Abstract
It is still controversial which cell types are responsible for synovial inflammation in osteoarthritic (OA) joints. The aim of this study was to quantify the mononuclear cell populations and their cytokines in patients with different knee OA subtypes. Synovial membrane (SM), synovial fluid (SF) and peripheral blood (PB) were harvested from patients with unicompartmental (UC) and bicompartmental (BC) knee OA. Frequencies of mononuclear cells were assessed by flow cytometry in PB and SM. Naive SF samples were analysed for a broad variety of cytokines by multiplex analysis. SM of both groups displayed a distinct mononuclear cell infiltration, with CD14+ macrophages being the major cell population, followed by CD4+ T cells and only small numbers of CD8+ T, CD19+ B and CD16+CD56+ natural killer (NK) cells. Between the two groups, SM of BC OA showed significantly higher amounts of mononuclear cells (135·7 ± 180 versus 805 ± 675 cells/mg, P = 0·0009) and higher CD4+ T cell presence (3·4 ± 4·6 versus 9·1 ± 7·5%, P = 0·0267). SF of BC OA displayed significantly higher concentrations for a number of proinflammatory cytokines [CXCL1, eotaxin, interferon (IFN)-γ, interleukin (IL)-7, IL-8, IL-9, IL-12]. UC and BC OA show significant differences in their synovial inflammatory pattern. Whereas in UC OA CD14+ macrophages are the predominant cell population, BC OA has a higher inflammatory profile and seems to be driven by CD14+ macrophages and CD4+ T cells. Inclusion of clinical information into the analysis of cellular and molecular results is pivotal in understanding the pathophysiology of OA.
Keywords: bicompartmental, inflammation, osteoarthritis, synovial membrane, unicompartmental
Introduction
Osteoarthritis (OA) is the most common type of arthritis and is a heterogeneous disease. Its pathophysiology is complex and far from understood. Although, clinically, subtypes can be defined by their aetiology, clinical presentation and radiographic evaluation, it remains unknown how this translates into the cellular and molecular pathways of joint degradation. While it is understood that the pathophysiology includes biomechanical, hereditary and molecular factors, none of these mechanisms have provided enough information to halt disease progression. Although a clinically relevant number of OA patients present with signs of inflammation, e.g. joint swelling and effusion, OA has long been interpreted as a ‘non-inflammatory’ disease. This has remained unchanged, despite the description of inflammatory cells and cytokines in OA joints through the last decades reaching back to the 1980s 1. These inflammatory processes were interpreted mainly as a bystander, and not as a driving force in OA pathogenesis. A set of new studies has raised interest in this topic and aimed to map these inflammatory processes more precisely, both in human OA 2–9 and in animal models 10,11. Magnetic resonance imaging (MRI) studies have shown that patients with inflammation show faster OA progression 12, confirming the hypothesis that inflammation has an impact on disease progression. This was supported further by reports in animal models, which showed that modulation of the inflammatory pathways have the potential to alter disease progression 13. Understanding the biology of synovial inflammation and the disturbed homeostasis in OA may ultimately reveal directions for new therapies. Further, it is pivotal to combine clinical and cellular parameters when trying to resolve the pathophysiology of this heterogeneous disease. To date, however, our knowledge of these inflammatory processes is insufficient and lacks answers to very basic questions, such as: which cell types are responsible for maintaining synovial inflammation in OA joints, and does the inflammatory pattern differ between OA subtypes? Studies by Sakkas et al. suggest that T cells are the predominant cell type in OA synovium and especially underline the role of T cells with an activated phenotype, as well as T helper type 1 (Th1) polarized cells 14,15. Conversely, the study by Bondeson et al. favoured the role of synovial macrophages and their main proinflammatory cytokines [interleukin (IL)-1, tumour necrosis factor (TNF)-α] in driving OA synovitis 16. The majority of previous reports either analysed synovial fluid (SF) samples or utilized histology, when synovial membrane (SM) samples were included. The SM is the main site of inflammation where cell–cell interaction takes place. Thus, it is of utmost interest to analyse this tissue in OA pathology. Clinical data of disease subtypes have received little attention when analysing OA joint samples for cellular and molecular parameters.
The overarching aim of the current project was to: (i) quantify the mononuclear cell infiltrate and their cytokines in the affected joints of OA patients; and (ii) assess how this differs in two common knee OA subtypes. Using SM samples in a unique study population we demonstrate, for the first time to our knowledge, that CD14+ macrophages are the major cell population in unicompartmental (UC) OA, whereas in bicompartmental (BC) OA CD14+ macrophages are accompanied by significantly higher CD4+ T cell concentrations. Furthermore, our study shows a significant increase of proinflammatory cytokines in BC compared to UC OA. Including clinical data in the interpretation of cellular and molecular analysis is pivotal in further resolving the complex pathophysiology of OA.
Material and methods
Study population
Fifty-nine patients with primary knee OA (35 women, 24 men) were enrolled into this study (Table 1). OA was diagnosed according to the criteria defined by the American College of Rheumatology. Based on anterior–posterior, sagittal and varus–valgus stress radiographs, two distinct groups were defined as medial UC OA and BC OA (Fig. 1). The Kellgren and Lawrence (K&L) scoring system was used to assess the radiographic severity of OA 17. The diagnosis was re-evaluated intra-operatively. Patients with UC OA were scheduled for UC and those with BC OA for total knee arthroplasty. None of the patients had an underlying inflammatory pathology or signs of systemic inflammation as determined by blood analysis. None of the patients were taking corticosteroids or any other kind of disease-modifying anti-rheumatic drugs. The local ethics committee of the University of Heidelberg approved the study (approval code: S333/2007) and informed consent from all patients was obtained prior to study enrolment.
Table 1.
Study population
| Total study population | UC OA | BC OA | P-values | |
|---|---|---|---|---|
| Number of patients n | 59 | 30 | 29 | |
| Gender, n (%) | ||||
| Male | 24 (40·7%) | 16 (53·3%) | 8 (27·5%) | 0·0641 |
| Female | 35 (59·3%) | 14 (46·7%) | 21 (72·5%) | |
| Age at surgery, years | 0·1024 | |||
| Mean ± s.d. (range) | 67·2 ± 10·6 (40–89) | 65·0 ± 10·7 (41–89) | 69·5 ± 10·1 (40–85) | |
| Operation side n (%) | ||||
| Right | 25 (42·3%) | 10 (33·3%) | 15 (51·7%) | 0·1923 |
| Left | 34 (57·7%) | 20 (66·6%) | 14 (48·3%) | |
| BMI kg/m2 | 0·3659 | |||
| Mean ± s.d. (range) | 30·3 ± 5·6 (19·8–43·2) | 29·6 ± 4·8 (20·6–40·1) | 30·9 ± 6·1 (19·8–43·2) | |
| Leucocytes cells/nl | 0·9999 | |||
| Mean ± s.d. (range) | 7·12 ± 1·6 (3·4–12) | 7·1 ± 1·2 (5·5–10·9) | 7·1 ± 1·9 (3·4–12) | |
| C-reactive protein mg/l | 0·1160 | |||
| Mean ± s.d. (range) | 4·5 ± 5·7 (2–39) | 3·3 ± 2·1 (2–9·6) | 5·6 ± 7·6 (2–39) | |
| K&L score, n (%) | 0·0797 | |||
| 3 | 50 (84·7%) | 28 (93·3%) | 22 (75·9%) | |
| 4 | 9 (15·3%) | 2 (6·7%) | 7 (24·1%) |
Demographic and clinical parameters of the study population are shown. Values are given as mean ± standard deviation (s.d.; range). Demographic parameters between study groups were compared using the unpaired t-test for parametric data [age, body mass index (BMI)] and the Fisher's exact test for proportions. All reported P-values are two-tailed. A P-value <0·05 was considered to show a statistically significant difference. OA = osteoarthritis; UC = unicompartmental; BC = bicompartmental; K&L score = Kellgren and Lawrence score.
Figure 1.
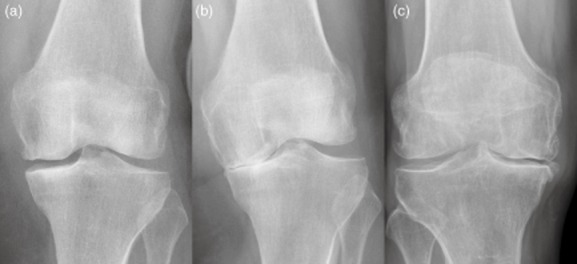
Radiographs of patients with unicompartmental and bicompartmental knee osteoarthritis (OA). Representative radiographs of patients with unicompartmental OA and bicompartmental OA (right) are shown. In unicompartmental OA the medial compartment is obliterated with (a) varus stress, and the lateral compartment is preserved with (b) valgus stress. In bicompartmental OA the medial and lateral compartment are affected (c), as shown by a reduced to obliterated joint space.
Sample collection and cell preparation
Peripheral blood (PB) samples were taken prior to surgery and joint samples at the time of surgery. SF was removed prior to arthrotomy by needle aspiration into heparinized tubes and stored at −80°C until further analysis. SM was taken from the suprapatellar pouch intra-operatively. SM samples were rinsed twice with phosphate-buffered saline (PBS), minced finely with sterilized scissors and digested with collagenase B (1 mg/ml; Roche Applied Science, Indianapolis, IN, USA) and bovine testicular hyaluronidase type IV (2 mg/ml; Sigma-Aldrich, St Louis, MO, USA) at 37°C for 2h in RPMI-1640 culture medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10 μg/ml penicillin–streptomycin (Invitrogen) and 10% fetal calf serum (FCS) (Biochrom AG, Berlin, Germany). The cell suspension was filtered through a 100 μm (BD Biosciences, Heidelberg, Germany) and a 40-μm pore-size cell strainer (EMD Millipore, Billerica, MA, USA) to remove any undigested tissue. The filtered cell suspension was washed twice with PBS. PB and SM mononuclear cells were isolated from heparin anti-coagulated whole blood and SM cell suspension using Ficoll-Paque™ PLUS (GE Healthcare, Cleveland, OH, USA) density gradient centrifugation.
Flow cytometry analysis and gating strategy
Multi-colour flow cytometry was used to identify mononuclear cells according to their cell surface markers. In brief, mononuclear cells were washed twice in magnetic affinity cell sorting (MACS) staining buffer, blocked with FCS blocking reagent and then stained (30 min at 4°C) with monoclonal antibodies (mAb) against CD4-allophycocyanin (APC)-cyanin 7 (Cy7) (BD clone: RPA-T4), CD8-VioBlue (Miltenyi clone: BW135/80), CD14-fluorescein isothiocyanate (FITC) (BD Pharmingen clone: M5E2), CD16-phycoerythrin (PE)-Cy7 (BD clone: 3G8), CD19-PE (Miltenyi clone: LT19) and CD56-APC (Miltenyi clone: AF12-7H3). The cells were washed again and taken into a final volume of 200 μl MACS staining buffer. Immediately before flow cytometric detection, cells were stained with 7-aminoactinomycin D (7-AAD; eBioscience, San Diego, CA, USA) with a final concentration of 0·5 μg/ml. A total of 105 events were assessed and analysed with a MACS-Quant flow cytometer (Miltenyi, Bergisch Gladbach, Germany). Data analysis was performed using FlowJo version 9·6 (TreeStar, Inc., Ashland, OR, USA). Cell debris and dead cells were excluded (7-AAD staining and forward-scatter profile) and mononuclear cells were gated based on their forward- and side-scatter profiles. Mononuclear cell subsets were defined by their surface marker expression as CD4+ T cells, CD8+ T cells, CD14+ macrophages, CD19+ B cells and CD16+CD56+ natural killer (NK) cells. The cut-off for all cell surface markers was defined based on isotype controls.
Multiplex cytokine analysis
The Pro-Human Cytokine Multiplex Assays (Bio-Rad, Munich, Germany) was used to analyse the cytokines in synovial fluid samples. The 27-plex analyses for IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8 (CXCL8), IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin (CCL11), macrophage colony-stimulating factor (M-CSF), interferon (IFN)-γ, monocyte chemotactic protein 1 (MCP-1; CCL2), macrophage inflammatory protein-1α (MIP-1α; CCL3), MIP-1β (CCL4), regulated upon activation normal T cell expressed and activated (RANTES) (CCL5), TNF-α and vascular endothelial growth factor (VEGF). The 21-plex contains, inter alia, IL-16, IL-18, leukaemia inhibitory factor (LIF) and macrophage migration inhibitory factor (MIF). Multiplex assays were carried out according to the manufacturers' instructions and run on the Luminex 200 platform. Bio-Plex Manager version 5·0 was used for data processing. Cytokine and chemokine concentrations were calculated by reference to the standard curve. The sensitivity of the multiplex kit was <5 pg/ml.
Statistical analysis
Demographic parameters between study groups were compared using the unpaired t-test for parametric data and Fisher's exact test for proportions. The unpaired t-test was used for analysis of mononuclear cell frequencies between study groups and the paired t-test for comparisons between concurrent PB and SM samples. The unpaired t-test was also performed to assess the differences between SF samples due to the predominantly Gaussian distribution. For cytokines which did not show Gaussian distribution, a Mann–Whitney U-test was performed. Correlation analysis between radiographic scores and inflammatory cells and cytokines was performed by Spearman's correlation analysis for non-parametric data and Pearson's correlation analysis for parametric data. All reported P-values are two-tailed. A P-value <0·05 was considered to show a statistically significant difference. Statistical analysis was performed using Prism version 5 software (GraphPad Software, Inc., San Diego, CA, USA) and spss version 22·0 (IBM SPSS, Inc., Chicago, IL, USA).
Results
Description of the study population
The demographic parameters and representative radiographs of the study population are shown in Table 1 and Fig. 1. The two study groups were comparable regarding clinical and demographic parameters. Even though there was a tendency towards higher K&L scores in the BC OA group, this was not statistically significant. The basic laboratory parameters [C-reactive protein (CRP), leucocytes] were within the standard range and did not show any signs of systematic inflammation at the time of surgery.
The SM shows a distinct mononuclear cell infiltration
Mononuclear cells of PB and the SM of the affected OA joints were evaluated for surface marker expression by flow cytometry; frequencies are shown in Fig. 2 . The frequency of mononuclear cells in PB did not show any significant difference between the two groups. In PB the distribution of mononuclear cells showed that CD14+ macrophages were the largest population (UC OA: 42·2 ± 4·7%; BC OA: 42 ± 2·8%), followed by CD4+ T helper cells (UC OA: 25·8 ± 6·2%; BC OA: 26·2 ± 8·1%) and CD8+ cytotoxic T cells (UC OA: 9·2 ± 2·4%; BC OA: 10·1 ± 4·4%). Frequencies of CD19+ B cells were 3·4 ± 3% in UC OA and 4 ± 3·2% in BC OA. CD16+CD56+ NK cells were within the same range, with 4 ± 2·5% in UC OA and 4·8 ± 0·9% in BC OA. All mononuclear cells displayed significantly smaller frequencies in SM samples when compared to concurrent PB samples in both groups, except for CD19+ B cells, as shown in Fig. 2a,b. As shown in Table 2 CD14+ macrophages and CD4+ T cells were the two main populations, followed by CD19+ B cells and CD8+ cytotoxic T cells. Hardly any CD16+CD56+ NK cells were detected in the SM.
Figure 2.
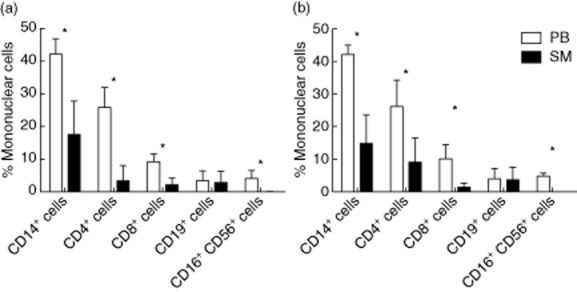
(a,b) Percentage of mononuclear cells in peripheral blood (PB) and synovial membrane (SM) of patients with unicompartmental and bicompartmental knee osteoarthritis (OA). Frequencies of the different cell populations are shown as the percentage of mononuclear cells for PB (white) and SM (black) for (a) unicompartmental and (b) bicompartmental OA. Mean values and standard deviation are plotted for both groups. Significant differences are marked with asterisks.
Table 2.
Frequencies of mononuclear cells in synovial membrane (SM) of unicompartmental and bicompartmental osteoarthritis (OA)
| UC OA | BC OA | P-values | |
|---|---|---|---|
| Sample volume (g) | 1·3 ± 0·7 (0·5–2·4) | 1·2 ± 0·4 (0·4–1·8) | 0·6988 |
| Total mononuclear cells, cells/mg | 135·7 ± 180 (7–731) | 805 ± 675 (12–1998) | 0·0009*** |
| CD14+ macrophages, % of mononuclear cells | 17·6 ± 10·2 (3·8–39·2) | 14·8 ± 8·9 (4·6–30) | 0·4983 |
| CD14+ macrophages, cells/mg | 28·1 ± 54·7 (0–227) | 112 ± 128 (2–416) | 0·0307* |
| CD4+ T cells, % of mononuclear cells | 3·4 ± 4·6 (0·3–16·9) | 9·1 ± 7·5 (0·8–22) | 0·0267* |
| CD4+ T cells, cells/mg | 2·6 ± 2·9 (0–9) | 85·1 ± 138 (1–440) | 0·0232* |
| CD8+ T cells, % of mononuclear cells | 2·1 ± 2·1 (0·4–8·1) | 1·4 ± 1·3 (0·34–4) | 0·3761 |
| CD8+ T cells, cells/mg | 2 ± 2·6 (0–10) | 9·9 ± 9·2 (0–25) | 0·0035** |
| CD19+ B cells, % of mononuclear cells | 2·8 ± 3·5 (0·02–13·4) | 3·7 ± 3·8 (0·2–12) | 0·5552 |
| CD19+ B cells, cells/mg | 3·8 ± 6·4 (0–24) | 39 ± 78 (0–246) | 0·0810 |
| CD16+CD56+ NK cells, % of mononuclear cells | 0·02 ± 0·04 (0–0·14) | 0·01 ± 0·01 (0–0·04) | 0·4722 |
| CD16+CD56+ NK cells, cells/mg | 0·0085 ± 0·0101 (0·0–0·0414) | 0·0679 ± 0·1045 (0·0016–0·2798) | 0·0313* |
Significant differences are indicated with asterisks: *P < 0·05; **P < 0·01; ***P < 0·001. Collected sample volumes and frequencies of the different cell populations are shown as the percentage of mononuclear cells and cell concentration (total cell counts per μl or μg) for peripheral blood (PB) and synovial membrane (SM). Values are shown as mean ± standard deviation (range). The unpaired t-test was used for analysis of mononuclear cell frequencies between study groups. All reported P-values are two-tailed. A P-value <0·05 was considered to show a statistically significant difference. OA = osteoarthritis; UC = unicompartmental; BC = bicompartmental; NK = natural killer.
Synovial mononuclear cells infiltration differs between OA subtypes
In order to assess the mononuclear infiltrate pattern between OA types we compared the SM of the affected joints. Dot-plots of representative SM samples from UC and BC OA are shown in Fig. 3. Total mononuclear cell infiltration of the SM was significantly higher in BC OA than UC OA (P = 0·0009) with a mean concentration of 805 ± 675 cells/mg in BC and 137·7 ± 180 cells/mg in UC OA (Table 2). There was a significant higher amount of CD4+ T cells in BC OA (9·1 ± 7·5%, P = 0·0267) when compared to UC (3·4 ± 4·6%). No significant differences were found for CD19+ B cells and CD16+CD56+ NK cells. Taking the higher amount of total mononuclear cells into account, the concentrations of all cell populations were higher in BC OA compared to UC OA, as shown in Table 3. Correlation analysis between the radiographic scores and the frequency of mononuclear cells did not show any significant correlation.
Figure 3.
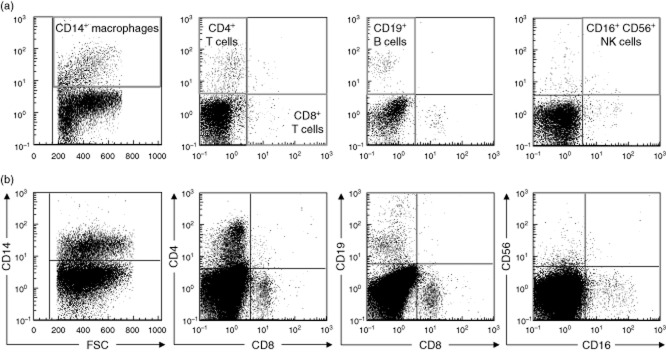
(a,b) Flow cytometry analysis of synovial mononuclear cells from unicompartmental and bicompartmental osteoarthritis (OA). Synovial membrane (SM) mononuclear cells were analysed by flow cytometry after staining with CD4–allophycocyanin (APC)–cyanin 7(Cy7) (BD clone: RPA-T4), CD8–VioBlue (Miltenyi clone: BW135/80), CD14-fluorescein isothiocyanate (FITC) (BD Pharmingen clone: M5E2), CD16–phycoerythrin (PE)–Cy7 (BD clone: 3G8), CD19–PE (Miltenyi clone: LT19) and CD56–APC (Miltenyi clone: AF12-7H3). Representative dot-plots for (a) one unicompartmental and (b) one bicompartmental OA patient are shown. Cell debris and dead cells were previously excluded [7-aminoactinomycin D (7-AAD) staining and forward-scatter profile] and mononuclear cells were gated based on their forward- and side-scatter profiles. Mononuclear cell subsets were defined by cell surface markers as CD4+ T cells; CD8+ T cells; CD19+ B cells; CD14+ macrophages; CD16+CD56+ natural killer (NK) cells, as indicated by the red gates. The cut-off for all cell surface markers was defined based on isotype controls.
Table 3.
Cytokines in synovial fluid
| Mediator | UC OA | BC OA | P-values |
|---|---|---|---|
| n = 14 | n = 20 | ||
| CXCL1 | 216·1 ± 438 (54·8–1 733) | 243·7 ± 206·4 (70·2–733·2) | 0·011* |
| Eotaxin | 5·93 ± 14·6 (0–40·7) | 14·17 ± 21·1 (0–82·3) | 0·045* |
| IFN-γ | 112·4 ± 37·3 (55·1–189) | 164 ± 85·1 (45·5–406·9) | 0·042* |
| IL-2 | 0·05 ± 0·2 (0–0·6) | 0·5 ± 1·1 (0–4·2) | 0·171 |
| IL-4 | 0·4 ± 0·2 (0·2–0·7) | 0·5 ± 0·2 (0·2-1·2) | 0·558 |
| IL-5 | 0·1 ± 0·2 (0–0·9) | 0·5 ± 1 (0–3·1) | 0·268 |
| IL-6 | 207·7 ± 273·1 (7·2–803·7) | 326·2 ± 423 (22·9–1 666) | 0·365 |
| IL-7 | 29·7 ± 9·1 (14·5–50·1) | 39·2 ± 14·2 (22·8–69·7) | 0·035* |
| IL-8 | 360 ± 923·3 (13·2–3 420) | 436·6 ± 502·9 (19·6–1 576) | 0·039* |
| IL-9 | 3·1 ± 1·5 (0–5·5) | 5·7 ± 4·6 (0·8–18) | 0·048* |
| IL-10 | 27·3 ± 8·1 (13·7–45·6) | 41·1 ± 18·6 (16·9–88·3) | 0·014* |
| IL-12 | 213·7 ± 61·1 (145·9–369·1) | 333·3 ± 174·9 (123·8–786·8) | 0·020* |
| IL-13 | 30·7 ± 11·4 (16·5–59·4) | 43·4 ± 17·9 (24–97·1) | 0·026* |
| IL-15 | 8·6 ± 4·9 (2·8–22·7) | 9·4 ± 3·9 (2·8–17·7) | 0·565 |
| IL-16 | 1127 ± 594·8 (421·1–2 672) | 1119 ± 868·6 (378·2–4256) | 0·975 |
| IL-18 | 90·3 ± 29·7 (48·6–152·1) | 108·1 ± 65·2 (43–321·6) | 0·349 |
| TNF-α | 4 ± 2·8 (0·5–10·5) | 5·6 ± 3·1 (1·7–12·2) | 0·135 |
| LIF | 44·4 ± 23·9 (16·4–85·8) | 49·1 ± 26·5 (10·1–108·6) | 0·602 |
| MCP-1 | 52·7 ± 39·7 (12·4–140·6) | 158 ± 378·7 (8·9–1 656) | 0·847 |
| M-CSF | 76 ± 63·9 (28·4–283·8) | 92·6 ± 41·9 (31·4–177·3) | 0·367 |
| MIF | 5177·5 ± 4499·4 (1461–14 415) | 8929·7 ± 25 029·5 (1000–114 827) | 0·753 |
| MIP-1α | 1·1 ± 1·2 (0–3) | 2·4 ± 2·8 (0–9·8) | 0·069 |
| MIP-1β | 33·3 ± 42·3 (12·8–59·2) | 44·6 ± 28·9 (16·24–133·1) | 0·190 |
| RANTES | 121·2 ± 211·6 (0·1–672·5) | 112·1 ± 397·5 (1·29–1 791) | 0·700 |
| VEGF | 1318·8 ± 655·5 (569·4–2 967) | 2783·3 ± 2519·2 (756·8–10 160) | 0·012* |
| IL-1α, IL-1β, IL-17 | Below detection level | Below detection level | n.a. |
Significant differences are indicated with asterisks: *P < 0·05; **P < 0·01; ***P < 0·001. The Pro-Human Cytokine Multiplex Assay (Bio-Rad) was used to analyse the cytokines in synovial fluid (SF) samples. Cytokine and chemokine concentrations were calculated by reference to the standard curve. The sensitivity of the multiplex kit was <5 pg/ml. The unpaired t-test was also performed to assess the differences between unicompartmental (UC) and bicompartmental (BC) osteoarthritis (OA) SF samples due to the predominantly Gaussian distribution. For cytokines which did not show Gaussian distribution, a Mann–Whitney U-test was performed. All reported P-values are two-tailed. A P-value <0·05 was considered to show a statistically significant difference. IFN = interferon; IL = interleukin; TNF = tumour necrosis factor; LIF = leukaemia inhibitory factor; MCP = monocyte chemotactic protein; M-CSF = macrophage colony-stimulating factor; MIP = macrophage inflammatory protein; RANTES = regulated upon activation normal T cell expressed and secreted; VEGF = vascular endothelial growth factor; n.a. = not applicable.
Higher proinflammatory cytokine concentration in synovial fluid of BC OA patients
Naive synovial fluid samples of patients (14 UC OA and 20 BC OA) were collected and analysed for their cytokine profile by multiplex analysis. The overall cytokine secretion pattern is shown in Fig. 4. IL-16 and VEGF showed the highest concentration, followed by CXCL1, IFN-γ, IL-6, IL-8, IL-12, IL-16, IL-18, MCP-1 and RANTES. Significant differences in cytokine expression were detected between the two patient groups, as shown in Table 3. Of the 28 measured cytokines, 10 were present at significantly higher levels in BC compared to UC OA. Seven of those 10 mediators possess proinflammatory characteristics (CXCL1, eotaxin, IL-7, IL-8, IL-9, IL-12, IFN-γ), while only two anti-inflammatory cytokines (IL-10, IL-13) were increased significantly in BC compared to UC OA. Other soluble mediators showed comparable levels between the groups. Only IL-16 was higher in UC than in BC OA, but this difference was not significant. Interestingly the Th17 cytokine, IL-17 and the predominantly macrophage-derived IL-1α and IL-1β were below detection levels. The cytokine concentrations did not show any significant correlation with the radiographic scores.
Figure 4.
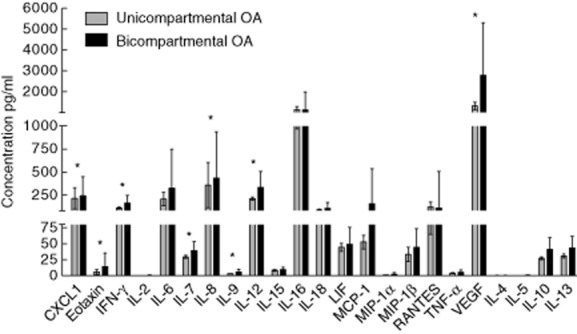
Cytokine pattern in synovial fluid (SF) of patients with unicompartmental and bicompartmental osteoarthritis (OA). The Pro-Human Cytokine Multiplex Assay (Bio-Rad) was used to analyse the cytokines in SF samples. Cytokine and chemokine concentrations were calculated by reference to the standard curve. The sensitivity of the multiplex kit was <5 pg/ml. Mean values and standard deviation are plotted for both groups. The unpaired t-test was also performed to assess the differences between unicompartmental and bicompartmental OA SF samples due to the predominantly Gaussian distribution. For cytokines, which did not show Gaussian distribution, a Mann–Whitney U-test was performed. All reported P-values are two-tailed. A P-value <0·05 was considered to show a statistically significant difference. Significant differences are indicated with asterisks.
Discussion
A growing body of evidence indicates that synovial inflammation is not only responsible for the main clinical symptoms but also contributes significantly to OA pathology. From RA treatment strategies we know that the modulation of inflammatory pathways holds a strong promise in opening new treatment avenues. In order to map the pattern of synovial inflammation in OA the analysis of joint samples, and in particular the SM, as the main site for mononuclear cell infiltration, is pivotal. Until the present, synovitis in OA has not been decoded sufficiently and our knowledge is based mainly on histological studies of end-stage OA patients 3,4. The heterogeneous nature of OA has not been addressed adequately, and studies analysing OA subgroups based on clinical and radiographic parameters are missing. In this study we provide flow cytometric data about the quantity of mononuclear cells in OA SM and compare this between two different OA subtypes.
Mononuclear cells in OA synovium
This is one of a very limited number of studies using flow cytometry to analyse the inflammatory pattern in the SM, which we consider to be the relevant site of cell interaction. We show, for the first time, that the inflammatory pattern of UC and BC knee OA differs significantly regarding the amount of mononuclear cells and the involved cell lines. Whereas, in UC OA, mononuclear cells displayed a concentration of 135·7 ± 180 cells/mg, this was increased significantly more than fivefold in BC OA with 805 ± 675 cells/mg. Further characterization of this mononuclear infiltrate showed that CD14+ macrophages were the largest cell population in UC OA, which were accompanied by a significant amount of CD4+ T cells in BC OA. Quantitative analysis showed that in UC OA, the macrophage population was almost sixfold as high as the CD4+ T cell population. This predominance was reduced in BC OA. While macrophages remained at the same level, the CD4+ T cells were increased significantly in bicompartmental OA to an average of 9·1 ± 7·5%.
Comparison of our data to previous reports is limited, due to utilization of different techniques and different study populations. Previous immunohistological studies indicated that CD68+ macrophages were detectable in the lining layer and CD4+ cells were present at the sublining layer of the synovium, and these were of higher frequency than CD8+ cells and CD19+ cells 3,4. Interestingly, a recent flow cytometric study of the infrapatellar fat pad showed that CD14+ macrophages were the most abundant immune cells present in both adipose tissues, followed by CD3+ T cells, mast cells and B cells 7. Our results amend these studies by identifying the different mononuclear cell populations and providing quantitative data about their frequency in OA synovium and by showing that the composition of inflammatory cells differs in OA subgroups. A previous study showed that macrophages contribute to OA inflammation by demonstrating that depletion of these cells in OA synovium led to a decrease of macrophage-derived cytokines (IL-1 and TNF-α), but also of fibroblast-derived cytokines [IL-6, IL-8, matrix metalloproteinases (MMP)-1 and MMP-3] 16. As shown by our results, the functional analysis performed in this study could be of relevance in UC OA, as macrophages were the predominant cell line. Interestingly, depletion of T cells in this study did not result in any changes which, to our understanding, is due primarily to the fact that the authors could not detect any T cells in OA synovium. This differs significantly from our findings in BC OA, where CD4+ T cells were the second-largest population. It would be of pivotal interest to analyse further the role of synovial CD4+ T cells through functional studies in BC OA, which will be the focus of further studies by our group. Our findings in BC OA are in support of some immunohistological reports of OA joint samples, which have shown the presence of activated phenotypes of CD4+ T cells in the SM 2,15. This was supported further by studies reporting clonal expansion of the T cell receptor, and a decreased expression of the CD3ζ chain in OA synovial membrane, which may reflect activation and chronic antigenic stimulation of T cells 2,14.
Role of cytokines in OA affected joints
The cellular changes detailed above accompanied a shift of the cytokine profile towards a proinflammatory milieu in BC OA, as shown by the analysis of naive SF samples. Here, a significant increase of a broad variety of proinflammatory cytokines was observed when comparing UC to BC OA. Some groups have proposed TNF-α as a key player 8,18 in OA pathogenesis, while others have suggested IL-1 and IL-6 6,19–21. We could not detect high concentrations of IL-1 or TNF-α in our samples, which is contrary to these reports. Interestingly, IL-1 as the main macrophage cytokine, and IL-17 as the main Th17 secreted cytokine, which had been reported by others to be involved in OA progression 9,22,23, were also not detectable in our samples. These cytokines could play a more relevant role at earlier stages of OA, and may have contributed to the expression of downstream mediators in late-stage OA, as described for CXCL1, eotaxin and IL-7 9,22,24,25. This hypothesis is strengthened further by a recent study showing that serum levels of IL-1β and TNF-α are increased in early compared to advanced OA 26,27, concluding that they play a more important role in developing OA, while subsiding with disease progression. The synovial analysis performed in our study suggests that the proinflammatory cytokines CXCL1, eotaxin, IL-7, IL-8, IL-9, IL-12 and IFN-γ are major players in maintaining and increasing the proinflammatory milieu in mid- to BC OA, thereby attracting the migration of inflammatory cells into the synovial membrane. Our data are in accordance with studies from other groups that have shown increased levels of CXCL1, eotaxin, IL-7, IL-8, IL-9, IL-10, IL-12 and IL-13 25,28–33 in OA compared to healthy controls. Furthermore, we detected a significant increase of the pro-angiogenetic growth factor VEGF, which might play a role in maintaining synovial hypertrophy and mononuclear infiltration by increasing blood flow in the joint tissues 34,35. Some specific pathogenic mechanisms have already been described for the above-mentioned mediators. CXCL-1 and IL-8 induce differentiation and calcification of articular cartilage, leading eventually to increased apoptosis and chondral degradation 36–39. IL-12 is produced by macrophages and is known to induce primarily IFN-γ production from T cells, thus enhancing the proliferation of Th1 cells 10,40. The significant increase of IL-12 in BC OA in our study could serve as an explanation for the increasing T cell response in end-stage OA, as shown above. It is also notable that the overall expression of a broad variety of proinflammatory mediators such as MIF, MIP, LIF and MCP1 is present in OA joints, although these cytokines did not increase significantly with OA stage.
UC and BC OA – different OA subtypes or stages of the same disease?
Until now it has been unknown if UC and BC knee OA are different stages of the same disease, or if they should be viewed as completely different OA subtypes with a different pathophysiology. Demographic data, and especially age at the time of surgery, were similar in both groups, which runs counter to the theory that these groups are consecutive stages of the same disease. Even though there was a tendency towards higher K&L scores in the BC OA group, this did not reach statistical significance. Interpretation of our data is dependent upon how these two knee OA subtypes are viewed. If they were viewed as different OA stages, then our data would provide a temporal pattern of mononuclear cell infiltration, showing that mononuclear cells increase with OA progression and the composition of cells is shifted from the macrophage lineage to a macrophage and lymphocyte interaction. The increased concentration of mononuclear cells from UC to BC OA and the fact that cytokine concentrations increased significantly in BC OA is in support of this hypothesis. Thus, assuming this, we hypothesize that synovial macrophages play a relevant role in OA initiation and are the first mononuclear cell lines in place. Studies analysing SM samples from healthy donors indicate that macrophages are part of the normal SM composition 19,21. These resident macrophages can be activated by mechanical, cellular or soluble stimuli 9,22. Thus, activation of resident macrophages could be the first part of the adaptive immune response in UC OA joints, which is supported by our data showing that macrophages were the major cell population in these joints. The slight decrease in BC OA could indicate that at later time-points macrophages play a less important role. Considering BC OA as the advanced stage of OA, it can be concluded that CD4+ T cells infiltrate the SM with disease progression and seem to contribute to OA pathology at later stages. From rheumatoid arthritis (RA) studies we know that macrophages and T lymphocytes contribute to the chronic inflammation by a very close bilateral interaction 13,36. A study by Shen et al. supports the hypothesis that T helper cells contribute to disease progression in OA by inducing the expression of MIP-1γ and nuclear factor-κB (NF-κB) 10,16. In this study CD4+ T cell knock-out mice showed less expression of MIP-1γ and slower cartilage degeneration.
Few studies have looked at the inflammatory pattern in different OA stages 3–5,41,42. The study by Benito et al. showed that the synovial membrane from patients with early OA demonstrated significantly greater CD4+ and CD68+ cell infiltration, blood vessel formation, VEGF and intercellular adhesion molecule-1 (ICAM-1) expression. The number of cells producing TNF-α and IL-1β were also significantly greater in early OA 3,4,41. This seems contrary to our results, but it should be taken into account that our study population utilized techniques different to these previous reports. In the study by Benito et al., the early study group was generated from patients undergoing arthroscopic procedures, which can be considered an even earlier stage of OA than in our study. This could mean that the initiating step of OA is accompanied by a high inflammatory pattern, which decreases in midterm OA and increases further towards BC OA. Nair et al. showed that the joint fluid sCD14 levels in early OA were comparable to BC OA 7,42. The study by Scanzello et al. suggested that IL-15 is elevated in early OA. In summary, these studies confirm that a temporal pattern exists in OA inflammation, which we think is of pivotal importance in order to understand OA pathophysiology.
Viewing these study groups as different OA subtypes with an individual pathophysiology could lead to the interpretation that BC OA is a significantly more inflammatory disease than UC OA, as shown by the higher presence of mononuclear cells and higher cytokine concentrations. Further, it suggests that inflammation in UC OA is primarily macrophage-driven, while in BC OA macrophages and CD4+ T cells are the predominant cell lines and contribute to disease progression. The fact that radiographic scoring showed comparable results in both groups would further support the hypothesis that the observed differences are due to a different pathophysiology rather than to progression of the disease. Conversely, it should be mentioned that there was a tendency towards higher K&L scores in BC OA. Further studies with a prospective set-up following the progression of the disease in patients with UC OA would be of particular interest to answer this question.
What triggers this infiltration is a question that arises immediately when acknowledging the infiltration of OA joints by inflammatory cells. Because the joint-derived samples did not mirror the PB, and the pattern of mononuclear cell infiltration was different in UC and BC OA, we believe that further distinct mechanisms are in place for the selective recruitment of mononuclear cells into the joints. Joint trauma has been shown to lead to an elevated level of chondrodestructive cytokines, such as IL-1β and TNF-α 2–9,26. These chondrodestructive cytokines may play an important role in the attraction of mononuclear cells and the development of an inflammatory milieu. Further, it is thought that the recruitment of effector cells is orchestrated in part by chemokines 10,11,28,29. A study by Scanzello et al. indicated that synovia of traumatic meniscal injury exhibit a unique chemokine signature, which could further initiate and drive synovial cell influx 12,34.
Low amounts of other mononuclear cells
The quantities of CD19+ B cells and CD8+ cytotoxic T cells were within the same range as CD4+ T cells in UC OA. The relative number of these cells (shown as percentage of mononuclear cells) remained constant, although with OA progression their total cell number increased as a result of the higher total mononuclear cell infiltration. Thus, CD8+ T, CD19+ B and CD16+CD56+ NK cells seem to be of minor importance for synovial inflammation if taken by their amounts in the joints. However, cell numbers alone probably do not translate directly to their role in OA pathology. The study by Da et al. reported on B cells in OA synovium and could detect these in half the patients. A higher T cell count was also shown in these patients, suggesting that infiltration perpetuates a chronic influx of other cells 16,43. The prevalence of autoantibodies to various arthritis-related proteins in early-stage knee OA supports the involvement of a specific immune response in initial cartilage degeneration in OA 2,15,44. Hardly any CD16+CD56+ NK cells were detected in our samples. The study by Huss et al. confirmed the presence of CD4+ T cells in OA joints but showed significantly higher numbers of NK cells, which is in striking contrast to our data 2,14,45.
Downstream effect of mononuclear cells and cytokines
Both the mononuclear cells described above and the cytokines detected in OA joints could not only have a direct effect on joint tissues, but could also lead to a downstream effect that perpetuates and promotes OA pathology. The study by Scanzello et al. demonstrated that MMP transcript levels in the synovium of OA patients correlated with CD3 and CD68 gene expression 5,10. The increasing inflammatory milieu in BC OA joints may be the end-point of a vicious cycle, in which release of cartilage breakdown forms the initial step leading to activation of inflammatory cells and the release of cytokines. In return, these cytokines may contribute to joint inflammation and destruction by promoting the influx and activation of inflammatory cells 5,41,42,46,47 and by the induction of chondrodestructive mechanisms through the release of matrix-degrading enzymes, such as MMPs 7,25,41, and the suppression of proteoglycan synthesis 39,41,48–50. The quantitative data concerning the cytokine pattern and the differences between OA subtypes provided in this study could help to further dissolve the inflammatory pattern in OA.
Strength and limitations
A major strength of this study is the generated study population, which included patients of different OA subtypes and shows for the first time, to the best of our knowledge, that the inflammatory pattern differs between OA subgroups. Even though we assessed and included all available clinical and radiographic data, it should be taken into account that human OA develops over decades and clinical assessment can lack accuracy regarding memorable numbers of joint traumas, etc. Nevertheless, the inclusion of clinical and radiographic parameters in the interpretation of cellular and molecular data is pivotal when analysing human samples. It would be of special interest to compare our findings to healthy controls, but this could not be performed due to ethical considerations and remains a limitation of this study.
Future prospects
The methodology established and described in this study can now be used to further analyse the subsets of these mononuclear cells in the affected joints. This is of utmost interest, as the polarization towards inflammatory or regulatory cells is critical in understanding the inflammatory pathways. Ishii et al. reported a Th1 polarization pattern in OA synovium 4,42, which was also confirmed by another study 8,43. In a recent study by our group, we report the significant accumulation of regulatory T cells into OA joints 44,51. Further, functional studies are needed in order to evaluate the role of these mononuclear cells in OA pathology and analyse how their quantity translates into functional properties.
Acknowledgments
The University of Heidelberg funded this study. We are grateful for the technical support of Patrick Göthlich.
Disclosure
The University of Heidelberg funded this study. The funding source did not have any involvement in study design, data collection, analysis and interpretation of data, writing of the report and the decision to submit the paper for publication. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. The authors have no potential or apparent conflicts of interest with regard to this work.
Device status/drug statement
The manuscript submitted does not contain information about medical device (s)/drug (s).
Author contributions
B. M., F. Z. and S. H. made substantial contributions to study conception and design. B. M., N. R. and E. T. carried out the flow cytometry study. B. M., N. R., E. T. and J. K. made substantial contributions to the data acquisition. B. M., N. R., A. B., F. Z. and S. H. made substantial contribution to data analysis and interpretation. B. M., N. R., T. G. and S. H. drafted the manuscript and E. T., J. K., A. B. and F. Z. revised it critically. B. M., N. R. and S. H. performed the statistical analysis. All authors read and approved the final manuscript.
References
- Goldenberg DL, Egan MS, Cohen AS. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982;9:204–209. [PubMed] [Google Scholar]
- Nakamura H, Yoshino S, Kato T, Tsuruha J, Nishioka K. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthr Cartil. 1999;7:401–402. doi: 10.1053/joca.1998.0224. [DOI] [PubMed] [Google Scholar]
- Saito I, Koshino T, Nakashima K, Uesugi M, Saito T. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthr Cartil. 2002;10:156–162. doi: 10.1053/joca.2001.0494. [DOI] [PubMed] [Google Scholar]
- Ishii H, Tanaka H, Katoh K, Nakamura H, Nagashima M, Yoshino S. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthr Cartil. 2002;10:277–281. doi: 10.1053/joca.2001.0509. [DOI] [PubMed] [Google Scholar]
- Scanzello CR, Umoh E, Pessler F, et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthr Cartil. 2009;17:1040–1048. doi: 10.1016/j.joca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Goekoop RJ, Kloppenburg M, Kroon HM, et al. Low innate production of interleukin-1beta and interleukin-6 is associated with the absence of osteoarthritis in old age. Osteoarthr Cartil. 2010;18:942–947. doi: 10.1016/j.joca.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851–857. doi: 10.1136/ard.2010.140046. [DOI] [PubMed] [Google Scholar]
- Yamada H, Nakashima Y, Okazaki K, et al. Preferential accumulation of activated Th1 cells not only in rheumatoid arthritis but also in osteoarthritis joints. J Rheumatol. 2011;38:1569–1575. doi: 10.3899/jrheum.101355. [DOI] [PubMed] [Google Scholar]
- Sohn DH, Sokolove J, Sharpe O, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P-C, Wu C-L, Jou I-M, et al. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthr Cartil. 2011;19:728–736. doi: 10.1016/j.joca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Lewis JS, Furman BD, Zeitler E, et al. Genetic and cellular evidence of decreased inflammation associated with reduced incidence of posttraumatic arthritis in MRL/MpJ mice. Arthritis Rheum. 2013;65:660–670. doi: 10.1002/art.37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer FW, Guermazi A, Felson DT, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–1809. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Rozelle AL, Lepus CM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas LI, Koussidis G, Avgerinos E, Gaughan J, Platsoucas CD. Decreased expression of the CD3zeta chain in T cells infiltrating the synovial membrane of patients with osteoarthritis. Clin Diagn Lab Immunol. 2004;11:195–202. doi: 10.1128/CDLI.11.1.195-202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas LI, Platsoucas CD. The role of T cells in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:409–424. doi: 10.1002/art.22369. [DOI] [PubMed] [Google Scholar]
- Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha-Scheepers S, Watt I, Slagboom E, et al. Innate production of tumour necrosis factor alpha and interleukin 10 is associated with radiological progression of knee osteoarthritis. Ann Rheum Dis. 2008;67:1165–1169. doi: 10.1136/ard.2007.084657. [DOI] [PubMed] [Google Scholar]
- Singh JA, Arayssi T, Duray P, Schumacher HR. Immunohistochemistry of normal human knee synovium: a quantitative study. Ann Rheum Dis. 2004;63:785–790. doi: 10.1136/ard.2003.013383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riyazi N, Slagboom E, de Craen AJ, et al. Association of the risk of osteoarthritis with high innate production of interleukin-1beta and low innate production of interleukin-10 ex vivo, upon lipopolysaccharide stimulation. Arthritis Rheum. 2005;52:1443–1450. doi: 10.1002/art.21014. [DOI] [PubMed] [Google Scholar]
- Smith MD. The normal synovium. Open Rheumatol J. 2011;5:100–106. doi: 10.2174/1874312901105010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofat N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int J Exp Pathol. 2009;90:463–479. doi: 10.1111/j.1365-2613.2009.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Deng Y, Tan Y, Qin J, Chen LB. Association between severity of knee osteoarthritis and serum and synovial fluid interleukin 17 concentrations. J Intern Med Res. 2014;42:138–144. doi: 10.1177/0300060513501751. [DOI] [PubMed] [Google Scholar]
- Long D, Blake S, Song X-Y, Lark M, Loeser RF. Human articular chondrocytes produce IL-7 and respond to IL-7 with increased production of matrix metalloproteinase-13. Arthritis Res Ther. 2008;10:R23. doi: 10.1186/ar2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-H, Hsieh M-S, Liang Y-C, et al. Production of the chemokine eotaxin-1 in osteoarthritis and its role in cartilage degradation. J Cell Biochem. 2004;93:929–939. doi: 10.1002/jcb.20239. [DOI] [PubMed] [Google Scholar]
- Marks PH, Donaldson MLC. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21:1342–1347. doi: 10.1016/j.arthro.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barker T, Rogers VE, Henriksen VT, et al. Serum cytokines are increased and circulating micronutrients are not altered in subjects with early compared to advanced knee osteoarthritis. Cytokine. 2014;68:133–136. doi: 10.1016/j.cyto.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Doodes PD, Cao Y, Hamel KM, et al. CCR5 is involved in resolution of inflammation in proteoglycan-induced arthritis. Arthritis Rheum. 2009;60:2945–2953. doi: 10.1002/art.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flytlie HA, Hvid M, Lindgreen E, et al. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine. 2010;49:24–29. doi: 10.1016/j.cyto.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Heard BJ, Fritzler MJ, Wiley JP, et al. Intraarticular and systemic inflammatory profiles may identify patients with osteoarthritis. J Rheumatol. 2013;40:1379–1387. doi: 10.3899/jrheum.121204. [DOI] [PubMed] [Google Scholar]
- Beekhuizen M, Gierman LM, van Spil WE, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthr Cartil. 2013;21:918–922. doi: 10.1016/j.joca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Vangsness CT, Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis-a pilot study. Bull NYU Hosp Joint Dis. 2011;69:122–127. [PubMed] [Google Scholar]
- Furuzawa-Carballeda J, Macip-Rodríguez PM, Cabral AR. Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin Exp Rheumatol. 2008;26:554–560. [PubMed] [Google Scholar]
- Scanzello CR, McKeon B, Swaim BH, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood L, McWilliams DF, Pearson CI, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- Gao S, Hao B, Yang XF, Chen WQ. Decreased CD200R expression on monocyte-derived macrophages correlates with Th17/Treg imbalance and disease activity in rheumatoid arthritis patients. Inflamm Res. 2014;63:441–450. doi: 10.1007/s00011-014-0716-6. [DOI] [PubMed] [Google Scholar]
- Olivotto E, Vitellozzi R, Fernandez P, et al. Chondrocyte hypertrophy and apoptosis induced by GROalpha require three-dimensional interaction with the extracellular matrix and a co-receptor role of chondroitin sulfate and are associated with the mitochondrial splicing variant of cathepsin B. J Cell Physiol. 2007;210:417–427. doi: 10.1002/jcp.20864. [DOI] [PubMed] [Google Scholar]
- Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171:4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- Borzì RM, Mazzetti I, Marcu KB, Facchini A. Chemokines in cartilage degradation. Clin Orthop Relat Res. 2004;(427 Suppl):S53–61. doi: 10.1097/01.blo.0000143805.64755.4f. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Kanda V, Bush-Joseph C, et al. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to toll-like receptor 4 and toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum. 2012;64:2268–2277. doi: 10.1002/art.34495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da R-R, Qin Y, Baeten D, Zhang Y. B cell clonal expansion and somatic hypermutation of Ig variable heavy chain genes in the synovial membrane of patients with osteoarthritis. J Immunol. 2007;178:557–565. doi: 10.4049/jimmunol.178.1.557. [DOI] [PubMed] [Google Scholar]
- Tsuruha J-I, Masuko-Hongo K, Kato T, et al. Autoimmunity against YKL-39: a human cartilage derived protein, in patients with osteoarthritis. J Rheumatol. 2002;29:1459–1466. [PubMed] [Google Scholar]
- Huss RS, Huddleston JI, Goodman SB, Butcher EC, Zabel BA. Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum. 2010;62:3799–3805. doi: 10.1002/art.27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J, Campagnaro S, Sallusto F, Lanzavecchia A. TCR-independent proliferation and differentiation of human CD4+ T cell subsets induced by cytokines. Adv Exp Med Biol. 2002;512:107–112. doi: 10.1007/978-1-4615-0757-4_14. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Smith RL. Degradative enzymes in osteoarthritis. Front Biosci. 1999;4:D704–712. doi: 10.2741/a388. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Nakamura H, Obata K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yammani RR, Long D, Loeser RF. Interleukin-7 stimulates secretion of S100A4 by activating the JAK/STAT signaling pathway in human articular chondrocytes. Arthritis Rheum. 2009;60:792–800. doi: 10.1002/art.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi B, Schnatzer P, Hagmann S, et al. CD4+CD25+/highCD127low/− regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints – analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther. 2014;16:R97. doi: 10.1186/ar4545. [DOI] [PMC free article] [PubMed] [Google Scholar]


