anti-native DNA and anti-ribonucleoprotein IgG on the cell cycle of activated T gamma cells. Clin Exp Immunol. 1983;52:365–371. [PMC free article] [PubMed] [Google Scholar]
- Vlahakos D, Foster MH, Ucci AA, Barrett KJ, Datta SK, Madaio MP. Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol. 1992;2:1345–1354. doi: 10.1681/ASN.V281345. [DOI] [PubMed] [Google Scholar]
- Yanase K, Smith RM, Cizman B, et al. A subgroup of murine monoclonal antideoxyribonucleic acid antibodies traverse the cytoplasm and enter the nucleus in a time- and temperature-dependent manner. Lab Invest. 1994;71:52–60. [PubMed] [Google Scholar]
- Anderson CL. Human IgG Fc receptors. Clin Immunol Immunopathol. 1989;53:S63–71. doi: 10.1016/0090-1229(89)90071-8. [DOI] [PubMed] [Google Scholar]
- Sato K, Nagai J, Mitsui N, Ryoko Y, Takano M. Effects of endocytosis inhibitors on internalization of human IgG by Caco-2 human intestinal epithelial cells. Life Sci. 2009;85:800–807. doi: 10.1016/j.lfs.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Deng SX, 1, Hanson E, Sanz I. In vivo cell penetration and intracellular transport of anti-Sm and anti-La autoantibodies. Int Immunol. 2000;12:415–423. doi: 10.1093/intimm/12.4.415. [DOI] [PubMed] [Google Scholar]
- Koscec M, Koren E, Wolfson-Reichlin M, et al. Autoantibodies to ribosomal P proteins penetrate into live hepatocytes and cause cellular dysfunction in culture. J Immunol. 1997;159:2033–2041. [PubMed] [Google Scholar]
- Koren E, Reichlin MW, Koscec M, Fugate RD, Reichlin M. Autoantibodies to the ribosomal P proteins react with a plasma membrane-related target on human cells. J Clin Invest. 1992;89:1236–1241. doi: 10.1172/JCI115707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Chapman GV, Chen SL, Penny R, Breit SN. Flow cytometry with crystal violet to detect intracytoplasmic fluorescence in viable human lymphocytes:demonstration of antibody entering living cells. J Immunol Methods. 1987;104:195–200. doi: 10.1016/0022-1759(87)90504-7. [DOI] [PubMed] [Google Scholar]
- Ma J, Chapman GV, Chen SL, Melick G, Penny R, Breit SN. Antibody penetration of viable human cells. I. Increased penetration of human lymphocytes by anti-RNP IgG. Clin Exp Immunol. 1991;84:83–91. doi: 10.1111/j.1365-2249.1991.tb08128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan TD, Gharavi AE, Elkon KB. Penetration of autoantibodies into living epithelial cells. J Invest Dermatol. 1993;100:316–322. doi: 10.1111/1523-1747.ep12469994. [DOI] [PubMed] [Google Scholar]
- Kurien BT, Newland J, Paczkowski C, Moore KL, Scofield RH. Association of neutropenia in systemic lupus erythematosus (SLE) with anti-Ro and binding of an immunologically cross-reactive neutrophil membrane antigen. Clin Exp Immunol. 2000;120:209–217. doi: 10.1046/j.1365-2249.2000.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kawakami A, Eguchi K. Mechanisms of autoantibody production and the relationship between autoantibodies and the clinical manifestations in Sjogren's syndrome. Transl Res. 2006;148:281–288. doi: 10.1016/j.trsl.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Dorner T, Hucko M, Mayet WJ, Trefzer U, Burmester GR, Hiepe F. Enhanced membrane expression of the 52 kDa Ro(SS-A) and La(SS-B) antigens by human keratinocytes induced by TNF alpha. Ann Rheum Dis. 1995;54:904–909. doi: 10.1136/ard.54.11.904. [DOI] [PMC free article] [PubMed] [Google Scholar]


