Abstract
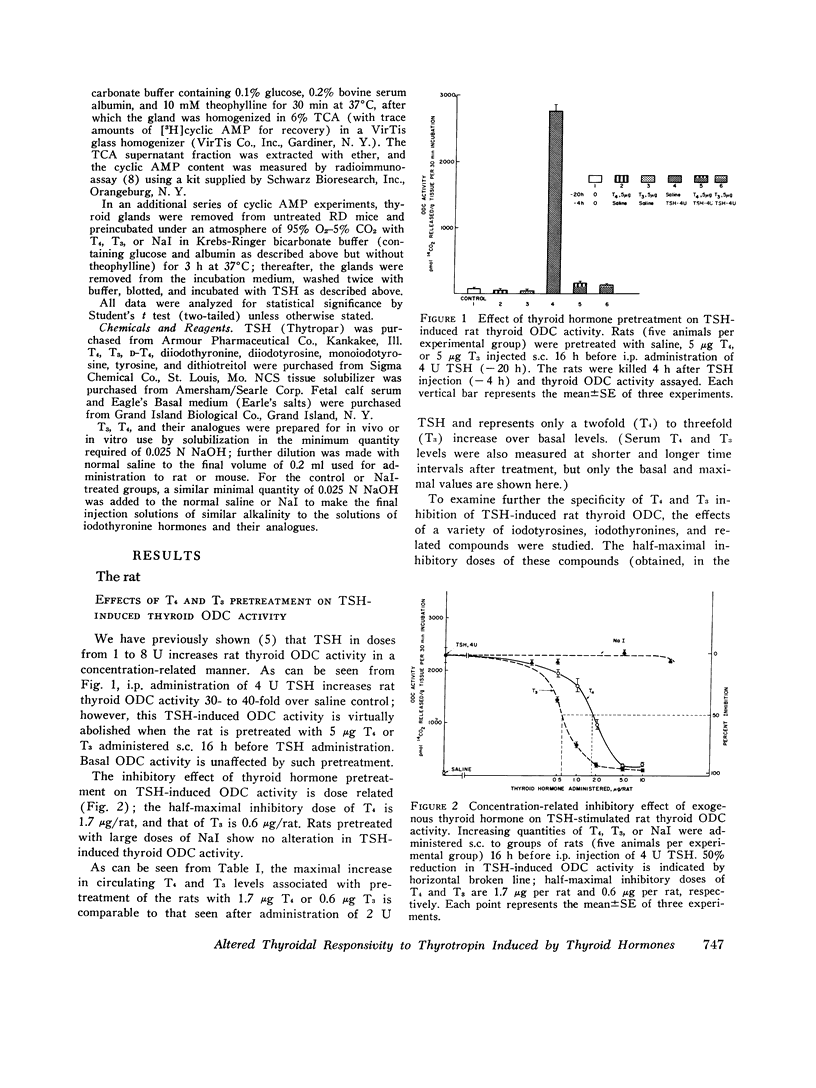
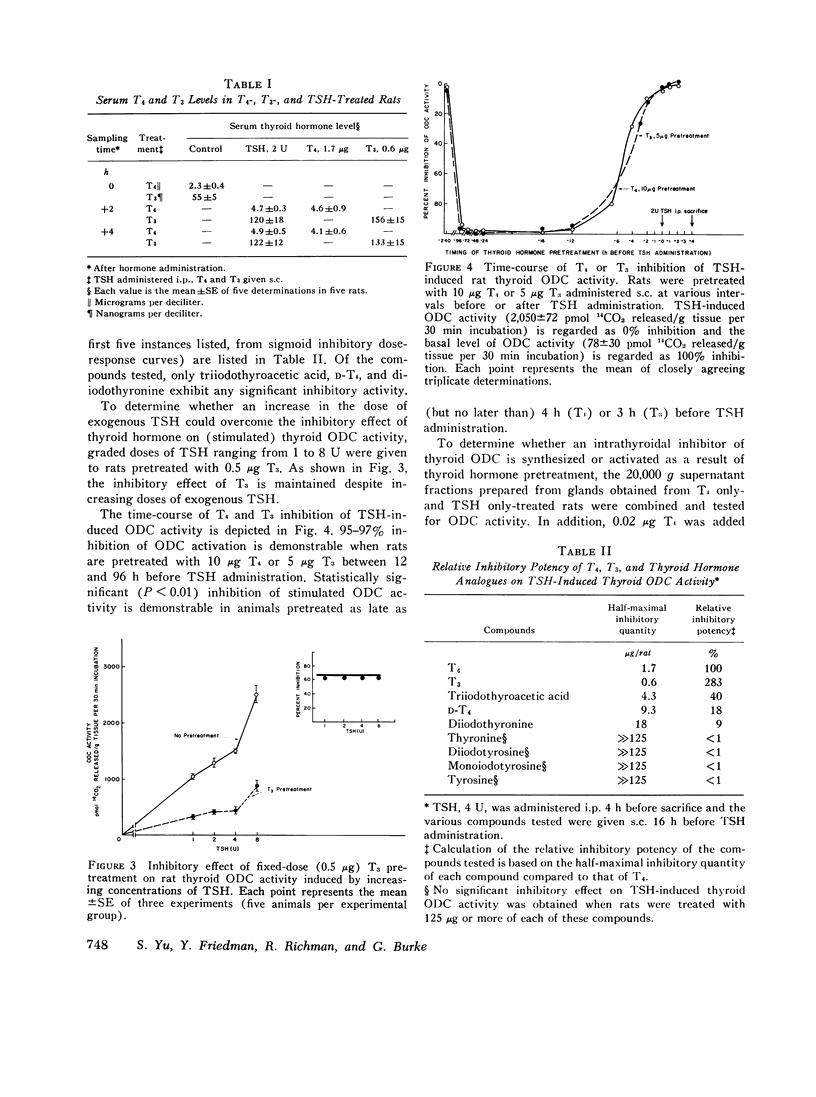
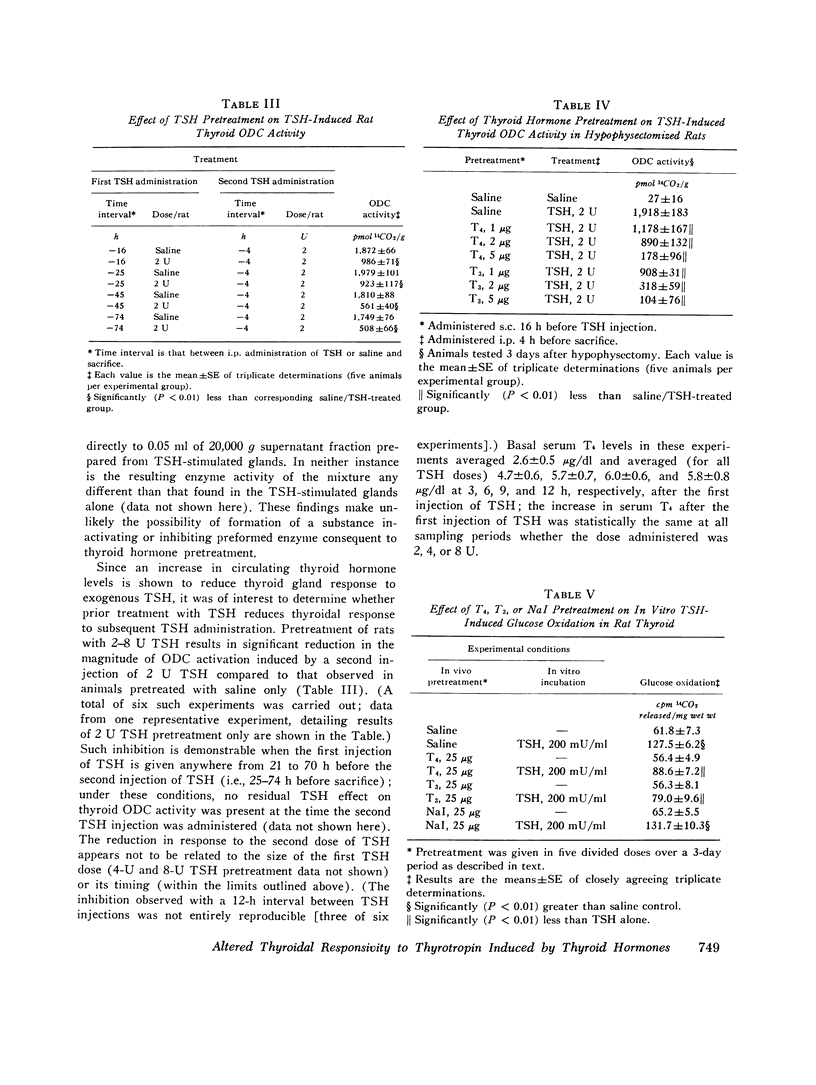
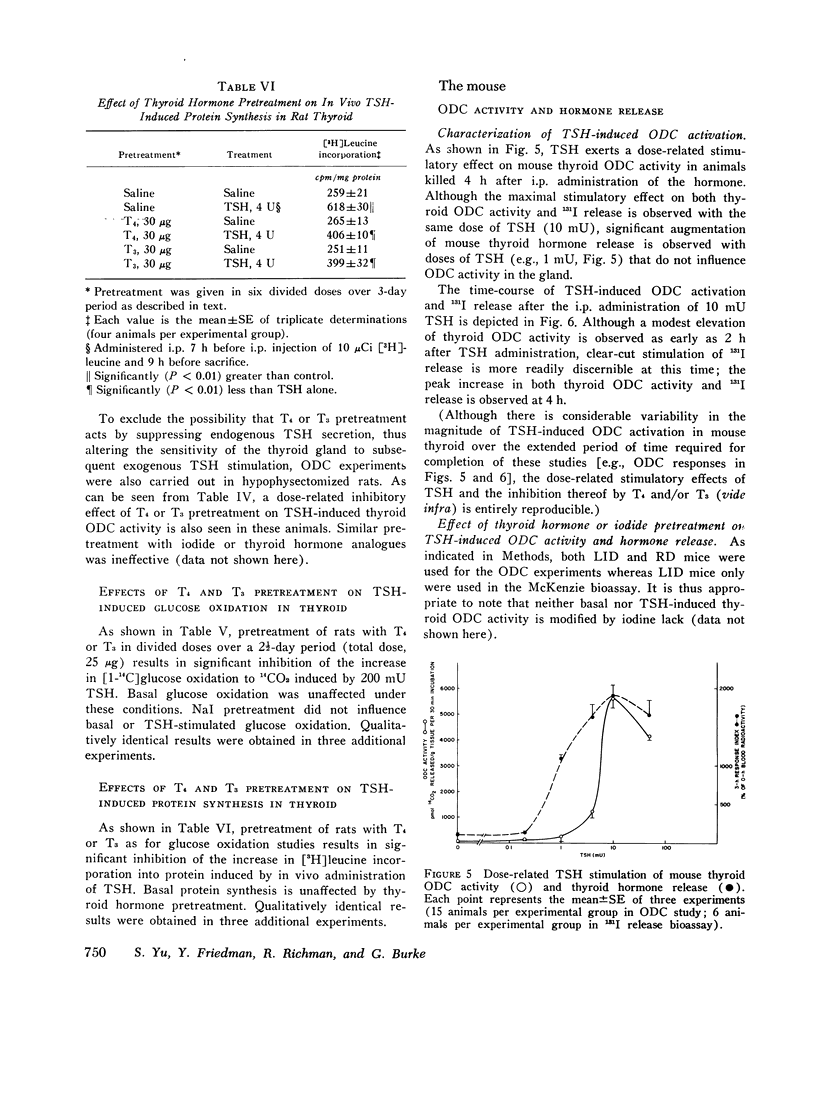
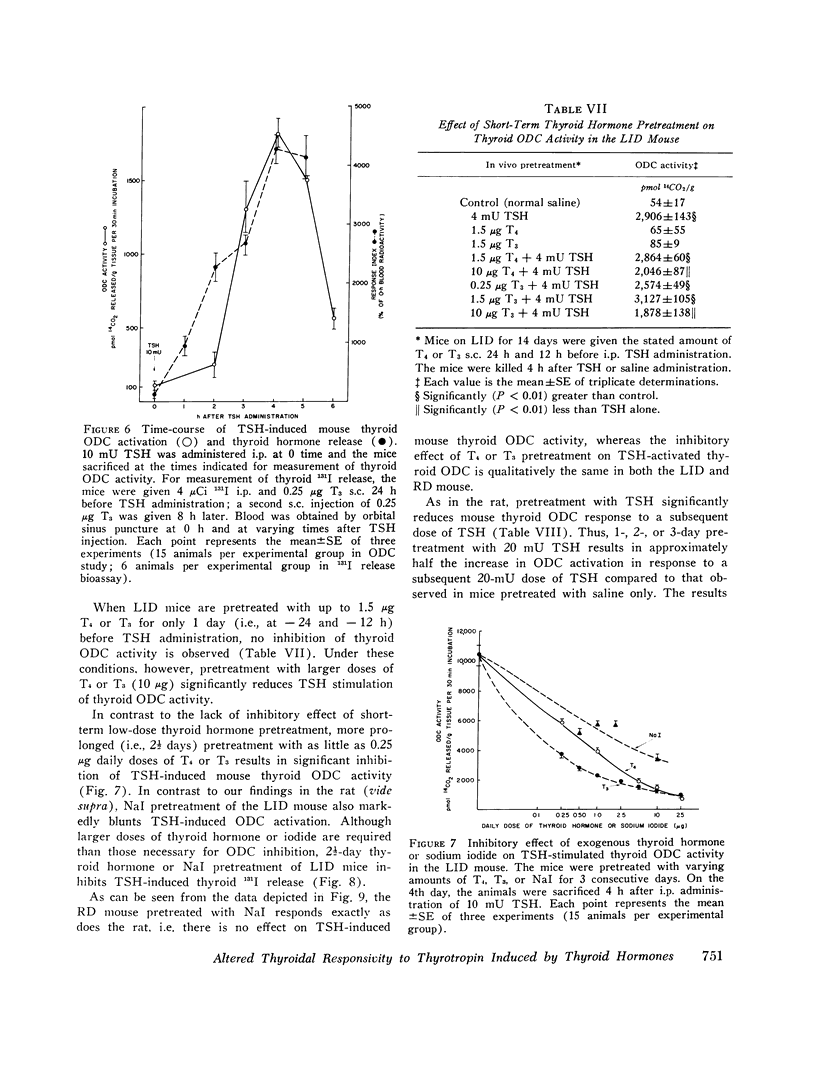
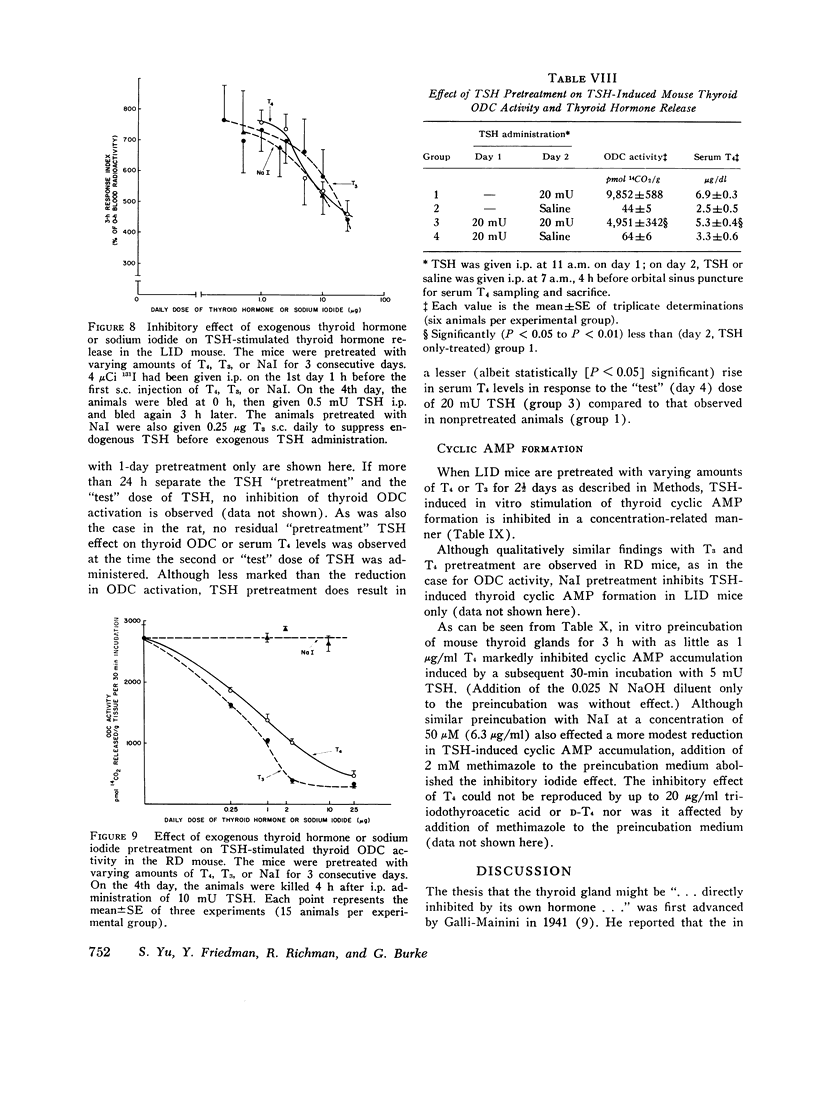
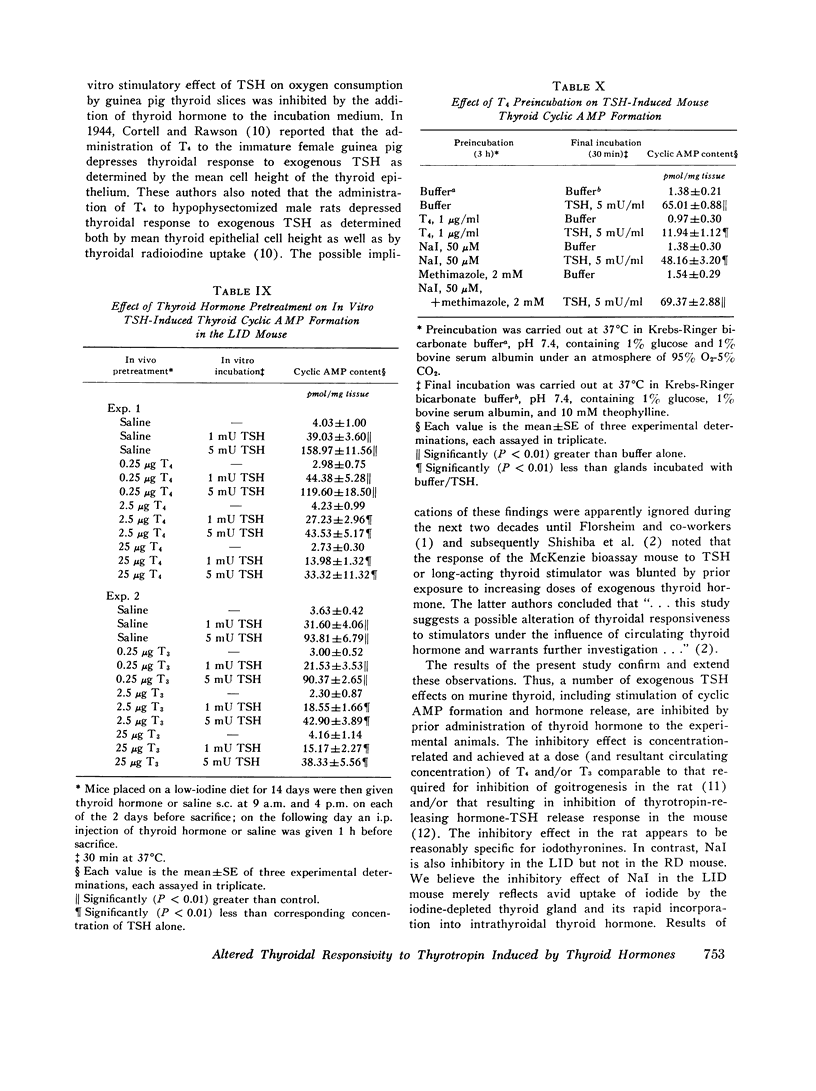
We studied the effect of thyroid hormone administration on responsivity of murine thyroid to exogenous thyrotropin (TSH) in order to explore the possibility that the thyroid gland might be directly inhibited by its own hormones. In the rat both L-thyroxine (T4) and 3,5,3'-L-triiodothyronine (T3) pretreatment inhibited TSH-induced thyroidal ornithine decarboxylase (ODC) activity in vivo in a dose-related manner (half-maximal inhibition, 1.7 mug/rat and 0.6 mug/rat, respectively). Other structurally related compounds exhibited the following inhibitory potencies compared to T4: T3, 283%; triiodothyroacetic acid, 40%; D-T4, 18%; 3,5-L-diiodothyronine, 9%. Monoiodotyrosine, diiodotyrosine, and iodide were not inhibitory. The full inhibitory effect of T4 or T3 was observed when thyroid hormone was administered from 96 to 12 h before TSH and was also seen in hypophysectomized animals. Pretreatment with T4 or T3 in divided doses over 2 1/2 days inhibited TSH-induced increase in [1-14C]glucose oxidation to 14C02 and [3H] leucine incorporation into protein in rat thyroid. In the mouse T4 or T3 pretreatment (0.25-25 mug daily) caused dose-related inhibition of both thyroidal ODC activity and 131I release induced by TSH in vivo. In mice on a low-iodine diet (LID) but not in animals on a regular diet (RD) NaI pretreatment also blunted TSH-induced thyroidal ODC activation and 131I release. When LID or RD mice were pretreated with 12.5-125 mug of T4 or T3 over 2 1/2 days, TSH-induced in vitro stimulation of thyroid cyclic 3',5'-adenosine monophosphate formation was inhibited in a dose-related manner; NaI pretreatment was inhibitory in the LID mouse only. Prior administration of exogenous TSH blunted the activation of thyroid ODC and thyroid hormone release induced by subsequent TSH administration in rat and mouse. These studies indicate altered thyroid responsivity to TSH under the influence of circulating thyroid hormones and suggest the existence of a "short-loop" negative feedback regulating thyroid function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastomsky C. H., Zakarija M., McKenzie J. M. Thyroid hydrolysis of cyclic AMP as influenced by thyroid gland activity. Biochim Biophys Acta. 1971 Feb 23;230(2):286–295. doi: 10.1016/0304-4165(71)90215-7. [DOI] [PubMed] [Google Scholar]
- Bowers C. Y., Schally A. V., Reynolds G. A., Hawley W. D. Interactions of L-thyroxine or L-triiodothyronine and thyrotropin-releasing factor on the release and synthesis of thyrotropin from the anterior pituitary gland of mice. Endocrinology. 1967 Oct;81(4):741–747. doi: 10.1210/endo-81-4-741. [DOI] [PubMed] [Google Scholar]
- Florsheim W. H., Williams A. D., Schönbaum E. On the mechanism of the McKenzie bioassay. Endocrinology. 1970 Nov;87(5):881–888. doi: 10.1210/endo-87-5-881. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuzaki S., Suzuki M. Thyroid function and polyamines. I. Rapid fluctuation of thyroid ornithine decarboxylase activity in response to change in circulating thyrotropin level in the rat. Endocrinol Jpn. 1974 Dec;21(6):529–537. doi: 10.1507/endocrj1954.21.529. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H. Possible clues in the continuing search for the subcellular basis of thyroid hormone action. Mt Sinai J Med. 1973 May-Jun;40(3):491–501. [PubMed] [Google Scholar]
- Richman R., Park S., Akbar M., Yu S., Burke G. Regulation of thyroid ornithine ornithine decarboxylase (ODC) by thyrotropin. I. The rat. Endocrinology. 1975 Jun;96(6):1403–1412. doi: 10.1210/endo-96-6-1403. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Shishiba Y. Effect of triiodothyronine or iodide on the thyroidal secretion in vitro: Inhibition of TSH- and dibutyryl-cyclic-AMP induced endocytosis. Endocrinol Jpn. 1975 Feb;22(1):55–60. doi: 10.1507/endocrj1954.22.55. [DOI] [PubMed] [Google Scholar]
- Shishiba Y., Yoshimura S., Shimizu T. Effect of dose of thyroid hormone on the sensitivity of the McKenzie bioassay. Endocrinology. 1974 Sep;95(3):922–925. doi: 10.1210/endo-95-3-922. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATA J. R. Inhibition of the biological action of thyroid hormones by actinomycin D and puromycin. Nature. 1963 Mar 23;197:1167–1168. doi: 10.1038/1971167a0. [DOI] [PubMed] [Google Scholar]
- TONG W. IN VITRO EFFECTS OF THYROTROPIN ON CERTAIN METABOLIC ACTIVITIES OF ISOLATED THYROID CELLS. Endocrinology. 1964 Oct;75:527–536. doi: 10.1210/endo-75-4-527. [DOI] [PubMed] [Google Scholar]
- Takasu N., Sato S., Tsukui T., Yamada T., Furihata R. Inhibitory action of thyroid hormone of the activation of adenyl cyclase-cyclic AMP system by thyroid-stimulating hormone in human thyroid tissues from euthyroid subjects and thyrotoxic patients. J Clin Endocrinol Metab. 1974 Oct;39(4):772–778. doi: 10.1210/jcem-39-4-772. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sande J., Grenier G., Willems C., Dumont J. E. Inhibition by iodide of the activation of the thyroid cyclic 3',5'-AMP system. Endocrinology. 1975 Mar;96(3):781–786. doi: 10.1210/endo-96-3-781. [DOI] [PubMed] [Google Scholar]
- Wilber J. F., Utiger R. D. Immunoassay studies of thyrotropin in rat pituitary glands and serum. Endocrinology. 1967 Jul;81(1):145–151. doi: 10.1210/endo-81-1-145. [DOI] [PubMed] [Google Scholar]
- Yamada T., Lewis A. E. An essential role of thyroxine and triiodothyronine balance in establishing normal pituitary-thyroid feedback control in goitrogen-treated rats. Endocrinology. 1968 Jan;82(1):91–99. doi: 10.1210/endo-82-1-91. [DOI] [PubMed] [Google Scholar]



