Abstract
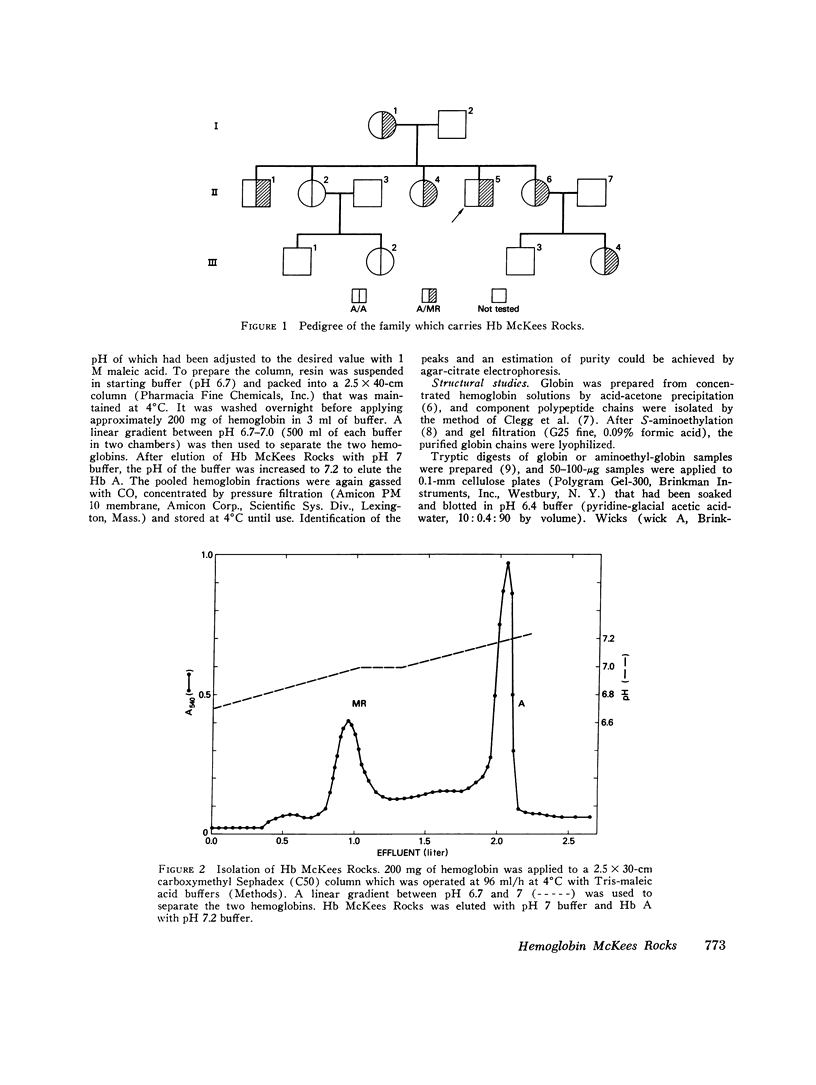
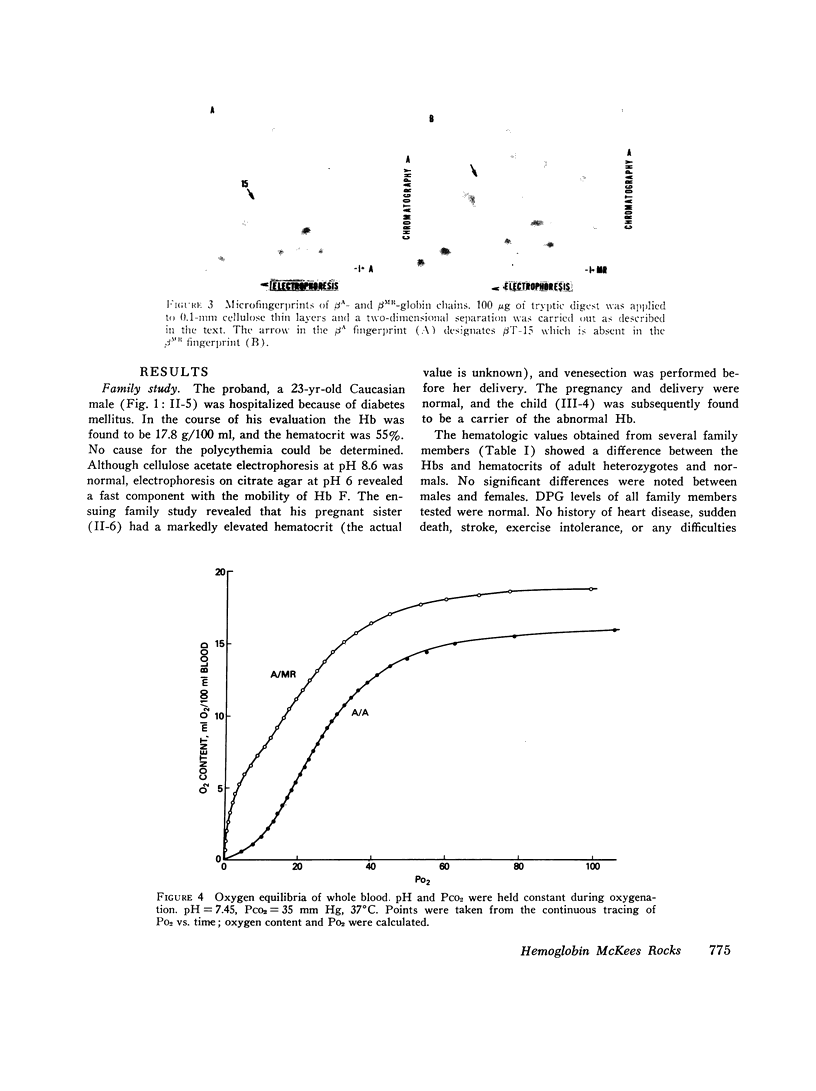
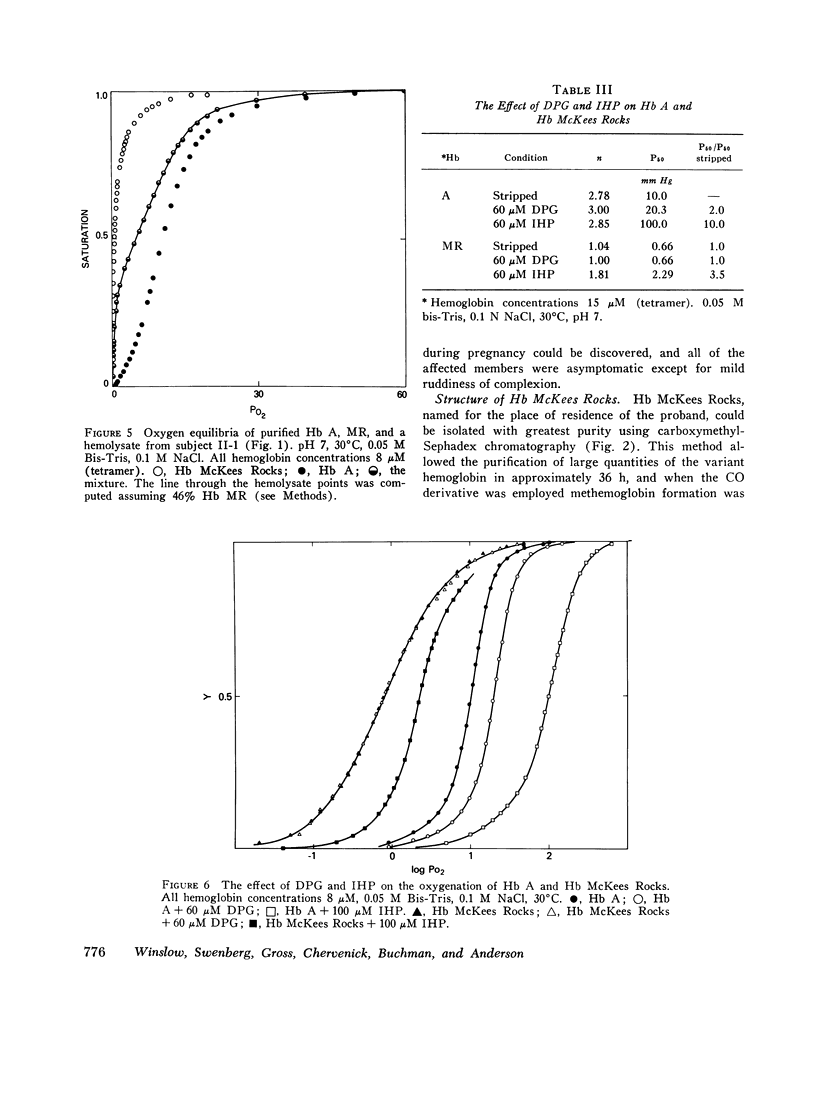
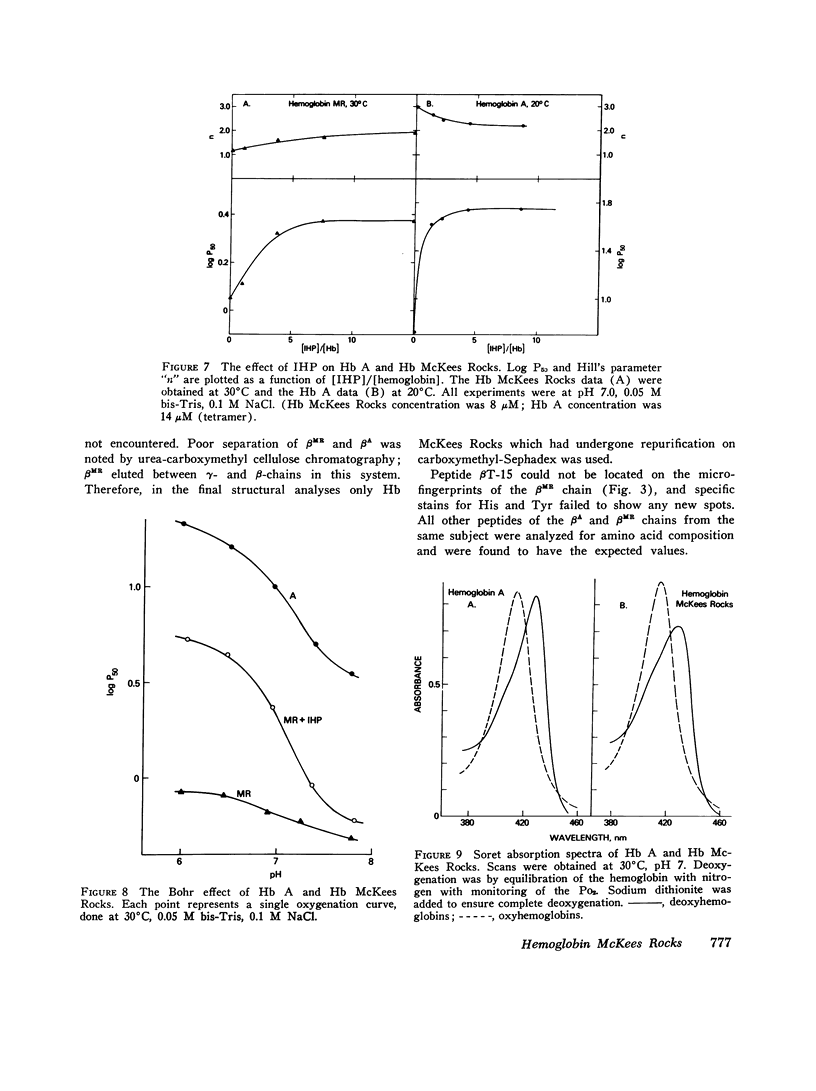
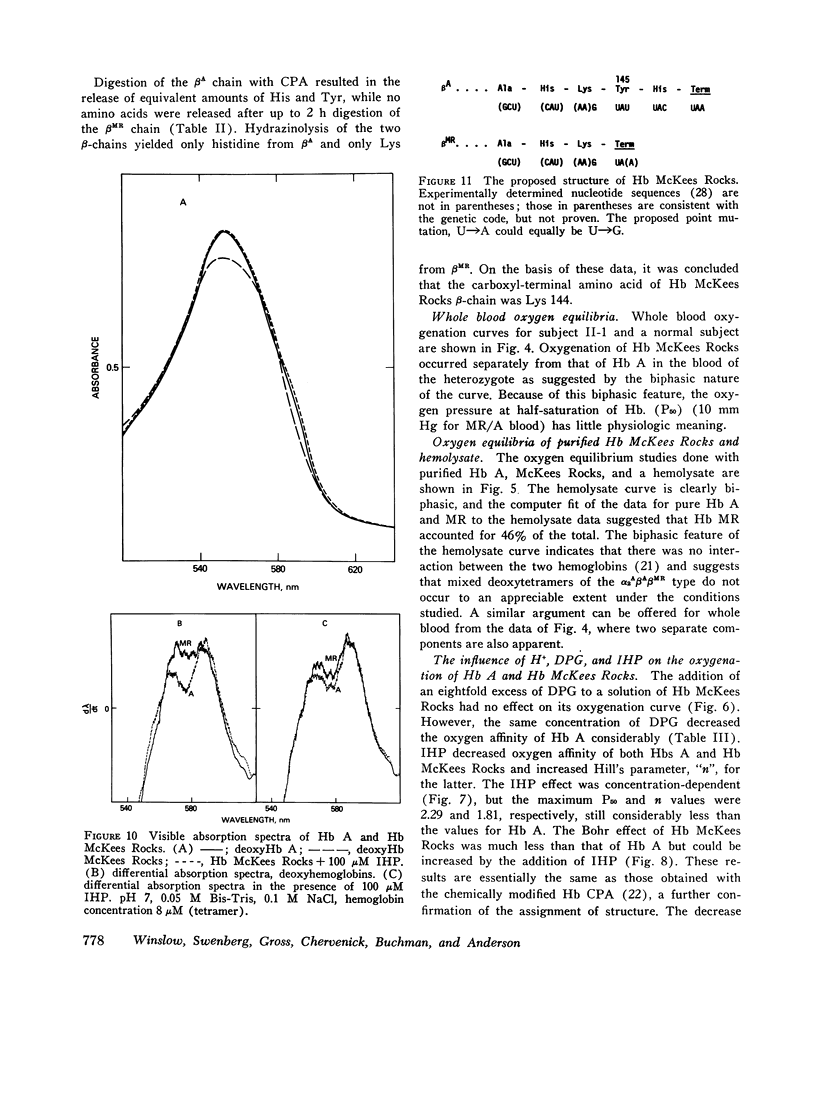
The first example of the premature termination of a polypeptide chain in man appears to be Hb McKees Rocks, beta145 Tyr leads to Term, discovered in polycythemic members of a Caucasian family. Point mutation has apparently occurred at the codon for Tyr beta145 from UAU to a "nonsense" codon, UAA or UAG, resulting in a shortened polypeptide chain with Lys 144 as its carboxyl-terminal amino acid. Evidence for this structural conclusion is the absence of tryptic peptide betaT-15 from "fingerprints" of the abnormal beta-chain, the finding of C-terminal Lys, and the similarity between the functional properties of this variant hemoglobin and those of des Tyr (145)-His(146)beta hemoglobin resulting from carboxypeptidase-A digestion of normal human hemoglobin. Hb McKees Rocks has markedly abnormal properties: its oxygen affinity is the highest of the human variants described to date; its Bohr effect is reduced; it is devoid of subunit cooperativity; and it is unaffected by 2,3-diphosphoglyceric acid. These properties are probably the consequences of decreased stability of the T quaternary conformation and are partially restored in the presence of the strong allosteric effector inositol hexaphosphate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., ZITO R., ROSSI-FANELLI A., CAPUTO A. Studies on carboxypeptidase digests of human hemoglobin. J Biol Chem. 1961 Sep;236:PC60–PC63. [PubMed] [Google Scholar]
- BAGLIONI C. An improved method for the fingerprinting of human hemoglobin. Biochim Biophys Acta. 1961 Apr 1;48:392–396. doi: 10.1016/0006-3002(61)90490-5. [DOI] [PubMed] [Google Scholar]
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
- Bare G. H., Alben J. O., Bromberg P. A., Jones R. T., Brinhall B., Padilla F. Hemoglobin Little Rock (beta143 (H21) His leads to Gln). Effects of an amino acid substitution at the 2,3-diphosphoglycerate binding site. J Biol Chem. 1974 Feb 10;249(3):773–779. [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Bonaventura C., Bonaventura J., Amiconi G., Tentori L., Brunori M., Antonini E. Hemoglobin Abruzzo (beta143 (H21) His replaced by Arg). Consequences of altering the 2,3-diphosphoglycerate binding site. J Biol Chem. 1975 Aug 25;250(16):6273–6277. [PubMed] [Google Scholar]
- Bonaventura J., Bonaventura C., Brunori M., Giardina B., Antonini E., Bossa F., Wyman J. Functional properties of carboxypeptidase-digested hemoglobins. J Mol Biol. 1974 Feb 5;82(4):499–511. doi: 10.1016/0022-2836(74)90244-7. [DOI] [PubMed] [Google Scholar]
- Bonaventura J., Bonaventura C., Giardina B., Antonini E., Brunori M., Wyman J. Partial restoration of normal functional properties in carboxypeptidase A-digested hemoglobin. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2174–2178. doi: 10.1073/pnas.69.8.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S. H., Hathaway P., Pascasio F., Bordley J., Orton C., Naughton M. A. Differences in the amino acid sequences of tryptic peptides from three sheep hemoglobin beta chains. J Biol Chem. 1967 May 10;242(9):2211–2232. [PubMed] [Google Scholar]
- Brown J. L., Ingram V. M. Structural studies on chick embryonic hemoglobins. J Biol Chem. 1974 Jun 25;249(12):3960–3972. [PubMed] [Google Scholar]
- Bunn H. F., Bradley T. B., Davis W. E., Drysdale J. W., Burke J. F., Beck W. S., Laver M. B. Structural and functional studies on hemoglobin Bethesda (alpha2beta2 145His), a varient associated with compensatory erythrocytosis. J Clin Invest. 1972 Sep;51(9):2299–2309. doi: 10.1172/JCI107040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Guidotti G. Stabilizing interactions in hemoglobin. J Biol Chem. 1972 Apr 25;247(8):2345–2350. [PubMed] [Google Scholar]
- Charache S., Brimhall B., Jones R. T. Polycythemia produced by hemoglobin Osler (beta-145 (HC2) Tyr yields Asp). Johns Hopkins Med J. 1975 Mar;136(3):132–136. [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Dozy A. M., Huisman T. H. Studies on the heterogeneity of hemoglobin. XIV. Chromatography of normal and abnormal human hemoglobin types on CM-Sephadex. J Chromatogr. 1969 Mar 11;40(1):62–70. doi: 10.1016/s0021-9673(01)96618-x. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Baltimore D., Benz E. J., Jr, Housman D., Lebowitz P., Marotta C. A., McCaffrey R. P., Skoultchi A., Swerdlow P. S., Verma I. M. Globin messenger RNA in the thalassemia syndromes. Ann N Y Acad Sci. 1974;232(0):76–87. doi: 10.1111/j.1749-6632.1974.tb20574.x. [DOI] [PubMed] [Google Scholar]
- Gacon G., Wajcman H., Labie D. Structural and functional study of Hb Nancy beta 145 (HC 2) Tyr replaced by Asp. A high oxygen affinity hemoglobin. FEBS Lett. 1975 Aug 1;56(1):39–42. doi: 10.1016/0014-5793(75)80106-2. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Shin M. An enzymic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim Biophys Acta. 1973 Jun 15;310(2):309–316. doi: 10.1016/0005-2795(73)90110-4. [DOI] [PubMed] [Google Scholar]
- Imai K. Correlation between narrow-banded ultraviolet spectra and oxygen equilibrium functions in native and chemically modified human hemoglobins. Biochemistry. 1973 Jan 2;12(1):128–134. doi: 10.1021/bi00725a021. [DOI] [PubMed] [Google Scholar]
- Imai K., Morimoto H., Kotani M., Watari H., Hirata W. Studies on the function of abnormal hemoglobins. I. An improved method for automatic measurement of the oxygen equilibrium curve of hemoglobin. Biochim Biophys Acta. 1970 Feb 17;200(2):189–196. doi: 10.1016/0005-2795(70)90163-7. [DOI] [PubMed] [Google Scholar]
- Imai K. Oxygen-equilibrium characteristics of abnormal hemoglobin Hiroshima (alpha-2 beta-2 143 Asp). Arch Biochem Biophys. 1968 Sep 20;127(1):543–547. doi: 10.1016/0003-9861(68)90260-9. [DOI] [PubMed] [Google Scholar]
- Imai K. Oxygen-equilibrium characteristics of abnormal hemoglobin Hiroshima (alpha-2 beta-2 143 Asp). Arch Biochem Biophys. 1968 Sep 20;127(1):543–547. doi: 10.1016/0003-9861(68)90260-9. [DOI] [PubMed] [Google Scholar]
- JONES R. T. STRUCTURAL STUDIES OF AMINOETHYLATED HEMOGLOBINS BY AUTOMATIC PEPTIDE CHROMATOGRAPHY. Cold Spring Harb Symp Quant Biol. 1964;29:297–308. doi: 10.1101/sqb.1964.029.01.032. [DOI] [PubMed] [Google Scholar]
- Jensen M., Oski F. A., Nathan D. G., Bunn H. F. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975 Mar;55(3):469–477. doi: 10.1172/JCI107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Hewitt J. A. The effect of removal of C-terminal residues on cooperative interactions in hemoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:311–314. doi: 10.1101/sqb.1972.036.01.041. [DOI] [PubMed] [Google Scholar]
- MARDER V. J., CONLEY C. L. Electrophoresis of hemoglobin on agar gels; frequency of hemoglobin D in a Negro population. Bull Johns Hopkins Hosp. 1959 Aug;105(2):77–88. [PubMed] [Google Scholar]
- Nagai M., Sugita Y., Yoneyama Y. Oxygen equilibrium and circular dichroism of hemoglobin-Rainer ( 2 2 1 45Tyr leads to Cys). J Biol Chem. 1972 Jan 10;247(1):285–290. [PubMed] [Google Scholar]
- Nygaard S. F., Rörth M. An enzymatic assay of 2,3-diphosphoglycerate in blood. Scand J Clin Lab Invest. 1969 Dec;24(4):399–403. doi: 10.3109/00365516909080179. [DOI] [PubMed] [Google Scholar]
- Secher D. S., Cotton R. G., Milstein C. Spontaneous mutation in tissue culture-chemical nature of variant immunoglobulin from mutant clones of MOPC 21. FEBS Lett. 1973 Dec 1;37(2):311–316. doi: 10.1016/0014-5793(73)80485-5. [DOI] [PubMed] [Google Scholar]
- Small K. A., Radford E. P., Frazier J. M., Rodkey F. L., Collison H. A. A rapid method for simultaneous measurement of carboxy- and methemoglobin in blood. J Appl Physiol. 1971 Jul;31(1):154–160. doi: 10.1152/jappl.1971.31.1.154. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Nagai M., Yoneyama Y. Circular dichroism of hemoglobin in relation to the structure surrounding the heme. J Biol Chem. 1971 Jan 25;246(2):383–388. [PubMed] [Google Scholar]
- Zak S. J., Brimhall B., Jones R. T., Kaplan M. E. Hemoglobin Andrew-Minneapolis alpha 2 A beta 2 144 Lys leads to Asn: a new high-oxygen-affinity mutant human hemoglobin. Blood. 1974 Oct;44(4):543–549. [PubMed] [Google Scholar]




