Abstract
Reparixin, a CXCR 1/2 antagonist, has been shown to mitigate ischaemia–reperfusion injury (IRI) in various organ systems in animals, but data in humans are scarce. The aim of this double-blinded, placebo-controlled pilot study was to evaluate the safety and efficacy of reparixin to suppress IRI and inflammation in patients undergoing on-pump coronary artery bypass grafting (CABG). Patients received either reparixin or placebo (n = 16 in each group) after induction of anaesthesia until 8 h after cardiopulmonary bypass (CPB). We compared markers of systemic and pulmonary inflammation, surrogates of myocardial IRI and clinical outcomes using Mann–Whitney U- and Fisher's exact tests. Thirty- and 90-day mortality was 0% in both groups. No side effects were observed in the treatment group. Surgical revision, pleural and pericardial effusion, infection and atrial fibrillation rates were not different between groups. Reparixin significantly reduced the proportion of neutrophil granulocytes in blood at the beginning [49%, interquartile range (IQR) = 45–57 versus 58%, IQR = 53–66, P = 0·035], end (71%, IQR = 67–76 versus 79%, IQR = 71–83, P = 0·023) and 1 h after CPB (73%, IQR = 71–75 versus 77%, IQR = 72–80, P = 0·035). Reparixin patients required a lesser positive fluid balance during surgery (2575 ml, IQR = 2027–3080 versus 3200 ml, IQR = 2928–3778, P = 0·029) and during ICU stay (2603 ml, IQR = 1023–4288 versus 4200 ml, IQR = 2313–8160, P = 0·021). Numerically, more control patients required noradrenaline ≥ 0·11 μg/kg/min (50 versus 19%, P = 0·063) and dobutamine (50 versus 25%, P = 0·14). Therefore, administration of reparixin in CABG patients appears to be feasible and safe. It concurrently attenuated postoperative granulocytosis in peripheral blood.
Keywords: cardiopulmonary bypass, CXCL8, CXCR1/2, postoperative inflammation, Phase II study
Introduction
Chemokines are chemotactic cytokines that cause directed migration of leucocytes, which are induced by inflammatory cytokines, growth factors and pathogenic stimuli 1. Chemokines are small, soluble peptides and interact with cells through specific chemokine receptors. In addition to chemotaxis, chemokines can activate integrins that mediate leucocyte adherence on endothelial cells. The diversity of chemokines and their specific receptors enable selective trafficking of different immune cells under normal and inflammatory conditions 2,3. The biological impact of chemokines is mediated by chemokine receptors such as class A G-protein coupled receptors (GPCRs) coupled with the Gαi class of heterotrimeric G proteins. As of today, 18 chemokine receptors with standard Gαi-dependent chemotactic activity have been identified in humans and mice. Five atypical (non-chemotactic, recycling or scavenging) chemokine receptors have also been described 4. CXCR1 and CXCR2 are the major chemokine receptors of neutrophils 5–7 and are also expressed on T lymphocytes and natural killer (NK) cells 8,9. The CXCR1 and CXCR2 receptors share 78% of their amino acid sequence and are activated by glutamic acid–leucine–arginine (ELR)+ chemokines, which contain the glutamic acid-leucine-arginine sequence 3. The latter are potent neutrophil chemoattractants and activators and can induce neutrophil mobilization from the bone marrow into the circulation 10. The CXCR1 chemokines are CXCL6, CXCL7, CXCL8 and acPGP (N-acetyl Pro-Gly-Pro), whereas ligands of the more ‘promiscuous’ CXCR2 are CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, CXCL8, acPGP and macrophage migration inhibitory factor (MIF) 4. Among the chemotactic factors generated at the site of inflammation, CXCL8 is considered to be the crucial mediator of leucocyte recruitment and activation 11,12, identified originally as neutrophil chemoattractant 13,14. Therefore, drug-induced modulation of CXCR1 and CXCR2 function is thought to be useful for the treatment of different inflammatory conditions in humans 12. Starting from the detection of a non-cyclooxygenase-dependent inhibitory effect of the known non-steroidal anti-inflammatory drugs ketoprofen and ibuprofen on CXCL8-induced granulocyte chemotaxis, a class of 2-arylphenylpropionic acid derivatives was developed, leading to the selection of acylmethanesulphonamide reparixin 12. Reparixin is a non-competitive allosteric blocker of the chemokine receptors CXCR1 and CXCR2 with higher efficacy in inhibiting CXCR1 activity than CXCR2 15. Reparixin was recently tested successfully in a Phase II study in patients with type 1 diabetes undergoing pancreatic islet transplantation. The authors found improved outcomes as measured by glycaemic control, decreased insulin requirement and appearance of detectable levels of C-peptide after 1 week of intravenous reparixin compared to standard care 16. In addition, a variety of animal studies suggest that reparixin attenuates ischaemia–reperfusion injury (IRI) in various organ systems 17–19, inhibits inflammatory responses in intestinal IRI 19 and prevents deterioration of kidney graft function due to IRI in rats 18.
Cardiac surgery provokes a marked inflammatory response by either direct ‘contact’ activation of the immune system following exposure of blood to surfaces of the cardiopulmonary bypass (CPB) circuit or by IRI of the heart, lungs, kidney and liver as a result of aortic cross- and declamping with concomitant release of different cytokines, such as interleukin (IL)-1β, interleukin (IL)-6, tumour necrosis factor (TNF)-α or the chemokine CXCL8 20–22. Restoration of perfusion after release of the aortic cross-clamp is associated with activation of key markers of the inflammatory response that involves massive polymorphonuclear cell infiltration into reperfused tissue 20,23, which is part of the pathogenesis of IRI 23,24. In coronary artery bypass grafting (CABG) surgery, in particular, adverse outcomes are related mainly to periprocedural myocardial injury 25. Attenuation of IRI and inflammatory response via non-competitive blockade of CXCR1 and CXCR2, therefore, might impact upon the prognosis of the patient and thus be a promising target for a pharmacological intervention.
As data regarding reparixin is based almost entirely on animal studies, no evidence for its use and safety to date exists in the setting of cardiac surgery with CPB. Therefore, we initiated this pilot trial to evaluate the safety and efficacy of reparixin to attenuate IRI and systemic and pulmonary inflammation in patients undergoing on-pump coronary artery bypass grafting (CABG).
Methods
Study design
This single-centre, double-blind, placebo-controlled, parallel-group study was conducted at a tertiary referral centre in Austria. Patients were assigned randomly to one of two groups to receive either reparixin or placebo. Randomization was achieved using blocked randomization with a computer-generated list of random numbers by one investigator who was not involved in the clinical part of the trial. This strategy ensured a predetermined ratio (1 : 1) and close balance of participants in each study group. After recruitment and obtaining patient consent, another investigator who was not involved in the recruitment process arranged the allocation consignment. Subsequently, local pharmacists prepared identical-appearing 50-ml syringes containing either reparixin or placebo according to the randomization. The attending anaesthesiologists, surgeons, nurses and data collectors were blinded to the allocation of participants. Ethical approval was obtained from the institutional review board, and all patients provided written informed consent before any trial-related intervention. The trial was registered within the EudraCT registry with the number 2004-001138-18.
Eligibility criteria
Eligible participants were adults aged 18–90 years undergoing first-time elective CABG surgery with CPB who had an American Society of Anesthesiologists (ASA) physical status ≤ IV and left ventricular ejection fraction ≥ 50%. Exclusion criteria were creatinine clearance < 70 ml/min, evidence of major pulmonary dysfunction, hypersensitivity towards ibuprofen and/or other non-steroidal anti-inflammatory drugs, a body mass index (BMI) > 35 and pregnancy.
Settings and locations
This study was conducted at the Division of Cardiothoracic and Vascular Anesthesia and Intensive Care at Vienna General Hospital. This university hospital is a tertiary health-care centre with more than 2100 beds and approximately 104 000 in-patients admitted per year. Our division is involved in the management of approximately 400 isolated CABG cases per year.
Interventions
Reparixin or placebo was administered intravenously (i.v.) via a central venous line. Reparixin was given immediately after the induction of anaesthesia starting with a loading dose of 4·5 mg/kg/h for 30 min followed by continuous infusion at 2·8 mg/kg/h until 8 h after the end of CPB. The placebo group received an equal volume of isotonic saline solution. Dosage was based on findings from previous Phase I trials, which showed that loading doses of 4·5–6·8 mg/kg/h for 30 min followed by a maintenance dose between 2·8-4·2 mg/kg/h for up to 47·5 h have been well tolerated in a group of young healthy male volunteers 26.
Primary and secondary outcome measures
The primary outcome measures were the proportion as well as the absolute number of neutrophil granulocytes in blood and bronchoalveolar lavage fluid (BALF) as a marker of recruitment and activation in the systemic circulation and their migration into the alveolar space. Neutrophils were determined in blood at baseline, every 30 min during CPB and 4 and 24 h after the end of CPB. In the BALF, neutrophils were counted at the beginning and 4 h after the end of CPB. The second primary outcome measures were serum creatine kinase (CK) and creatine phosphokinase MB isoenzyme (CKMB) as markers of myocardial injury measured 4, 8 and 24 h after the end of CPB.
Secondary outcome measures were serum levels of IL-8 at baseline and 4 and 24 h after the end of CPB, BALF levels of IL-8 at baseline and 4 h after CPB, C-reactive protein (CRP) at baseline and 4 and 24 h after termination of CPB, and the presence of neutrophilic alveolitis (categorized; see below) as surrogate of pulmonary IRI, as well as the maximum intraoperative dose of noradrenaline, the need for inotropes, the perioperative fluid balance and the duration of CPB as surrogates for myocardial IRI. The safety end-point was the deterioration of renal and hepatic function as measured by creatinine and aspartate-amino-transferase (ASAT) 4 and 24 h after the end of CPB. The following clinical outcome parameters were also collected: duration of intensive care unit (ICU) and hospital stay, 30- and 90-day mortality, surgical re-exploration, pleural and pericardial effusion, infection and atrial fibrillation rates, requirements for the transfusion of red blood cells and the output from chest drains in the ICU.
Management of anaesthesia and postoperative care
Anesthaesia was administered according to a standardized protocol via bolus injection of 0·2–0·3 mg/kg etomidate i.v. and continuous infusion of 0·1–0·5 μg/kg/min remifentanil for a 5-min period during mask ventilation with 100% oxygen. Tracheal intubation was facilitated with 0·2 mg/kg cis-atracurium. Patients were then ventilated mechanically with a mixture of 50% oxygen in air. Anaesthesia was maintained by continuous infusion of remifentanil at 0·15–0·4 μg/kg/min and titration of inhaled sevoflurane to a target bispectral (BIS) index of 40 ± 10 27. Cefazolin (2 g) was administered before the beginning and at the end of surgery. Transoesophageal echocardiography was employed routinely during surgery to guide fluid management in addition to the use of inotropes and vasopressors. Postoperatively, patients were admitted to the ICU and extubated after having met standardized extubation criteria. Haemodynamically stable patients were transferred to the step-down unit the day after surgery.
Management of ECB
Buckberg blood cardioplegia, i.e. a mixture of native blood and a commercially available crystalloid solution (Köhler Chemie, Alsbach-Hähnlein, Germany), at a ratio of 4 : 1, was administered in all patients. Extracorporeal circulation during normothermic CPB was performed using an S III multi-flow roller pump (Stoeckert SIII, Munich, Germany) with non-pulsatile flow at a flow rate of 2·5 l/min/m−2. In addition, a membrane oxygenator (Monolyth, Sorin, Italy) and arterial line filter (Dideco, Mirandola, Italy) were used. During CPB, haematocrit > 21% was maintained. Heparin (400 U/kg) i.v. was used for anti-coagulation, and activated clotting times ≥ 480 s were maintained.
Bronchoalveolar lavage
BAL was performed immediately after induction of anaesthesia and 4 h after termination of CPB with a fibreoptic bronchoscope (Olympus CLV-U20; Olympus Co., Tokyo, Japan). Sterile saline solution (5 × 20 ml) was instilled randomly into a subsegment of the left lower, right lower or middle lobe. The total cell count and percentage of neutrophils were determined in the lavage fluid (SYSMEX Counter, Milton Keynes, UK). Similarly, a cell-free supernatant was prepared from lavage fluid and stored at −80°C for subsequent analysis of CXCL-8 with a highly sensitive chemiluminescence assay (R&D Systems, Abingdon, UK) 28. BALF at baseline and 4 h after the end of CPB was also examined histopathologically. The degree of inflammation was categorized as either no specific inflammation or low-, medium- or high-grade neutrophilic alveolitis.
Blood sampling and laboratory analyses
Blood samples were obtained at baseline before infusion of the study drug, after the induction of anaesthesia, immediately after the start of surgery, every 30 min during surgery and at the end of CPB as well as 4, 8 and 24 h after CPB. Blood was drawn from the arterial line into sterile evacuated tubes (Vacutainer; BD Biosciences, Heidelberg, Germany). Differential white blood cell counts were analysed using an automated counter (Sysmex XE' 2100). Blood was centrifuged immediately after collection and stored at −80°C until batch analysis of CXCL-8 levels. Cardiac troponin T (TnT), CK, CK-MB, CRP and other parameters (e.g. creatinine) were determined at the laboratory of the Clinical Institute of Medicinal and Chemical Laboratory Diagnostic.
Statistical analysis
The sample size calculation was based on an intended 30% reduction of the peak neutrophil count in blood with an α-level of 0·05 and power of 80%. Data were reviewed for completeness, plausibility and outliers before analysis. If values were missing, alternative data sources were explored. If data recovery was unsuccessful, values were replaced by appropriate subgroup medians as long as fewer than 5% were missing. Baseline characteristics included age, sex, BMI, cardiovascular co-morbidities and risk factors, renal function and Euroscore II 29. Characteristics and outcome variables were described using medians with interquartile range (IQR) or absolute numbers (percentages), as appropriate. To minimize bias, continuous variables were generally assumed to be non-parametric and compared using the Mann–Whitney U-test. Proportions were compared using Fisher's exact test. All tests were two-sided and differences considered significant if P < 0·05. Statistical analyses were performed using spss version 22·0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 (GraphPad, San Diego, CA, USA).
Results
A total of 473 patients were screened for eligibility; 108 were eligible for inclusion in the study, but 61 patients declined to participate and another 15 patients could not be included due to organizational problems. Thirty-two patients provided informed consent (Fig. 1; 11 females, 21 males, n = 16 per study group). Baseline characteristics are provided in Table 1 and intraoperative data in Table 2. No significant differences were found between the study groups at baseline. The reparixin group included more smokers than the placebo group (six versus one; P = 0·08). In-hospital and 30-day mortality was 0% in both groups.
Figure 1.
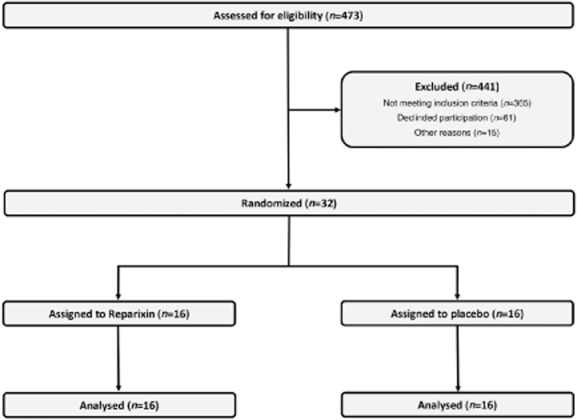
Study flowchart.
Table 1.
Baseline characteristics.
| Variables | Reparixin (n = 16) | Placebo (n = 16) | P-value |
|---|---|---|---|
| Age (years) | 65 (58;69) | 66 (60;73) | 0·564† |
| BMI (kg/m2) | 28 (25;31) | 28 (26;29) | 0·809† |
| LVEF (%) | 50 (50;50) | 50 (50;50) | 0·564† |
| EuroScore II (%) | 0·83 (0·75;1·17) | 1·05 (0·71;1·35) | 0·59† |
| Female | 6 (31·3) | 5 (37·5) | 1* |
| ASA | 0·439* | ||
| II | 1 (6·2) | 3 (18·8) | |
| III | 12 (75) | 12 (75) | |
| IV | 3 (18·8) | 1 (6·2) | |
| Diabetes | 0·613* | ||
| No | 11 (68·8) | 8 (50) | |
| Non-IDDM | 3 (18·8) | 6 (37·5) | |
| IDDM | 2 (12·4) | 2 (12·5) | |
| History of MI | 1* | ||
| No | 11 (68·8) | 12 (75) | |
| Yes | 5 (31·2) | 4 (25) | |
| Nicotine abuse | 0·172* | ||
| No | 11 (68·8) | 15 (93·8) | |
| Smoker | 5 (31·2) | 1 (6·2) |
Fisher's exact or χ2 tests
Mann–Whitney U-test. Given are median with interquartile range (IQR) or absolute numbers (percentages). IDDM = insulin-dependent diabetes mellitus; MI = myocardial infarction; ASA = American Society of Anesthesiologists; BMI = body mass index; LVEF = left ventricular ejection fraction.
Table 2.
Intraoperative data.
| Variables | Reparixin (n = 16) | Placebo (n = 16) | P-value |
|---|---|---|---|
| Number of grafts | 0·27* | ||
| Two | 4 (25) | 2 (12·5) | |
| Three | 10 (62·5) | 14 (87·5) | |
| Four | 2 (12·5) | 0 (0) | |
| Time aortic clamping (min) | 52 (35;71) | 61 (46;72) | 0·564† |
| CPB time (min) | 95 (66;110) | 94 (74;113) | 0·491† |
| Anaesthesia time (min) | 290 (280;327) | 347 (318;374) | 0·017† |
| Total of Ringer's lactate (ml) | 1100 (1000;1838) | 1550 (1075;2500) | 0·043† |
| Fluid balance (ml) | 2575 (2027;3080) | 3200 (2928;3778) | 0·029† |
| Maximum noradrenaline ≥ 0·11 μg kg−1 min−1 | 3 (18·8) | 8 (50) | 0·063* |
| Use of dobutamine | 4 (25) | 8 (50) | 0·14* |
Fisher's exact or χ2 tests
Mann-Whitney U-test. Given are median with interquartile range (IQR) or absolute numbers (percentages); min = minutes; CBP= cardiopulmonary bypass.
Impact of reparixin on systemic inflammation
No significant differences were found in the absolute numbers of neutrophils between the two groups at any time-point (Figs 2, 3). Notably, at the beginning of CPB the neutrophil count trended low in the reparixin group with a median of 2·4 × 103/μl (IQR = 2–3) compared to 2·8 × 103/μl in the placebo group (IQR = 2·6–3·7, P = 0·086). However, reparixin significantly reduced the proportion of neutrophil granulocytes in the peripheral blood compared to placebo at the start of CPB (49%, IQR = 45–57 versus 58%, IQR = 53–66, P = 0·035), the end of CPB (71%, IQR = 67–76 versus 79%, IQR = 71–83, P = 0·023) and 1 h after the end of CPB (73%, IQR = 71–75 versus 77%, IQR = 72–80, P = 0·035). Two h after the end of CPB this effect was lost (70%, IQR = 67–74 versus 73%, IQR = 70–77, P = 0·094, Fig. 4). At baseline, IL-8 levels were not significantly different between groups (3·1 pg/ml, IQR = 1·6–5·4 versus 3·3 pg/ml, IQR = 1·9–4, P = 0·6). IL-8 levels peaked 4 h after the end of CPB in both groups, with an almost 11-fold increase for the reparixin group and 14-fold increase for the placebo group. At this time-point IL-8 levels tended to be lower in the reparixin group (35 pg/ml, IQR = 21–57 versus 45 pg/ml, IQR = 27–60, P = 0·4). CRP values were not significantly different 24 h after the end of CPB but tended to be lower in the reparixin group (14, IQR = 12–20 versus 16, IQR = 14–22, P = 0·4).
Figure 2.
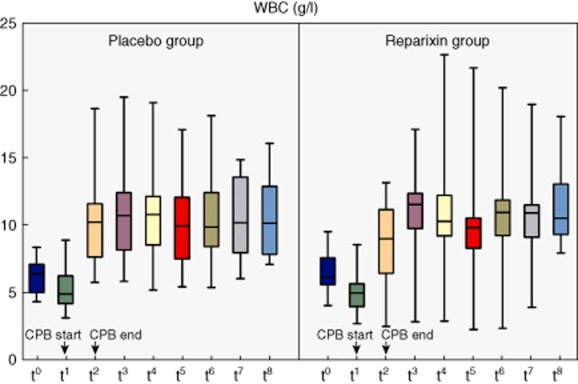
White blood count in systemic differential blood count at different time-points [t0 = baseline, t1 = start cardiopulmonary bypass (CPB), t2 = end CPB, t3 = 1 h after CPB, t4 = 2 h after CPB, t5 = 3 h after CPB, t6 = 4 h after CPB, t7 = 8 h after CPB, t8 = 24 h after CPB]. Mann–Whitney U-test was used for comparison; non-significant P-values are not indicated.
Figure 3.
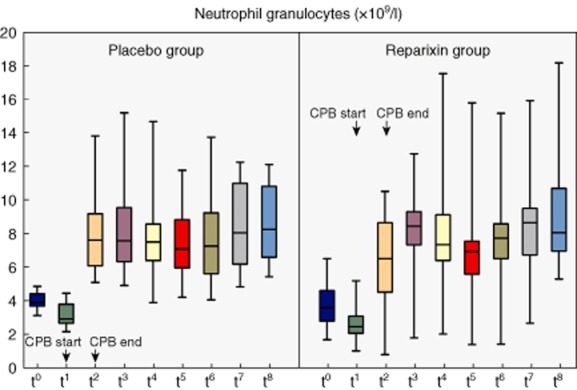
Absolute numbers of neutrophil granulocytes in systemic differential blood count at different blood count at different time-points [t0 = baseline, t1 = start cardiopulmonary bypass (CPB), t2 = end CPB, t3 = 1 h after CPB, t4 = 2 h after CPB, t5 = 3 h after CPB, t6 = 4 h after CPB, t7 = 8 h after CPB, t8 = 24 h after CPB]. Mann–Whitney U-test was used for comparison; non-significant P-values are not indicated.
Figure 4.
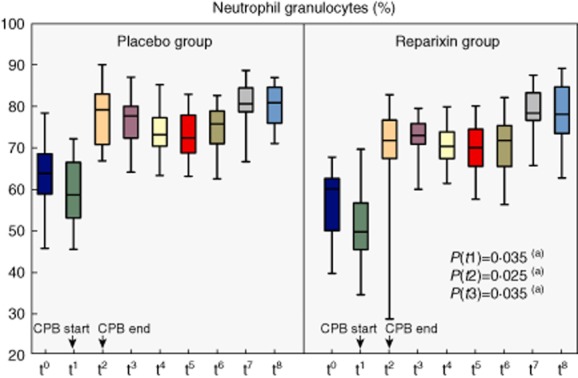
Percentage of neutrophil granulocytes in systemic differential blood count at different time-points [t0 = baseline, t1 = start cardiopulmonary bypass (CPB), t2 = end CPB, t3 = 1 h after CPB, t4 = 2 h after CPB, t5 = 3 h after CPB, t6 = 4 h after CPB, t7 = 8 h after CPB, t8 = 24 h after CPB].(a) Mann–Whitney U-test; non-significant P-values are not indicated.
BALF examinations
The absolute number and the proportion of neutrophil granulocytes as well as IL-8 levels were not significantly different between the two groups at baseline and 4 h after the end of CPB. For IL-8, median values in the reparixin group tended to be lower than the placebo group at baseline (15 pg/ml, IQR = 1·6–39 versus 28 pg/ml, IQR = 9–65, P = 0·2) and 4 h after CPB (33 pg/ml, IQR = 12–220 versus 95 pg/ml, IQR = 38–309, P = 0·21). Histopathological examination revealed deterioration of alveolitis in 57% of placebo patients compared to 27% of reparixin patients (P = 0·099).
Myocardial ischaemia–reperfusion injury
Median CK and CK-MB serum levels were also numerically lower in the reparixin group than the placebo group at every time-point, but the differences were not significant. We observed the most distinct differences between groups for CK (605 U/l, IQR = 538–1069 versus 473 U/l, IQR = 284–1073, P = 0·086) and CK-MB (37 U/l, IQR = 33–53 versus 30 U/l, IQR = 7–46, P = 0·094) 8 h after the end of CPB. Equally, TnT values were numerically, but not significantly lower in the reparixin group 24 h after the end of CPB (0·29, IQR = 0·2–0·45 versus 0·32, IQR = 0·26–0·5, P = 0·5).
In addition, significantly less Ringer's lactate was used (1550 ml, IQR = 1075–2500 versus 1100 ml, IQR = 1000–1838, P = 0·043) and a less positive intraoperative fluid balance was maintained (3200 ml, IQR = 2928–3778 versus 2575 ml, IQR = 2027–3080, P = 0·029) in the reparixin group compared to the placebo group (Fig. 5). The effect on fluid balance was sustained and even more distinct between groups during ICU stay (2603 ml, IQR = 1023–4288 versus 4200 ml, IQR = 2313–8160, P = 0·021, Table 3). Furthermore, control patients appeared to receive high-dose noradrenaline (≥ 0·11 μg/kg/min) more frequently than reparixin patients (n = 8 versus 3, P = 0·063). Control patients also seemed to require dobutamine more often than reparixin patients (n = 8 versus 4, P = 0·14). CPB time (94 min, IQR = 74–113 versus 95 min, IQR = 66–110, P = 0·491) and duration of aortic cross-clamping (61 min, IQR = 46–72 versus 52 min, IQR = 35–71, P = 0·564) were comparable.
Figure 5.
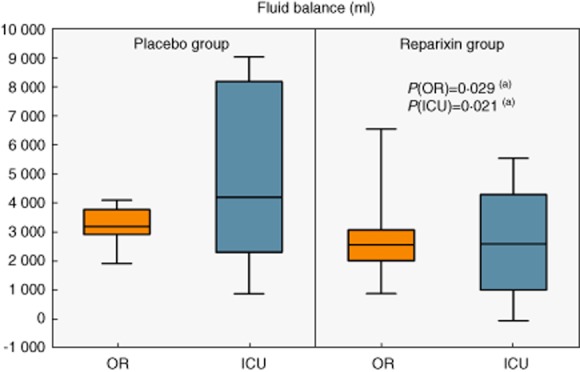
Fluid balance in operating room (OR) and intensive care unit (ICU);(a) Mann–Whitney U-test.
Table 3.
Clinical outcome.
| Variables | Reparixin (n = 16) | Placebo (n = 16) | P-value |
|---|---|---|---|
| Atrial fibrillation | 5 (31·3) | 2 (12·5) | 0·394* |
| Infections | 1* | ||
| Wound (vene harvesting) | 2 (12·5) | 2 (12·5) | |
| Wound (sternal) | 1 (6·3) | 0 (0) | |
| Post-procedural transfusion requirements | 0·069* | ||
| Units of PRBC (n = 2) | 0 (0) | 4 (25) | |
| Units of PRBC (n = 4) | 0 (0) | 1 (6·3) | |
| Units of PRBC (n = 6) | 1 (6·3) | 0 (0) | |
| Pleural effusion | 0·333* | ||
| No need for punction | 1 (6·3) | 4 (25) | |
| Need for punction | 1 (6·3) | 0 (0) | |
| Need for surgical revision | 1* | ||
| Pericardial effusion | 1 (6·3) | 0 (0) | |
| Wound (sternal) | 1 (6·3) | 0 (0) | |
| Others | 0 (0) | 1 (6·3) | |
| Chest tube drainage while ICU stay (ml) | 615 (323;977) | 675 (413;898) | 0·985† |
| Fluid balance while ICU stay (ml) | 2603 (1023;4288) | 4200 (2313;8160) | 0·021† |
| Hospital stay (days) | 8·5 (7;11·5) | 8 (7;8) | 0·239† |
| ICU stay (h) | 19·6 (16;23·4) | 20·6 (15·9;22) | 0·809† |
| 30 days mortality | 0 (0) | 0 (0) | 1* |
| 90 days mortality | 0 (0) | 0 (0) | 1* |
Fisher's exact or χ2 tests
Mann–Whitney U-test; given are median with interquartile range (IQR) or absolute numbers (percentages). ICU = intensive care unit; PRBC = packed red blood cells.
Safety end-points
In this setting, reparixin was well tolerated by all patients at the dosage we used. We did not observe any adverse events related to drug administration. Creatinine values were not significantly different between the groups at baseline (1·0 mg/dl, IQR = 0·8–1·1 versus 1·1 mg/dl, IQR = 0·9–1·1, P = 0·753), 4 h after CPB (0·9 mg/dl, IQR = 0·8–1·2 versus 1·0 mg/dl, IQR = 0·8–1·1, P = 0·78) and 24 h after CPB (0·8 mg/dl, IQR = 0·7–1·0 versus 0·9 mg/dl, IQR = 0·8–1·1, P = 0·423). Administration of reparixin was not associated further with any bleeding complications.
Clinical outcome
Length of ICU stay was similar in both groups (20 h, IQR = 16–23 for reparixin versus 21 h, IQR = 16–22 for placebo, P = 0·809). In addition, length of hospital stay was not significantly different between the groups (9 days, IQR = 7–12 for reparixin versus 8 days, IQR = 7–8 for placebo, P = 0·239); 30- and 90-day mortality was 0% in both groups. Surgical re-exploration, pleural and pericardial effusion, infection and atrial fibrillation occurred almost equally as frequently in both groups (Table 3). Postoperative requirements for the transfusion of red blood cells were more frequent in the placebo group, but not significantly different from the reparixin group (Table 3). Chest tube drainage in the ICU was similar in both groups (615 ml, IQR = 323–977 versus 675 ml, IQR = 413–898, P = 0·985, Table 3).
Discussion
We have demonstrated for the first time, to our knowledge, that intravenous reparixin, a small molecular weight allosteric CXCR1/2 inhibitor, can be administered safely to patients undergoing elective CABG surgery with CPB. Reparixin significantly reduced the proportion of neutrophils in peripheral blood at the beginning, end and 1 h after CPB in relation to placebo. Furthermore, it resulted in significantly less positive fluid balance intraoperatively and during the ICU stay.
Interestingly, we did not observe sustained effects of reparixin on neutrophil counts and laboratory indices of myocardial injury. Our observations can be explained by recently described regulatory mechanisms of neutrophil mobilization from bone marrow. According to these mechanisms, the release of neutrophils is regulated antagonistically by the CXC chemokine receptors CXCR2 and CXCR4, which are both expressed on neutrophils 30,31. The CXCR4 ligand stromal cell-derived factor (SDF-1 or CXCL12) functions to retain neutrophils in the bone marrow, whereas the CXCR2 ligands KC (CXCL1) and macrophage inflammatory protein (MIP-2 or CXCL2) promote their release. SDF-1, CXCL1 and MIP-2 are both expressed constitutively by endothelial cells and osteoblasts. Osteoblasts are the major source of SDF-1, whereas endothelial cells are the major cellular source of CXCR2 ligands in the bone marrow. Thus, release or retention of neutrophils is a function of the ratio of CXCR4 and CXCR2 ligands in the bone marrow. Consequently, the mobilization of neutrophils from the bone marrow by CXCR2 ligands can be described as inhibition of CXCR4-mediated retention 30,31. As noted above, reparixin is a non-competitive allosteric blocker of both CXCR1 and CXCR2 with an almost 400-fold 32 higher efficacy in inhibiting CXCR1 activity than CXCR2, due to a less favourable environment through the isobutyl group and the lack of specific hydrophobic interactions between reparixin and CXCR2 15. Thus, overwhelmingly blocking the CXCR1 axis and keeping the CXCR2 axis almost unimpaired could result in an unimpeded ratio of CXCR4 and CXCR2 ligands and less effect on the regulation of release or retention of neutrophils from bone marrow. Otherwise, CXCR2 expression is down-regulated significantly during and after CPB and correlates with CXCL8 levels, but CXCR1 expression remains unchanged 33. This effect may be due to desensitization and down-regulation of the CXCR1 and CXCR2 receptors, whereby the former internalizes slowly but recovers rapidly, while the latter internalizes rapidly but recovers slowly at the cell surface 34. Conversely, this difference could shift the ratio to more CXCR4-mediated retention of neutrophils during CPB.
Based on the multiplicity of CXCR1 and CXCR2 ligands and the more selective inhibition of the CXCR1-related pathway via reparixin, the impact of reparixin could be counterbalanced through redundant mechanisms of neutrophil chemotaxis, resulting in perpetuation of inflammation independently of the initial signal. For example, the Duffy antigen receptor for chemokines (DARC), which is considered a ‘silent’ chemokine receptor, was shown recently to act as a regulator of bioavailability for several chemokines, including CXCL1, CXCL2, CXCL5 and CXCL8, resulting in DARC-enhanced neutrophil trafficking into tissues 35,36. The CXCR2 ligand CXCL5 in particular regulates the availability of binding sites for other ELR+ CXC chemokines released during inflammation through its interaction with erythrocyte DARC. CXCL5 inhibits chemokine scavenging, at least in part, through its homeostatic and high-affinity binding with erythrocyte DARC. Thus, in the absence of CXCL5, DARC scavenges proinflammatory chemokines, contributing to reshaping the chemokine gradients for neutrophil influx into, for example, the lung. During a severe inflammatory response, such as in a model of Escherichia coli-induced pneumonia, further expression of CXCL5 by alveolar type 2 cells inhibits the chemokine scavenging capability of DARC at a time when the production of CXCL1 and CXCL2 increases dramatically, resulting in marked increases in circulating plasma concentrations of these chemokines and adverse consequences for the efficient accumulation of neutrophils 37. This example shows the complexity and alternative pathways of neutrophil recruitment and regulation independent of overwhelmingly blocking the CXCR1 pathway.
Cardiac dysfunction has been associated with elevated circulating chemokine levels in both animals and humans. One of the first chemokines to be detected in the myocardium in response to IRI was CXCL8. Therefore, CXCL8 has been hypothesized to participate in neutrophil-induced myocardial injury. However, several other chemokines have been detected early in animal models of myocardial injury, including CCL2 and CXCL10. Cardiac myocytes also have other receptors such as CXCR2, CCR2 and the SDF-1 (or CXCL12) receptor CXCR4. These receptors are expressed constitutively and up-regulated following oxidative stress both in vivo and in vitro 38. In an animal model of IRI, Tarzami et al. 38 found a direct myocardial protective action of CXCR2 during ischaemia–reperfusion, but the magnitude of this protective effect was smaller than the damaging effect of CXCR2-mediated inflammatory cell recruitment. The authors assumed that this was due to different CXCR2-induced signalling between the two different cell types, mainly cardiac myocytes and neutrophils 39. In addition, new data refer to the relevance of the CXCL12/CXCR4 axis in myocardial IRI. CXCR4 over-expression in vivo worsened cardiac function in an animal model of IRI, enhancing the recruitment of inflammatory cells, TNF-α production and cell death. The detrimental effect of CXCR4 over-expression in vivo following ischaemia–reperfusion may be a consequence of increased production of CXCL12 38,40. Furthermore, under conditions of stress, the paracrine and/or autocrine activation of CXCR4 by its ligand may trigger intracellular signalling pathways that exacerbate contractile dysfunction. CXCR4 activation also modulates the β2AR structure, and downstream signalling in ventricular cardiac myocytes and has been shown to be a key mediator of calcium handling in cardiac myocytes in adult rats 41. Thus, independently of the action of reparixin, myocardial IRI may be triggered and maintained by different chemokine/chemokine-receptor inter-relations (e.g. the CXCL12/CXCR4 axis), which could be an explanation of the lack of significant differences in primary end-points for myocardial IRI in our study.
With regard to haemodynamics, we found a significantly lower need for crystalloids and a tendency towards reduced vasopressor and inotrope use in patients treated with reparixin. The intraoperative volume-sparing effect was even sustained and more impressive during the patients' ICU stay. Recently, evidence is accumulating that volume overload is associated with increased morbidity and mortality 42,43. Silva et al., for example, reported that a high intraoperative fluid balance is an independent risk factor for death [odds ratio (OR) =1·024 per 100 ml additional fluid] 43. Volume overload can result in pulmonary oedema with impaired gas exchange, reduced compliance and increased breathing work, myocardial oedema with impaired contractility, diastolic dysfunction and conduction disturbances, hepatic congestion with, among others, impaired synthetic function, gut oedema with malabsorption and ileus and renal interstitial oedema with acute renal failure 42. These patients are also at risk for pulmonary failure and more susceptible to lung and wound infections by microcirculatory derangement 42,43. Remarkably, with increasing duration of reparixin administration, the volume-sparing effect was more distinct between groups. This finding could indicate that reparixin affects capillary leakage beneficially due to partial inhibition of neutrophil diapedesis into the tissue. According to established models of neutrophil migration into the tissue, neutrophils prioritize the chemotactic signals by distinguishing between ‘intermediary’ (e.g. CXCL8) and ‘end target’ (e.g. C5a) chemoattractants with distinctly different intracellular signalling pathways, avoiding ‘distraction’ in a complex milieu of chemoattractants 35,44. With regard to the order of a chemoattractants in the hierarchy, CXCL8 was suggested to be the most potent among the host-derived chemoattractants 45. Neutrophils transmigrate preferentially at endothelial cell–cell junctions and work in concert with endothelial junction molecules to disassemble vascular endothelial (VE)–cadherin complexes at the cell–cell junctions, forming gaps and allowing neutrophils to squeeze between endothelial cells. Thus, the VE–cadherin complex acts as a gatekeeper during transmigration 35. Among CXCL8 receptors, evidence indicates that CXCR1 could play a dominant role in mediating the CXCL8 chemotaxis of neutrophils that express both CXCL8 receptors 12,46. Preferential inhibition of the CXCR1-mediated CXCL8 effect in chemotaxis and endothelial diapedesis at cell–cell junctions using reparixin could attenuate the disassembly of VE–cadherin complexes at cell–cell junctions, leaving the endothelial barrier function intact. Additionally, diminished release of vasodilating agents, cytotoxic proteases (e.g. elastase), myeloperoxidase and reactive oxygen species, resulting in less vasodilation and less damage to the vascular endothelium and surrounding tissues, could result in less neutrophil recruitment and diapedesis 20. Conversely, the smaller volume requirement and reduced vasopressor and inotropic use in the reparixin group could be explained by an attenuated inflammatory response consequently mitigating myocardial stunning and cardiovascular dysfunction. Because wall motion abnormalities in the left ventricle and myocardial ischaemic episodes after CPB have been shown to correlate with IL-6 and CXCL-8 levels 20,47, at least the effect of CXCL-8 could be diminished partly by CXCR1 inhibition. Otherwise, as noted above, the CXCL12/CXCR4 axis and CXCR2 with its ligands could have a greater impact upon the pathogenesis of myocardial IRI so that, even in the presence of reparixin-induced partial inhibition, ligands other than CXCL8 could trigger intracellular signalling pathways that exacerbate contractile dysfunction.
As our surrogate variables for myocardial IRI point in the same direction, our findings could be explained by a positive drug effect, although we cannot rule out that the trial was underpowered as sample size calculation was based on detection of a 30% reduction in the peak neutrophil count in blood.
Conversely, we could have missed a larger effect of reparixin in our study population because of the low incidence of CPB times > 100 min 48. The moderate drug effect on the heart may also be explained by an effective cardioprotective regimen via mixed cardioplegia in our study patients.
As noted above, we used inhaled sevoflurane for anaesthesia in both groups. Recently, sevoflurane has been shown to interfere with neutrophil inflammatory pathways, leading to decreased neutrophil migration and reduced expression of β2-integrin CD11b. The site of action of volatile anesthetics is located upstream of protein kinase C and downstream of CXCR2. Therefore, interference with CXCR2 signalling may contribute to the well-established anti-inflammatory effect of volatile anaesthetics for the development of IRI 49. This could be a confounder in our results, because even the placebo group received sevoflurane, an agent that acts instantaneously with the same receptors as reparixin, making interpretation of our results more delicate.
With respect to the BALF results, we found a tendency for less frequent deterioration of alveolitis grading for reparixin patients. In particular, the CXCR2 receptor plays a major role in chemotaxis of neutrophils into the lung. Reutershan et al. showed in a CXCR2–/– murine model of acute lung injury, that CXCR2 is crucial for transendothelial and transepithelial migration of polymorphonuclear leucocytes (PMNs) into the lung and proposed that CXCR2-independent PMN migration is negligible 3,50. In addition, CXCR2–/– mice were better protected from hyperoxia-induced PMN infiltration, lung oedema and vascular protein leakage 51. In contrast, PMNs also accumulate in the pulmonary vasculature in vivo even in the absence of CXCR2, suggesting the contribution of other CXCR2-independent chemoattractants 50. Furthermore, Zarbock et al. showed that reparixin is effective for reducing transendothelial and transepithelial migration of neutrophils in a murine model of lipopolysaccharide (LPS)-induced pulmonary inflammation and acid-induced acute lung injury (ALI), but does not regulate neutrophil accumulation in the intravascular compartment in the lung in response to aerosolized LPS 52. However, the results of murine models may not be directly transferable to human studies due to the different expression of CXCR subtypes 3,4. Furthermore, because the reparixin group included more smokers, we observed higher alveolitis grades in this group at baseline, which could be a confounder when interpreting our BALF results. We considered excluding smokers and chronic obstructive pulmonary disease (COPD) patients when defining eligibility criteria for this study, but we refrained from doing so because smokers and patients who quit smoking recently may not have different degrees of alveolitis.
Conclusion
We report for the first time the use of the CXCR1/2 antagonist reparixin in patients undergoing CABG surgery with CPB. We demonstrated that administration of reparixin with the intention to diminish the systemic inflammatory reaction is feasible, and not associated with increased morbidity compared to placebo. Reparixin appears to have the potential to reduce the recruitment and diapedesis of neutrophils, and to attenuate haemodynamic alterations and potentially myocardial IRI after on-pump CABG surgery. The interest in this topic is still ongoing. Currently, more specific inhibitors of CXCR2 are being tested in healthy humans 53. However, in order to assess the value of reparixin on non-pathogen-associated inflammation, such as CPB, further clinical trials using large sample sizes are needed.
Acknowledgments
We thank Dompe's.p.a., L'Aquila, Italy, for kindly supplying us with the study drug.
Disclosure
None declared.
Author contributions
U. D. and P. O. helped to design the study; recruited and managed the patients; collected, analysed and interpreted the data; and prepared the manuscript. A. F., J. W. and D. S. recruited the patients and collected and interpreted the data. A. Z. and M. D. helped to recruit and manage the patients, collected and interpreted the data and critically reviewed the manuscript. B. J. and B. S. designed the study; recruited and managed the patients; collected, analysed and interpreted the data; and prepared the manuscript.
References
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- Konrad FM, Reutershan J. CXCR2 in acute lung injury. Mediators Inflamm. 2012;2012:740987. doi: 10.1155/2012/740987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- Ahuja SK, Lee JC, Murphy PM. CXC chemokines bind to unique sets of selectivity determinants that can function independently and are broadly distributed on multiple domains of human interleukin-8 receptor B. Determinants of high affinity binding and receptor activation are distinct. J Biol Chem. 1996;271:225–232. doi: 10.1074/jbc.271.1.225. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- Tani K, Su SB, Utsunomiya I, Oppenheim JJ, Wang JM. Interferon-gamma maintains the binding and functional capacity of receptors for IL-8 on cultured human T cells. Eur J Immunol. 1998;28:502–507. doi: 10.1002/(SICI)1521-4141(199802)28:02<502::AID-IMMU502>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Burdon PC, Martin C, Rankin SM. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br J Haematol. 2008;142:100–108. doi: 10.1111/j.1365-2141.2008.07018.x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–420. doi: 10.1016/s0167-5699(00)01672-8. [DOI] [PubMed] [Google Scholar]
- Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther. 2006;112:139–149. doi: 10.1016/j.pharmthera.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Matsushima K, Morishita K, Yoshimura T, et al. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LM, Maxwell PJ, Waugh DJ. Rationale and means to target pro-inflammatory interleukin-8 (CXCL8) signaling in cancer. Pharmaceuticals. 2013;6:929–959. doi: 10.3390/ph6080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini R, Allegretti M, Bizzarri C, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA. 2004;101:11791–11796. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citro A, Cantarelli E, Maffi P, et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122:3647–3651. doi: 10.1172/JCI63089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri B, Mosca M, Ramadori P, et al. Neutrophil recruitment in the reperfused-injured rat liver was effectively attenuated by repertaxin, a novel allosteric noncompetitive inhibitor of CXCL8 receptors: a therapeutic approach for the treatment of post-ischemic hepatic syndromes. Int J Immunopathol Pharmacol. 2005;18:475–486. doi: 10.1177/039463200501800307. [DOI] [PubMed] [Google Scholar]
- Cugini D, Azzollini N, Gagliardini E, et al. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int. 2005;67:1753–1761. doi: 10.1111/j.1523-1755.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Souza DG, Bertini R, Vieira AT, et al. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132–142. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Erik Botker H, Condorelli G, et al. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013;98:7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- Liakopoulos OJ, Schmitto JD, Kazmaier S, et al. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thorac Surg. 2007;84:110–118. doi: 10.1016/j.athoracsur.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Francischetti I, Moreno JB, Scholz M, Yoshida WB. Leukocytes and the inflammatory response in ischemia–reperfusion injury. Rev Bras Cir Cardiovasc. 2010;25:575–584. doi: 10.1590/s0102-76382010000400023. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Kleinbongard P, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- Leitner JM, Mayr FB, Firbas C, et al. Reparixin, a specific interleukin-8 inhibitor, has no effects on inflammation during endotoxemia. Int J Immunopathol Pharmacol. 2007;20:25–36. doi: 10.1177/039463200702000104. [DOI] [PubMed] [Google Scholar]
- Puri GD, Murthy SS. Bispectral index monitoring in patients undergoing cardiac surgery under cardiopulmonary bypass. Eur J Anaesthesiol. 2003;20:451–456. doi: 10.1017/s026502150300070x. [DOI] [PubMed] [Google Scholar]
- Derhaschnig U, Bergmair D, Marsik C, Schlifke I, Wijdenes J, Jilma B. Effect of interleukin-6 blockade on tissue factor-induced coagulation in human endotoxemia. Crit Care Med. 2004;32:1136–1140. doi: 10.1097/01.ccm.0000126265.08175.be. [DOI] [PubMed] [Google Scholar]
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–744. doi: 10.1093/ejcts/ezs043. discussion 44–5. [DOI] [PubMed] [Google Scholar]
- Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriconi A, Cesta MC, Cervellera MN, et al. Design of noncompetitive interleukin-8 inhibitors acting on CXCR1 and CXCR2. J Med Chem. 2007;50:3984–4002. doi: 10.1021/jm061469t. [DOI] [PubMed] [Google Scholar]
- Chishti AD, Dark JH, Kesteven P, et al. Expression of chemokine receptors CXCR1 and CXCR2 during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;122:1162–1166. doi: 10.1067/mtc.2001.116559. [DOI] [PubMed] [Google Scholar]
- Singh V, Raghuwanshi SK, Smith N, Rivers EJ, Richardson RM. G Protein-coupled receptor kinase-6 interacts with activator of G protein signaling-3 to regulate CXCR2-mediated cellular functions. J Immunol. 2014;192:2186–2194. doi: 10.4049/jimmunol.1301875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruenster M, Mudde L, Bombosi P, et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10:101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Liu Y, Dai N, et al. CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity. 2010;33:106–117. doi: 10.1016/j.immuni.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarzami ST. Chemokines and inflammation in heart disease: adaptive or maladaptive? Int J Clin Exp Med. 2011;4:74–80. [PMC free article] [PubMed] [Google Scholar]
- Tarzami ST, Miao W, Mani K, et al. Opposing effects mediated by the chemokine receptor CXCR2 on myocardial ischemia–reperfusion injury: recruitment of potentially damaging neutrophils and direct myocardial protection. Circulation. 2003;108:2387–2392. doi: 10.1161/01.CIR.0000093192.72099.9A. [DOI] [PubMed] [Google Scholar]
- Chen J, Chemaly E, Liang L, et al. Effects of CXCR4 gene transfer on cardiac function after ischemia–reperfusion injury. Am J Pathol. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Schwarzkopf M, Altman P, et al. beta2-Adrenergic receptor signaling in the cardiac myocyte is modulated by interactions with CXCR4. J Cardiovasc Pharmacol. 2010;56:548–559. doi: 10.1097/FJC.0b013e3181f713fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- Silva JM, Jr, de Oliveira AM, Nogueira FA, et al. The effect of excess fluid balance on the mortality rate of surgical patients: a multicenter prospective study. Crit Care. 2013;17:R288. doi: 10.1186/cc13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit B, Robbins SM, Downey CM, et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- Kim D, Haynes CL. Neutrophil chemotaxis within a competing gradient of chemoattractants. Anal Chem. 2012;84:6070–6078. doi: 10.1021/ac3009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cioccio V, Strippoli R, Bizzarri C, et al. Key role of proline-rich tyrosine kinase 2 in interleukin-8 (CXCL8/IL-8)-mediated human neutrophil chemotaxis. Immunology. 2004;111:407–415. doi: 10.1111/j.1365-2567.2004.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennein HA, Ebba H, Rodriguez JL, et al. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108:626–635. [PubMed] [Google Scholar]
- Hausenloy D, Kunst G, Boston-Griffiths E, et al. The effect of cyclosporin-A on peri-operative myocardial injury in adult patients undergoing coronary artery bypass graft surgery: a randomised controlled clinical trial. Heart. 2014;100:544–549. doi: 10.1136/heartjnl-2013-304845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Edenborn B, Frick R, Piegeler T, et al. Volatile anaesthetics reduce neutrophil inflammatory response by interfering with CXC receptor-2 signalling. Br J Anaesth. 2015;114:143–149. doi: 10.1093/bja/aeu189. [DOI] [PubMed] [Google Scholar]
- Reutershan J, Morris MA, Burcin TL, et al. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sue RD, Belperio JA, Burdick MD, et al. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol. 2004;172:3860–3868. doi: 10.4049/jimmunol.172.6.3860. [DOI] [PubMed] [Google Scholar]
- Zarbock A, Allegretti M, Ley K. Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br J Pharmacol. 2008;155:357–364. doi: 10.1038/bjp.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz O, Khalilieh S, Ludwig-Sengpiel A, et al. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J. 2010;35:564–570. doi: 10.1183/09031936.00048509. [DOI] [PubMed] [Google Scholar]


