Abstract
Previous studies have shown developmental differences in smooth muscle tone of the airways. Differences in airway mechanics may be based upon cellular differences between animals of different ages. We developed a method [JAP 86(1): 427–35, 1999] for isolating ovine tracheal smooth muscle cells and for measuring shortening velocity. This technique was used to study the change in contractile response of the airway smooth muscle cell during development. Here we report differences between preterm (110–124 or 125–140 days post-conception), newborn (3–7 days postnatal) and adult (9–36 months) cells. These cells were compared with respect to morphometry, shortening velocity, and percent shortening. The neonatal cells were shorter and narrower than the adult cells. Maximum shortening velocity was faster for adult (45.1 μm/sec) than for neonatal cells (range 11.1 to 25.1 μm/s). When velocity was normalized to the cell length, there was no difference between the adult and preterm cells, but there was a significant difference between the newborn (0.30 sec−1) and adult (0.54 sec−1) cells. The percent shortening did not show any significant difference with age. Within the neonatal groups, there were no significant differences in morphometry, shortening or velocity. To facilitate comparison between ASM tissues of different sizes with different sized cells, we also expressed percent shortening and velocities relative to a hypothetical 1mm segment of tissue. Represented this way, the amount of shortening for all age groups was the same, but the predicted maximum velocity of the hypothetical preterm tissue (125–140 days) was significantly greater than for newborn.
Keywords: single cell isolation, ovine airway smooth muscle, respiratory mechanics, development, bronchoconstriction
Introduction
The ability to treat neonatal respiratory problems has improved significantly with the advent of infant intensive care units, new mechanical ventilation techniques, exogenous surfactant treatment, positive end-distending pressure, and advanced monitoring systems. Because of these techniques, a greater number of smaller (<1500 grams) and more immature (<30 weeks gestation) infants are being treated, survive respiratory distress and recover uneventfully. However, as many as a third of those infants who require prolonged mechanical ventilation develop chronic respiratory problems1,2. Specifically, airway injury and dysfunction is a hallmark of bronchopulmonary dysplasia (BPD) and the presumably greater assisted ventilatory requirements of premature infants relative to the older infants precipitates an age-related predisposition for airway deformation and damage3.
To better understand the problems of airway injury during early development, animal tracheae have been used extensively as a model to study mechanisms that determine maturational changes in airway function4–8. These studies have demonstrated that the highly compliant immature airway is relatively more susceptible to deformational changes associated with barotrauma as compared to the less compliant airway of the older infant or adult. With development, as the airways stiffen they become more stable, thus less collapsible conferring less resistance to airflow.
However, developmental age is not the only factor that affects tracheal compliance. It has been shown previously that increased muscle tone can increase the rigidity of the trachea in the full-term newborn lamb4,8. This finding is suggestive of a mechanism by which ventilation-induced alterations of the airway can be ameliorated. If the ability of ASM to increase tone in response to pharmacologic stimulation is age- and/or regionally-dependent, then prophylactic treatment prior to ventilation may have differential effects on the proximal versus peripheral airways of the developing animal. This intervention could conceivably lessen the damage caused by ventilation. It follows that a thorough understanding of the airways and all their component structures would advance the development of such a clinical approach.
Over the last several decades, considerable progress has been made in characterizing developmental differences in the physiology of the trachea with respect to pressure-volume and pressure-flow relationships, the effects of mechanical ventilation, and pharmacologic stimulation4,6,8,9–13. These studies, conducted using in vivo, in vitro, and muscle bath preparations, lend valuable insight into the developmental differences in airway function.
More recent studies on structural maturation of airway smooth muscle at the cellular, ultrastructural, and protein expression levels have elucidated structural mechanisms for developmental changes in function14–16. While the individual lengths of isolated tracheal smooth muscle cells increased from prematurity to adulthood, a linear increase in cell length was not demonstrated during the neonatal period, precluding cell length changes as a mechanism for functional development14. Similarly, developmental alterations in the electron microscopic ultrastructure of the tracheal smooth muscle could not be identified as potential mechanisms. At the protein expression level, the expression of total myosin content, SM1 and SM2 myosin heavy chain isoforms, increased across development. This increase correlates with the developmental increase in tracheal smooth muscle force development, providing one possible mechanism for the functional maturation of this airway smooth muscle.
Because contraction of ASM tissues is modulated by paracrine agents released by epithelial cells17, study of the isolated ASM cells allows cellular contractile responses to be measured without the influence of substances released from epithelial cells and nerve endings. In addition, measurement of some contractile properties, such as determination of the unloaded shortening rate, are actually easier with single cells than with muscle strips because the responses are not affected by passive tissue elements (collagen and elastin). A further advantage is that when isolated cells are allowed to contract freely, the series elastic component plays a minimal role in the response because the cells have not been forced to develop high levels of isometric force, as they would in a muscle strip that was being studied by an afterloaded quick release protocol. Further, use of an isolated ASM cell preparation can provide direct insight regarding airway responsiveness to agonists and dimensional changes to stretch, stimuli associated with in vivo challenges such as mechanical ventilation. As such, the isolated ASM preparation should provide further understanding of basic biological development of the airway which can facilitate continual advancement of neonatal, pediatric, and adult clinical respiratory management.
In the past, we described methods for isolating contractile smooth muscle cells from smooth muscle tissue, and measuring single cell shortening velocity18. The response of the isolated cells that can most readily be studied is unloaded shortening, which is described by the unloaded shortening rate constant and the percent shortening. The rate constant reflects the myosin cross-bridge cycling rate, while percent shortening illustrates the intrinsic shortening ability of the unloaded cell19. Therefore, in the present study, in order to further understand the differences in developing airway smooth muscle, we determined the maximum and average shortening velocities, in microns/second, of isolated cells as a function of developmental age.
Furthermore, we examined developmental differences at the level of the individual smooth muscle cells. Of particular interest was whether or not changes in the contractile response of cells could be tied to cellular changes that take place during development. The previous study looked only at the extremes of age, preterm and adult. We believe that, just as there is significant postnatal development of the lung, there are also major changes occurring in the airway at this time. Thus, as previously reported14, we classified animals into four groups: preterm (110–124 or 125–140 days post-conception, where 147 ± 3 days is full-term), newborn (3–7 days postnatal), and adult (9–36 months). We hypothesized that there would be an age-related pattern of contractile responses, showing no difference in rate constant or the percent shortening, but greater contractile velocities in older animals because of their longer cells.
Methods
Full methods were described previously17, 18. Tracheae were harvested from preterm, newborn, and adult sheep following a protocol that was approved by the Temple University Institutional Animal Care Committee in accordance with National Institutes of Health guidelines. The trachea were placed in cold collecting solution (117.8mM NaCl, 0.027mM Na2EDTA, 6.0mM KCl, 1.2mM MgSO4, 24.3mM 3-(N-Morpholino) propane-sulfonic acid (MOPS) [pH 7.5], 1.6mM CaCl2, and 1.2mM NaH2PO4). Tracheae from adult sheep were also received from a local butcher and placed in solution. The tissue was then refrigerated (4°C) overnight.
The smooth muscle layer was dissected from the intact tracheae, using a calcium-free MOPS-PSS solution (117.8mM NaCl, 0.027mM Na2EDTA, 6.0mM KCl, 1.2mM MgSO4, 24.3mM MOPS [pH 7.5], and 5.6mM glucose) to keep the tissue moist. Trachealis was then digested in a warmed (37°C) solution containing papain (Sigma, P-4762) and DTE for 10–30 minutes. Tissue was removed from solution when it appeared soft and swollen. It was rinsed and placed in a petri dish containing calcium-free MOPS-PSS.
Individual cells were released into solution when muscle strips were teased using forceps. Viable cells appeared bright under a dissecting microscope using transmitted light. Cells selected for study met the following criteria18: cells were elongated, that they remain elongated in millimolar concentrations of Ca2+, that they contract within seconds of being exposed to contractile agonists, and that the contractions be substantial (ie about half of the cell’s starting length). In our experience with both artery and airway smooth muscle cells we found that trypan blue exclusion is not a very stringent criterion of viability. For that reason in the current study we did not routinely pre-test the cells with trypan blue but instead used an operational definition of viability (ie. Ca2+ tolerance and prompt and substantial contraction when exposed to agonist). Cells were placed in a perfusion chamber (37°C) containing MOPS-PSS solution with calcium (CaCl2) at a concentration of 1.6 mM. Once cells had settled, solution was allowed to flow. Isolated cells were monitored when agonist-containing solution was allowed to flow. Carbachol was present at a concentration of 100 μM with calcium still at 1.6 mM. This solution also contained trypan blue so the flow of solution could be visualized. Brightfield optics were used for videomicroscopy. All contractions were recorded on videotape for analysis later. Cell length was measured and the corresponding time noted using the BioQuant image analysis system (R&M Biometrics, Nashville, TN, USA). A nonlinear fitting program was used to calculate the shortening rate, constant, maximum and average velocities, and percent shortening.
Nature of shortening response
Expressing the shortening response of an isolated smooth muscle cell in a meaningful way requires some care, because a single number cannot completely describe the response. We characterized the response in terms of rate, the amount of shortening, and maximum and average velocity.
Fit of cell lengths
A plot of cell length as a function of time was fit to the following equation, as in previous work18.
The parameter “rate” was the exponential rate constant, in units of reciprocal seconds (s−1) and describes how fast the cell length changes between its two limits, starting length (Lmax) and final length (Lmin). Fitting the cell lengths to a single exponential was empirical, and did not imply an underlying exponential process. “Lag” was a time offset of the experimental apparatus that reflects the delay involved for the flow of agonist solution to reach the cell.
However, the parameter “rate” did not take into account the fact that cells shorten different amounts. Furthermore, “rate”, expressed in s−1, did not provide a value of shortening velocity in absolute units of microns/second. Expressing shortening velocity in absolute units was valuable because it allowed comparison to other types of experimental data.
Percent shortening was calculated by the following equation: .
Instantaneous shortening velocity between two cell length measurements was calculated by dividing the change in cell length by the time difference between the measurements. It had units of microns/second, but it is an inherently noisy measurement, and sometimes even changes sign. This made it difficult to use instantaneous velocity as a reliable representation of unloaded shortening velocity.
Predicted maximum velocity
After the cell length measurements were fit to a single exponential, the time derivative of this function gave the predicted maximum shortening velocity in microns/second:
Predicted maximum velocity is a calculated parameter that by definition is greatest at the value of time that is equal to the estimated parameter “lag”. The fitting program estimates the parameter “lag” by successively eliminating early time points and testing the goodness-of-fit of the remaining time points. When the fitting program converges on a solution it essentially tells us when the contraction started. We used the value of predicted maximum velocity to represent the maximum shortening velocity of each cell. We calculated the predicted maximum velocity based on a “mathematical” model rather than risk observer bias associated with graphic assessment.
Average velocities
As a cell shortened, velocity, in microns/second, decreased. An average velocity for the entire response was the total amount of shortening divided by the amount of time required to shorten that distance. Since the cell lengths were fit to a single exponential, this could be used to describe average velocities over various multiples of the time constant. The time constant, τ, was the reciprocal of the rate constant and had units of seconds. Averaging the shortening response over 5 time constants included over 99% of the total shortening, as determined by the difference between the initial length (Lmax) and best-fit value of the final length (Lmin). The average velocity over 5 time constants was calculated as:
Specifying an average velocity this way, together with the initial and final length, allowed measurement on the single cell to be extrapolated to multicellular tissues.
Tissue velocities and shortening
To compare our results with cells to what might happen in vivo, for each cell we calculated properties of a hypothetical tissue composed of identical cells. The hypothetical 1 mm long tissues consisted of cells of equal length (the Lmax of each cell) placed end to end. The following equations, based on the formulas explained for single cells, were used to measure tissue shortening and velocities:
From this we calculated the tissue shortening, tissue predicted maximum velocity, and tissue average velocity for every cell.
Statistical Analysis
Nonlinear fitting and statistical analysis were performed using the JMP program (SAS Institute, Cary, NC, USA). The rate constant was estimated by nonlinear fitting of cell length to the exponential equation described previously. A goodness-of-fit test was routinely performed as part of the nonlinear fitting of the cell length data to the exponential model. Values of R2 for the non-linear fits were ≥ 0.9 when the program’s convergence criterion was satisfied. All the data points in the rapid phase of contraction were included. R2 = 1-(SSE/TSS) where SSE is sum of squares for error and TSS is corrected total sum of squares. Length measurements before shortening started were omitted because this allowed the entire shortening response to be fit better by the equation. Predicted maximum and average velocities were then calculated. Data was then grouped by age of the animal and an analysis of variance was performed. The Tukey-Kramer test was then performed to determine significant differences between the means of each group. This test was chosen based on its conservative nature in differentiating differences in group means. Differences were considered statistically significant at P<0.05.
Results
Morphometry
The mean length of isolated adult cells was more than twice the length of neonatal cells, averaging 173 μm versus 85 μm for the 125–140 day group (Table 1). In addition, the adult cells were wider, perhaps due to an increase in cellular material. We presumed that the cells were circular in cross-section and that the width therefore was the diameter. Cell volumes were calculated assuming a cylindrical geometry. The greater width and length of the adult cells meant that the volume of adult cells was about six times greater than neonatal cells. When the neonatal groups were compared to each other, there were no significant differences in calculated volumes. If cell volumes were calculated by treating each cell as two cones joined at the base, the volumes would be one-third the cylindrical values.
Table 1.
Morphometry of isolated ASM cells
| Age group | Cell Lmax, (μm) | Width, μm | Volumecyl, (pl) | SA: Vol, μm2/μm3 |
|---|---|---|---|---|
| PT110 (n=9) | 73.1 + 32.0 | 4.8 + 0.83 | 1.3 + 0.5 | 0.89 + 0.14 § |
| PT125 (n=9) | 84.7 + 19.3 | 5.8 + 1.79 | 2.5 + 1.5 | 0.83 + 0.46 |
| NB (n=10) | 74.0 + 19.1 | 5.0 + 0.94 | 1.4 + 0.5 | 0.85 + 0.14 |
| Adult (n=10) | 172.8 + 46.0 * | 8.4 + 2.88* | 10.7 + 8.1* | 0.54 + 0.20 § |
PT = preterm; NB = newborn; SA:Vol = surface area to volume ratio. These cells were measured in 1.6 mM CaCl2.
Considered significantly different from all other groups at p < 0.05.
Significantly different from each other only at p < 0.05.
Surface to volume ratios were calculated for each cell using the cylindrical model, and were expressed in μm2 area/μm3 volume. The surface to volume ratios were similar for the neonatal groups (0.83 to 0.89 μm2/μm3) but the surface to volume ratio for the adult cells was less (0.54 μm2/μm3), although this value was only significantly different from the 110–124 day group.
Nature of shortening response
Cell contractions were observed in a flow-through chamber. Cells that were Ca2+ tolerant (remaining elongated in 1.6 mM Ca2+) were exposed to agonist solution containing 100 μM carbachol. Cells from all groups contracted within 2–3 seconds of contact with the agonist. Cell length measurements were made from the videotape and were plotted as a function of time.
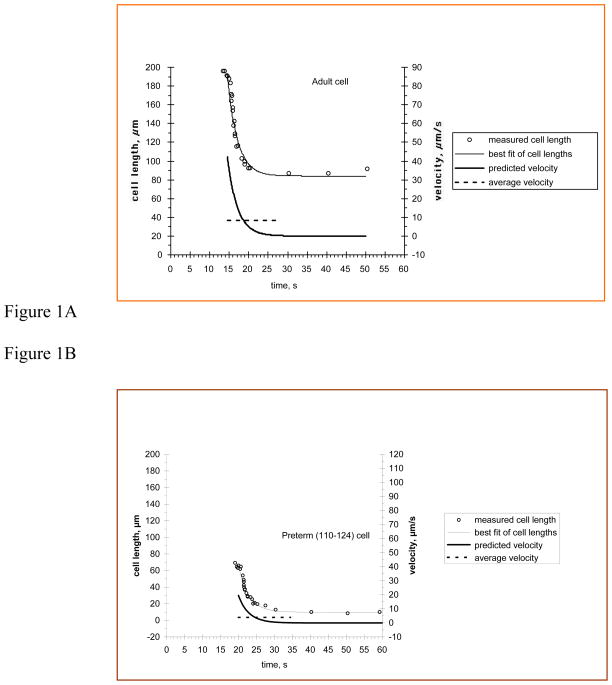
Figure 1A (upper trace) represents the contraction of an adult ASM cell and illustrates important aspects of the shortening response. First, the isolated smooth muscle cells did not shorten at a constant rate; they shortened rapidly at first, and then slowed as they approached their final length. Additionally, cells were different lengths, a distinction that was particularly important when comparing adult and neonatal cells. Finally, not all cells shortened the same amount, even when shortening was normalized to starting lengths. Because of these factors, expressing the shortening response of an isolated smooth muscle cell in a meaningful way required some care, because a single number could not completely describe the response. We characterized the shortening response in terms of its rate, the amount of shortening, and maximum and average velocity.
Figure 1. Contraction of ASM cells.
Cell length is plotted as a function of time during shortening. Agonist solution was 100 μM carbachol. Circles represent measured length of the cell at times indicated. The dashed line is the best fit of these points using our exponential model. Solid line represents the average shortening velocity to five time constants and the dashed-dotted line is the predicted maximum shortening velocity. A: An adult cell during contraction. B: A preterm (110–124 days) cell during contraction.
The smooth curve drawn through the cell length measurements was fit to the equation:
The fit of the equation represented the exponential rate constant, which normalized velocity to the cell length change. The rate constant for the adult cell shown in Figure 1A was 0.39 s−1, slightly less than the mean value for adult cells (0.54 s−1). There were no significant differences in rate constant between any of the neonatal groups (0.44 s−1, 0.47 s−1, 0.30 s−1). As seen in Table 2, only the newborn cells (0.30 s−1) had a rate constant significantly different from the adult cells (0.54 s−1). Cells from both groups of preterm animals and the adults completed their contractions with similar kinetics, but the newborn cells were significantly slower than the adults.
Table 2.
Contraction of isolated ASM cells
| Age group | % Shortening | Rate (1/sec) | PMV (μm/sec) | AV (μm/sec) |
|---|---|---|---|---|
| PT110 (n=9) | 58.3 + 11.4 | 0.44 + 0.24 | 17.8 + 13.0 | 3.53 + 2.59 |
| PT125 (n=9) | 62.7 + 9.49 | 0.47 + 0.15 | 25.1 + 11.3 | 4.99 + 2.23 |
| NB (n=10) | 50.7 + 11.9 | 0.30 + 0.10 § | 11.1 + 5.17 | 2.20 + 1.03 |
| Adult (n=10) | 51.1 + 7.67 | 0.54 + 0.24 § | 45.1 + 17.4 * | 8.95 + 3.46 * |
PT = preterm; NB = newborn; PMV = predicted maximum velocity; AV = average velocity. All measurements were done in the presence of 1.6 mM CaCl2.
Considered significantly different from all other groups at p < 0.05.
Significantly different from each other only at p < 0.05.
Table 2 also shows that there were no significant differences in the percent shortening when normalized to the initial length. These values ranged from 50.7 to 62.7 % of the starting lengths. However, since the starting lengths varied significantly between the adult and neonatal cells, it was important to examine the dependence of tissue responses on the cell length.
Since the initial length of the cell was an important variable between individual cells within an age group and particularly between adult and neonatal cells, we calculated predicted maximum and average velocities, which were not normalized to cell length. The predicted velocity was the time derivative of the equation fitting the cell length to a single exponential, and is shown by the dot-dash curve in the lower trace of Figure 1A. Predicted velocity (microns/second) described the actual shortening velocity with which one end of the cell approached the other. The maximum value of the predicted velocity for such a model occurs at the beginning of the shortening response. Predicted maximum shortening velocities are also shown in Table 2. The mean predicted maximum shortening velocity for the adult cells (45.1 μm/s) was greater than that for any of the groups of neonatal cells. There were no significant differences in predicted maximum shortening velocities between the neonatal groups (range 11.1 to 25.1 μm/s), nor was there a trend of increasing velocity with age since the lowest value was found with newborn cells.
Since the shortening velocity decreased during a contraction, we also calculated average shortening velocities for each contraction. After fitting the cell length to the single exponential function, the parameter estimates were used to calculate average velocity over 5 time constants. This value includes over 99% of the total shortening. The average velocity calculated this way was approximately one-fifth of the predicted maximum velocity as shown in Table 2.
Figure 1B represents the contraction of a preterm cell from the 110–124 day group. It illustrates that the contraction of these smaller cells was also well described by fitting cell length to a single exponential. The rate constant for the cell shown here was 0.35 s−1, slightly less than the mean value for the group, but similar to that of the adult cell shown in Figure 1. The initial length of the adult cell was about three times that of the preterm cell shown, and the predicted maximum and average velocities (right axis) of the adult cell were about twice those of the preterm cell.
Tissue velocities and shortening
Examining the contraction of single isolated cells was a good way to study the contractile process, but it was also important to be able to relate such findings to intact multicellular tissues. Because individual cells had different lengths, shortened different percentages of their starting length, and shortened at different rates, we attempted to model the responses of a hypothetical tissue made of many identical cells. For each cell, we calculated the shortening, predicted maximum velocity, and average velocity of a hypothetical 1 mm long tissue made of cells laid end-to-end. Table 3 shows these results. Although shortening velocities (both predicted maximum and average) were greater for longer cells, the longer the cell was, the fewer cells were in series in a 1 mm segment. Because of this, the hypothetical adult tissues did not have the highest velocities. It was noteworthy that the 125–140 day preterm group had the highest velocities, although the only significant difference in velocities occurred between this group and the newborns. Finally, we calculated a parameter called “shortening time” by dividing the tissue shortening by the tissue average shortening velocity. This represented how long it would take the hypothetical tissue to complete shortening. Noting the extremes, the shortening time was least for the adult cells (10.7 sec) and was 75% greater for the newborns (18.7 sec), but these differences were not significant at the 0.05 level. The hypothetical tissue properties we calculated may be a useful starting point in understanding how cellular responses relate to observed tissue properties.
Table 3.
Contraction of hypothetical 1mm tissue segments
| Age group | Cell Lmax (μm) | Tissue shortening (μm) | Tissue PMV (μm/sec) | Tissue AV (μm/sec) | Shortening time (sec) |
|---|---|---|---|---|---|
| PT110 (n=9) | 73.1 + 32.0 | 583 + 114 | 257 + 132 | 51.0 + 26.3 | 16.0 + 3.7 |
| PT125 (n=9) | 84.7 + 19.3 | 627 + 94.9 | 301 + 130 § | 59.8 + 25.8 § | 11.8 + 1.3 |
| NB (n=10) | 74.0 + 19.1 | 507 + 119 | 147 + 48.5 § | 29.3 + 9.63 § | 18.7 + 2.1 |
| Adult (n=10) | 172.8 + 46.0 * | 511 + 76.7 | 274 + 118 | 54.3 + 7.44 | 10.7 + 1.1 |
PT = preterm; NB = newborn; PMV = predicted maximum velocity; AV = average velocity.
Considered significantly different from all other groups at p < 0.05.
Significantly different from each other only at p < 0.05.
Discussion
Recent studies on maturation of ASM have elucidated structural mechanisms for developmental changes in function14–16. While the individual lengths of isolated fixed tracheal smooth muscle cells increased from prematurity to adulthood, a linear increase in cell length was not demonstrated during the neonatal period, thus precluding cell length alone as a mechanism for functional development14. Similarly, developmental alterations in the electron microscopic ultrastructure of ASM could not be identified as a potential mechanism for functional changes. However, at the protein expression level, the expression of SM1 and SM2 myosin heavy chain isoforms increased across development. This increase correlates with the developmental increase in tracheal smooth muscle force development, providing a likely mechanism for the functional maturation of this ASM.
Building upon previous work that compared preterm cells to adult cells, we expanded our study of isolated cells from ovine tracheae. In this study, we divided neonates into three groups based on age: preterms 110–124 days post-conception, preterms 125–140 days post-conception, and newborns 3–7 days postnatal. This age grouping was based on previous developmental airway studies in the sheep model14. In addition to the division of neonates, we developed an analytical approach to more completely describe the shortening response of a single cell and used this process to more thoroughly compare the contraction of cells from animals of different ages. Finally, we used these measurements of single cell responses to predict the shortening responses of hypothetical tissue segments.
In our previous work, we used the rate constant, in sec−1, as the major parameter for assessing contractile performance of isolated cells. The additional complexity of comparing the responses of cells from animals of such different stages of development required us to devise additional ways of expressing cellular contractile performance. Here we used predicted maximum and average shortening velocities to compare cells from four age groups in addition to the rate constant of shortening. This form of analysis could now be used to compare cells not only from different age groups of the same tissue but also to contrast cells from different tissues and species.
The shortening rate constant was the lowest in the newborn cells. This value was significantly different from the adult value, but not from the other neonatal groups. The mean predicted maximum shortening velocity for the adult cells was significantly greater than that for all the neonatal groups. Since the percent shortening was not significantly different between any of the groups, the greater predicted maximum velocity of the adult cells, therefore, was due solely to the longer lengths of the adult cells and not any differences in the contractile ability of the preterm cells. Taken together, these findings suggest that, when normalized to cell length, the newborn cells shorten as much as the other cells, but more slowly. Possible explanations for this center on factors which might make the contractile response intrinsically slower or which might cause an increased cellular resistance to shortening. Intracellular resistance to shortening might be proportionally greater in the newborn cells and a potential cause of their slower shortening as found.
With regard to the first possibility, several candidates exist such as altered myosin isoform expression, altered calcium handling, and altered activity of protein kinases and phosphatases. It is tempting to explain the slower shortening of the newborns by invoking mechanisms involving altered cellular geometry and increased diffusion distances for Ca2+ entering cells through sarcolemmal channels, but we found no significant differences in cell width or surface-to-volume ratios between any of the neonatal groups, making such geometrical explanations unlikely. The smaller surface-to-volume ratio we calculated for adult cells suggests there might be some important differences in function. However, such measurements were merely approximations. It has long been known that the surface of smooth muscle cells is highly irregular, having many infoldings and caveolae, making accurate volume calculations difficult. The smaller surface-to-volume ratio of the adult cells suggests that the adult cells might be more dependent on the SR as a source of calcium than on an influx through extracellular calcium channels. However, we believe the contractions are caused primarily by Ca2+ release from the SR rather than Ca2+ influx through sarcolemmal channels because caffeine causes contractions similar to those caused by carbachol20.
At least two forms of myosin heavy chain are expressed in smooth muscle, one of which contains a seven amino acid insert in the motor domain that is associated with faster shortening velocities in tissues and cells21. Rovner et al. reported that myosin with this insert propelled actin filaments in the in vitro motility assay with a velocity twice as fast as myosin lacking the insert could22. As noted by Cullen et al., there are documented alterations in myosin heavy chain during early development, and these changes have been linked to functional alterations14.
Differences in Ca2+ handling do not seem to be a likely explanation for the slower velocity of newborn cells, since we reported that caffeine (10 mM) causes prompt contraction of neonatal ASM cells18,20. This implies that the ability of the SR to accumulate and release calcium is already developed at birth. Certainly, differences in protein kinase and phosphatase activities could explain slower shortening, and as the role of protein phosphorylation in modulating contraction is further elucidated, it will undoubtedly be examined in neonatal cells. In canine trachealis, Gunst showed that shortening could lead to a progressive reduction of contraction; thus, there is precedent for a length-dependent reduction in activation23. Finally, it should be pointed out that a slower velocity with the same percent shortening can result when contraction of a cell is not synchronous, i.e., if some regions contract before others. However, there was no evidence of that in the video records of cells used here.
Another factor that could explain a lower shortening velocity is an increased internal resistance to shortening. If such an internal resistance to shortening exists and becomes more pronounced at birth, it could lead to slower shortening velocities, even with isolated cells. Meiss and co-workers have addressed the nature of the resistance to shortening seen in multicellular tissues. Recently they presented evidence that the resistance to shortening arose because as the tissue shortened, the constant volume behavior of the tissue stretched radially-oriented structures24. However, it is not clear if this mechanism exists at the single-cell level, or whether it is solely a function of the elastic and connective tissue between cells of a multicellular smooth muscle preparation. Intracellular sources of an internal resistance are also likely. It is possible that components of the cytoskeleton, such as intermediate filaments and dense bodies, impede the shortening of the cells. We find that when cells are briefly stimulated with lower concentrations of agonists, they sometimes elongate after the agonist is washed out. We interpret this is to mean that there is an internal spring that has been compressed by the shortening of the contractile apparatus, and after the cross-bridges stop cycling, this spring elongates the cell partially.
It is unclear why newborn cells act differently than both the preterm and adult cells. One possibility is that the newborn airway, just like the newborn lungs, is undergoing significant developmental changes. Perhaps this state of transition means that cells cannot operate maximally while the cell is remodeling.
In addition to filament changes, calcium handling may differ during cellular development. The size of the SR calcium load may not be the same for neonates as for adults. It is possible that preterm cells use a higher percentage of calcium from the extracellular space than adult cells do. This could be because of less SR calcium pumps or just a smaller SR calcium pool. Again, if the newborn cells are representative of a transition period, moving from extra- to intracellular sources, calcium handling may not be efficient, resulting in the decreased shortening rate as well as the lowest of the predicted maximum velocities.
The responses we calculated for the hypothetical 1 mm tissues should allow researchers who work with tracheal strips and rings to relate our finding to their work. However, some important differences should be kept in mind. The first is that in vivo, the constraints placed upon smooth muscle cells by intercellular collagen, elastin, and connections to other cells would affect the tissue responses, as Meiss has demonstrated24. Secondly, cell lengths in a smooth muscle are not uniform, but our tissue calculations assume all the cells are identical. It is not clear how this would complicate the responses, if at all. Finally, our results focus on the unloaded shortening of cells, but in vivo the tracheal smooth muscle shortens against the resistance of the tracheal cartilage and other structures. Nevertheless the responses we measure document the activity of the ASM contractile system and reflect its ability to develop force or resist elongation.
In vivo, several tissue properties of the airways are of importance. They include compliance (both resistance to distension and resistance to compression), the force-generating ability of the ASM, its shortening capacity, its velocity of shortening, and the extent to which ASM activation alters compliance. In addition, hypersensitivity of the ASM to normal excitatory input will alter the effect of ASM activation on tissue properties.
When activated, tracheal smooth muscle can develop force and shorten. In vivo, this can lessen the diameter of the trachea and thereby increase resistance to airflow. By decreasing the diameter of the airway, contraction also lessens (through the Laplace relationship) the distending tension placed on the airway by a given distending pressure. Clearly the ability of the muscle cells to shorten, the amount of shortening and the velocity of shortening is relevant to airway function. Force development by smooth muscle cells is also important because it determines if the ASM will be able to shorten against a given distending tension25. At constant diameter, activation of smooth muscle will increase the stiffness of the airway, making it less susceptible to over-distension (and associated damage) and will make the airway more resistant to collapse. Paradoxically in arteries, activation of vascular smooth muscle, at a constant distending pressure, can sometimes decrease the stiffness because it constricts the artery so that more of the load is born by the smooth muscle and less by the stiffer connective tissue26. It is not known if this happens with airways.
A number of factors should be taken into consideration when comparing our results with isolated cells to the large body of published work on the force-velocity relationship of multicellular airway smooth muscle strips27–33. At first glance, it may seem that the time course of the cell shortening responses we report here disagrees with findings from studies made on tracheal and bronchial strips; however, with careful consideration of boundary conditions, findings from the isolated cell preparation can be extrapolated to estimates of unloaded shortening velocity and shortening capacity of tracheal and bronchial strips.
Study of the isolated ASM cells allows cellular contractile responses to be measured without the influence of unknown substances released from epithelial cells and nerve endings. In addition, measurement of some contractile properties, such as determination of the unloaded shortening rate, are actually easier with single cells than with muscle strips because the responses are not affected by passive tissue elements (collagen and elastin). A further advantage is that when isolated cells are allowed to contract freely, the series elastic component plays a minimal role in the response because the cells have not been forced to develop high levels of isometric force, as they would in a muscle strip that was being studied by an afterloaded quick release protocol. Consequently the isolated cell can allow unloaded shortening to be measured readily without the involved and specialized apparatus needed to perform force-velocity measurements. Furthermore it demonstrates the potential for rapid shortening inherent in the airway smooth muscle tissue.
Stimulation methods are also preparation specific and provide insight to contractile behaviors. The most common way to activate tracheal and bronchial strips in the force-velocity studies is to use electric field stimulation (EFS), which releases neurotransmitters from nerve endings in the tissue. The transmitters only have to diffuse a short distance to receptors on the muscle cells, and consequently the muscle responds very rapidly. A disadvantage of the EFS method is that it is not physiological, and the mix of neurotransmitters released is not known (ACh, norepinephrine, peptides, nitric oxide, etc.). Muscle strips are stimulated and contract isometrically for various times until being quickly released to shorten isotonically against various afterloads. During the isometric portion of the contraction the contractile element (CE) shortens while stretching the series elastic element (SEC). When released to a constant afterload there is a rapid elastic recoil of the SEC, and after oscillations subside the measured shortening of the strip represents the active shortening by the contractile element. One of the many important findings that have emerged from studies such as those30–35 that, when the strips are stimulated and held isometrically for 10 seconds, the force-velocity curves obtained have much lower velocities than those obtained after about 3 seconds of isometric contraction, a fact that is attributed to slowing of the myosin crossbridge cycle during the longer isometric contraction. Thus, while these techniques are a powerful tool to study the dynamics of crossbridge cycling in an airway smooth muscle, they, like all methodologies, have limitations. As such, the mode of stimulation and the mechanical maneuvers imposed may not completely represent in vivo conditions.
In contrast, we measured the shortening of cells lying on a cover slip in a flow chamber. Because length could not be fixed when stimulated, the cell was free to shorten without restraint. Thus, we have referred to this as unloaded shortening because we did not impose any external loads on the cells. Under these conditions, the contractile element of the cell would have to shorten only a very small amount to stretch the SEC enough to develop sufficient force to overcome these sources of resistance. In both in vitro and in vivo whole airways, there would always be some type of external load due to other tissue elements. However, under the conditions of our experiments the cells would demonstrate the upper limit of shortening velocity that could be expected in vivo.
There are also differences in the method of agonist delivery. In our experiments the agonist and its concentration are known and chosen by the experimenter. The chamber we used provided smooth laminar flow of the agonist-containing solution over the cells but this probably does not provide as rapid delivery of agonist to receptors as would occur with EFS stimulation of a muscle strip. We calculated that it in our apparatus it could take up to 500 ms for the agonist solution to flow over the whole cell; therefore it is possible that one end of the cell could have been contracting before the other. This may be why the velocity, in some case, may have appeared to increase in the first few seconds. One of the important advantages of our approach is the ability to study different concentrations of different agonists in a relatively simple apparatus and to record one of the most fundamental properties of muscle tissue, shortening. The simplicity of the approach opens up a world of possibilities to researchers studying airway function in normal and pathophysiological situations.
As noted above, we do not think that our results contradict those of muscle strip force-velocity studies which have provided insight into crossbridge dynamics following instantaneous maximal activation of the tissue. Furthermore, we submit that our measurements of single cell shortening velocity provide a more realistic view of the maximum shortening velocity that is possible in airways in vivo.
In summary, we utilized isolated airway smooth muscle cells to investigate contractile differences that occur developmentally in cells from preterm, newborn, and adult animals. Surprisingly, the shortening rate constant of the newborn cells was the lowest. The predicted maximum shortening velocity of adult cells was significantly different from that of both newborn and preterm cells. These distinctions were not evident using only the rate constant normalized to cell length. Importantly, no significant difference in percent shortening existed between any age group of cells, demonstrating that all cells, of all age groups, have the same intrinsic ability to shorten.
Acknowledgments
The authors are grateful to Robert Roache for his aid in harvesting tissue. This study was partially supported by a Temple University Grant-in-Aid of Research (to Steven P. Driska), an American Heart Association Grant (to Marla R. Wolfson), and NIH COBRE grant 1 P20 RR 020173 to Thomas H. Shaffer.
Abbreviations
- PSS
physiological salt solution
- NB
newborn
- SR
sarcoplasmic reticulum
- DTE
dithioerythritol
- ASM
airway smooth muscle
- PT
preterm
References
- 1.Northway WA, Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.O’Brodovich HM, Mellins RB. Bronchopulmonary dysplasia (State of the Art) Am Rev Resp Dis. 1985;132:684–709. doi: 10.1164/arrd.1985.132.3.694. [DOI] [PubMed] [Google Scholar]
- 3.Bhutani VK, Ritchie WG, Shaffer TH. Acquired tracheomegaly in very preterm neonates. Am J Dis Child. 1986;140:449–452. doi: 10.1001/archpedi.1986.02140190059026. [DOI] [PubMed] [Google Scholar]
- 4.Shaffer TH, Bhutani VK, Wolfson MR, Penn RB, Tran NN. In vivo mechanical properties of the developing airway. Pediatr Res. 1989;25:143–146. doi: 10.1203/00006450-198902000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Bhutani VK, Rubenstein D, Shaffer TH. Pressure-induced deformation of immature airways. Pediatr Res. 1981;15:829–832. [PubMed] [Google Scholar]
- 6.Bhutani VK, Koslo R, Shaffer TH. The effect of tracheal smooth muscle tone on neonatal airway collapsibility. Pediatr Res. 1986;20:492–495. doi: 10.1203/00006450-198606000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TM, Mitchell RW, Blake JS, Mack MM, Kelly EA, Munoz NM, Leff AR. Expression of airway contractile properties and acetylcholinesterase activity in swine. J Appl Physiol. 1989;67:174–180. doi: 10.1152/jappl.1989.67.1.174. [DOI] [PubMed] [Google Scholar]
- 8.Penn RB, Wolfson MR, Shaffer TH. Effect of tracheal smooth muscle tone on collapsibility of immature airways. J Appl Physiol. 1988;65:863–869. doi: 10.1152/jappl.1988.65.2.863. [DOI] [PubMed] [Google Scholar]
- 9.Panitch HB, Deoras KS, Wolfson MR, Shaffer TH. Maturational changes in airway smooth muscle structure-function relationships. Pediatr Res. 1992;31:151–156. doi: 10.1203/00006450-199202000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Sparrow MP, Mitchell HW. Contraction of smooth muscle of pig airway tissues from before birth to maturity. J Appl Physiol. 1990;68:468–477. doi: 10.1152/jappl.1990.68.2.468. [DOI] [PubMed] [Google Scholar]
- 11.Fisher JT. Airway smooth muscle contraction at birth: In vivo versus in vitro comparisons to the adult. Can J Physiol Pharmacol. 1992;70:590–596. doi: 10.1139/y92-075. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez RJ, Dreshaj IA, Kumar G, Miller MJ, Martin RJ. Maturation of the cholinergic response of tracheal smooth muscle in the piglet. Pediatr Pulmonol. 1994;18:28–33. doi: 10.1002/ppul.1950180108. [DOI] [PubMed] [Google Scholar]
- 13.Haxhiu-Poskurica B, Ernsberger P, Haxhiu MA, Miller MJ, Cattarossi L, Martin RJ. Development of cholinergic innervation and muscarinic receptor subtypes in piglet trachea. Am J Physiol. 1993;264:L606–L614. doi: 10.1152/ajplung.1993.264.6.L606. [DOI] [PubMed] [Google Scholar]
- 14.Cullen AB, Cooke PH, Driska SP, Wolfson MR, Shaffer TH. Correlation of airway smooth muscle function with structure and protein expression during early development. Pediatr Pulmonol. 2007;42(5):421–432. doi: 10.1002/ppul.20494. [DOI] [PubMed] [Google Scholar]
- 15.Booth RJ, Sparrow MP, Mitchell HW. Early maturation of force production in pig tracheal smooth muscle during fetal development. Am J Respir Cell Mol Biol. 1992;7:590–597. doi: 10.1165/ajrcmb/7.6.590. [DOI] [PubMed] [Google Scholar]
- 16.Kuo KH, Herrera AM, Seow CY. Ultrastructure of airway smooth muscle. Respir Physiol Neurobiol. 2003;137(2–3):187–208. doi: 10.1016/s1569-9048(03)00147-2. [DOI] [PubMed] [Google Scholar]
- 17.Panitch HB, Wolfson MR, Shaffer TH. Epithelial modulation of preterm airway smooth muscle contraction. J Appl Physiol. 1993;74:1437–1443. doi: 10.1152/jappl.1993.74.3.1437. [DOI] [PubMed] [Google Scholar]
- 18.Driska SP, Laudadio RE, Wolfson MR, Shaffer TH. A method for isolating adult and neonatal airway smooth muscle cells and measuring shortening velocity. J Appl Physiol. 1999;86(1):427–435. doi: 10.1152/jappl.1999.86.1.427. [DOI] [PubMed] [Google Scholar]
- 19.Sims SM, Vivaudou MB, Clapp LH, Lassignal NL, Walsh JV, Jr, Singer JJ. Neurotransmitter regulation of ionic channels in freshly dissociated smooth muscle cells. Annals New York Acad Sci. 1988;527:346–59. doi: 10.1111/j.1749-6632.1988.tb26991.x. [DOI] [PubMed] [Google Scholar]
- 20.Laudadio RE, Driska SP, Wolfson MR, Shaffer TH. Caffeine-induced contraction of adult airway smooth muscle (ASM) cells with different exposures to calcium. FASEB J. 1998;11:534. Abstract. [Google Scholar]
- 21.Eddinger TJ, Meer DP. Expression of smooth muscle myosin heavy chains and unloaded shortening in single smooth muscle cells. Am J Physiol. 1998;273:C1259–C1266. doi: 10.1152/ajpcell.1997.273.4.C1259. [DOI] [PubMed] [Google Scholar]
- 22.Rovner A, Freyzon Y, Trybus KM. An insert in the motor domain determines the functional properties of expressed smooth muscle myosin isoforms. J Muscle Res Cell Mot. 1997;18(1):103–10. doi: 10.1023/a:1018689102122. [DOI] [PubMed] [Google Scholar]
- 23.Gunst SJ. Effect of length history on contractile behavior of canine tracheal smooth muscle. Am J Physiol. 1986;250 (19):C146–C154. doi: 10.1152/ajpcell.1986.250.1.C146. Cell Physiol. [DOI] [PubMed] [Google Scholar]
- 24.Meiss RA. Influence of intercellular tissue connections on airway muscle mechanics. J Appl Physiol. 1999;86(1):5–15. doi: 10.1152/jappl.1999.86.1.5. [DOI] [PubMed] [Google Scholar]
- 25.Penn RB, Wolfson MR, Shaffer TH. Developmental differences in tracheal cartilage mechanics. Pediatr Res. 1989;26:429–433. doi: 10.1203/00006450-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Dobrin PB, Rovick AA. Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am J Physiol. 1969;217 (6):1644–1651. doi: 10.1152/ajplegacy.1969.217.6.1644. [DOI] [PubMed] [Google Scholar]
- 27.Stephens NL, Kroeger E, Mehta JA. Force-velocity characteristics of respiratory airway smooth muscle. J Appl Physiology. 1960 Jun;26(6):685–92. doi: 10.1152/jappl.1969.26.6.685. 1969. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell RW, Stephens NL. Maximum shortening velocity of smooth muscle: zero load-clamp vs. afterloaded method. J Appl Physiol: Respiratory, Environmental & Exercise Physiology. 1983 Nov;55(5):1630–3. doi: 10.1152/jappl.1983.55.5.1630. [DOI] [PubMed] [Google Scholar]
- 29.Stephens NL. Force-velocity constants in smooth muscle: afterloaded isotonic and quick-release methods. Can J Physiol & Pharmacol. 1985 Jan;63(1):48–51. doi: 10.1139/y85-008. [DOI] [PubMed] [Google Scholar]
- 30.Stephens NL, Kagan ML, Packer CS. Time dependence of shortening velocity in tracheal smooth muscle. Am J Physiol. 1986 Sep;251(3 Pt 1):C435–42. doi: 10.1152/ajpcell.1986.251.3.C435. [DOI] [PubMed] [Google Scholar]
- 31.Seow CY, Stephens NL. Force-velocity curves for smooth muscle: analysis of internal factors reducing velocity. Am J Physiol. 1986 Sep;251(3 Pt 1):C362–8. doi: 10.1152/ajpcell.1986.251.3.C362. [DOI] [PubMed] [Google Scholar]
- 32.Seow CY, Stephens NL. Velocity-length-time relations in canine tracheal smooth muscle. J Appl Physiol. 1988 May;64(5):2053–7. doi: 10.1152/jappl.1988.64.5.2053. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Jiang H, Stephens NL. A modified force-velocity equation for smooth muscle contraction. J Appl Physiol. 1994 Jan;76(1):253–8. doi: 10.1152/jappl.1994.76.1.253. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Seow CY, Kepron W, Stephens NL. Mechanical alterations in sensitized canine saphenous vein. J Appl Physiol. 1990;69:171–178. doi: 10.1152/jappl.1990.69.1.171. [DOI] [PubMed] [Google Scholar]
- 35.Ford LE, Gilbert SH. Mechanism and Significance of Early, Rapid Shortening in Sensitized Airway Smooth Muscle. Can J Physiol Pharmacol. 2007;85:747–753. doi: 10.1139/Y07-049. [DOI] [PubMed] [Google Scholar]