Abstract
In the present study, the detrimental effect of β-emission on pig skin was evaluated. Skin injury was modeled in mini-pigs by exposing the animals to 50 and 100 Gy of β-emission delivered by 166Ho patches. Clinicopathological and immunohistochemical changes in exposed skin were monitored for 18 weeks after β-irradiation. Radiation induced desquamation at 2~4 weeks and gradual repair of this damage was evident 6 weeks after irradiation. Changes in basal cell density and skin depth corresponded to clinically relevant changes. Skin thickness began to decrease 1 week after irradiation, and the skin was thinnest 4 weeks after irradiation. Skin thickness increased transiently during recovery from irradiation-induced skin injury, which was evident 6~8 weeks after irradiation. Epidermal expression of nuclear factor-kappa B (NF-κB) differed significantly between the untreated and irradiated areas. One week after irradiation, cyclooxygenase-2 (COX-2) expression was mostly limited to the basal cell layer and scattered among these cells. High levels of COX-2 expression were detected throughout the full depth of the skin 4 weeks after irradiation. These findings suggest that NF-κB and COX-2 play roles in epidermal cell regeneration following β-irradiation of mini-pig skin.
Keywords: β-ray, irradiation, pig, skin
Introduction
Skin injury is not only a common side effect of exposure to radiation for therapeutic purposes but is also the most common symptom associated with accidents that involve unintentional exposure to radiation [10,12]. Skin damage can occur without additional symptoms, especially after acute exposure to β-irradiation such as when radioactive materials contaminate a patient's skin or clothes. A previous study provided information relevant to protecting skin from radiological damage after localized exposure of the skin to radiation [2,12]. Although the basic concepts of radiation-induced skin damage and recovery are known [2,12], the underlying biological mechanisms have not been extensively studied.
Most studies of radiation-induced skin injury have focused on radiation dermatitis as a side effect of radiotherapy and primarily used an experimental design involving either γ- or X-rays [14]. Given that β-irradiation does not penetrate the deep dermis, clinical significance of this type of radiation was not sufficiently evaluated before the Chernobyl nuclear power plant accident [5]. Health reports of people with acute radiation syndrome caused by the Chernobyl incident [18] indicated that at least 19 of the related deaths were caused primarily by infection of large areas of skin damaged by beta burns. Accordingly, skin injury has been identified as one of the main factors that contribute to death caused by radiation accidents. Fatal beta burns caused by the Chernobyl tragedy underscore the need to better understand the mechanisms that govern the clinicopathology of β-irradiation of skin.
β-irradiation severely damages skin epithelial cells. Unlike other types of radiation (e.g., neutrons, X-rays, and γ-rays), β-irradiation cannot penetrate very far into living matter and causes negligible damage to layers beneath the epidermis. For pig skin exposed to a β-emitter, variations in the dose administered to epidermis kill cells in the basal layer of the skin; this is usually associated with the development of moist desquamation [11].
Nuclear factor-kappa B (NF-κB) plays crucial roles in immune and inflammatory responses by regulating the expression of genes that encode pro-inflammatory cytokines, adhesion molecules, chemokines, growth factors, and inducible enzymes such as cyclooxygenase-2 (COX-2) [4]. Factors that induce COX-2 expression include proinflammatory agents and mitogens. COX-2 is expressed in inflammatory cells, such as neutrophils, and epidermal cells at a wound site, and its generation increases transiently during the inflammatory phase of healing [1,25]. Both NF-κB and COX-2 are associated with tumorigenesis in cases of skin cancer [1], and are thought to have a radiosensitizing effect [9,17,32]. Animal studies of mucositis of the oral [27] and gastrointestinal [8,30,31] tracts as well as of ultraviolet (UV)-induced skin damage [3,23] have elucidated the roles of NF-κB and COX-2 in radiation-induced damage. However, the roles of NF-κB and COX-2 in the response to β-irradiation of skin have not been investigated.
Small animals, such as mice or rats, are frequently used for studies on irradiation-associated tissue damage. However, experiments using small animals are limited by anatomical and pathophysiological differences between these animals and humans. Given the physiological similarities between pigs and humans, the pig should be an excellent animal model of human skin damage and wound healing [28]. Accordingly, porcine models provide excellent tools for evaluating therapeutic agents to treat human skin damage [26,28].
In the light of the clinical importance of beta burns revealed by the aftermath of the Chernobyl accident, we used mini-pigs, a popular animal model for evaluating human wounds [28], to evaluate the sequence of clinicopathological changes in β-irradiated skin. Additionally, the levels of NF-κB and COX-2 expression in serial biopsy samples were analyzed. The objectives of this study were to establish a mini-pig model of acute β-irradiation-induced skin damage caused by a 166Ho skin patch, and to evaluate the possible effects of β-irradiation on NF-κB and COX-2 expression in the skin.
Materials and Methods
Animal experiments
Six male Göttingen mini-pigs (mean weight: 19 kg; weight range: 18~20 kg, ages: 6~7 months) were obtained from PWG Genetics (Korea). The mini-pigs were randomly assigned to a sham group or irradiated group (n = 3 per group). The pigs were fed a standard animal diet (Cargill Agri Purina, USA). All of the animal experiments followed a protocol approved by the Institutional Animal Care and Use Committee of the Korea Institute Radiological and Medical Sciences (KIRAMS).
β-irradiation of porcine skin
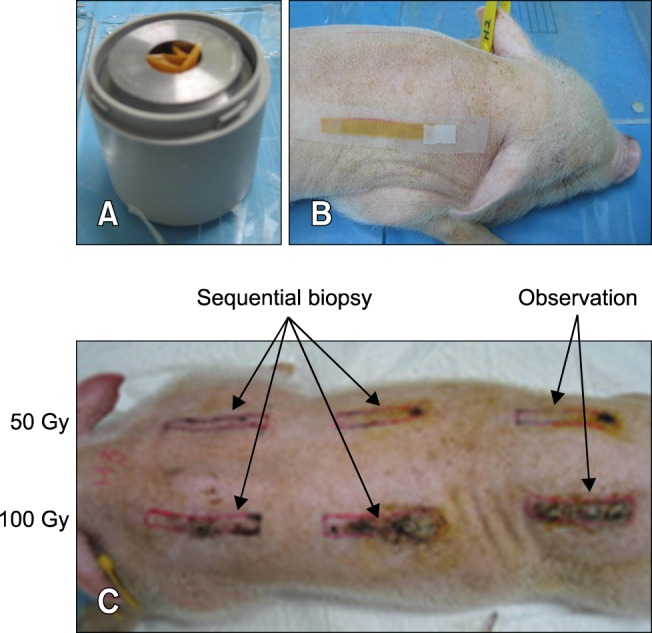
165Ho(NO3)3·5H2O (231 g) and tetrahydrofuran (246 mL) were purchased from Merck (USA) and completely dissolved in a solvent mixture containing 235 mL dimethylformamide (Merck), 2 L tetrahydrofuran (Merck), and 462 g polyurethane (Dow Chemical, USA) at 23 ± 2℃. The solution was applied uniformly onto adhesive tape to form a layer approximately 500-µm thick. The patches were placed in a nuclear reactor (Donghwa, Korea) with a neutron flux of 1 × 1013 n/cm2 sec to convert the 165Ho to the β-emitter 166Ho, which has an Emax of 1.84 MeV and a half-life of 26.9 h. 166Ho also emits γ photons (5.4% of 0.081 MeV and 0.9% of 1.38 MeV). To assess the effects of 166Ho-derived β-emission onto the mini-pig skin, the animals were irradiated by attaching the 166Ho patches onto the surface of the back skin of each experimental pig. Three to 4 days prior to irradiation, the hair was clipped from the areas to be exposed and the positions of the irradiated fields were marked with India ink. Six rectangular patches (1 cm × 7 cm) were applied separately to each pig of irradiated group. Adhesive tape was used to firmly affix the patches for either 23 min or 46 min to deliver a total surface radiation dose of 50 or 100 Gy, respectively. Each animal had three 50-Gy irradiating patches and another three 100-Gy irradiating patches (Fig. 1). During all of the procedures, the animals were anesthetized with tiletamine and zolazepam (Zoletil 50; Virbac, France) along with medetomidine (Domitol; Pfizer Animal Health, USA). Sham-irradiated mice were anesthetized and immobilized for the same period of time without irradiation.
Fig. 1. β-irradiation exposure using 166Ho patches to induce mini-pig dorsal skin lesions. (A) The 166Ho patch. (B) The 166Ho patch applied to dorsal skin of a mini-pig. (C) β-irradiated lesions (caused by 50 or 100 Gy) with the separate observation fields and punch biopsy sites. a; sequential biopsy, b; observation.
Skin scoring
The pigs were carefully evaluated for 18 weeks after irradiation, and the skin reactions were scored using a clinical status scoring system for the observed irradiated field (panel C in Fig. 1). A scoring system was based on a previously described skin damage model [29]: grade 1.0, normal; grade 1.5, minimal erythema and mild dry skin; grade 2.0, moderate erythema and dry skin; grade 2.5, marked erythema and dry desquamation; grade 3.0, dry desquamation and minimal dry crusting; grade 3.5, dry desquamation, dry crusting, and superficial minimal scabbing; grade 4.0, patchy moist desquamation and moderate scabbing; grade 4.5, confluent moist desquamation, ulcers, and large deep scabs; grade 5.0, open wounds and full-thickness skin loss; and grade 5.5, necrosis.
Skin tissue sampling
At the time of β-irradiation, three skin biopsy samples were obtained and pinned to cork to maintain their original sizes (panel C in Fig. 1). After anesthesia had been induced, a 5-mm biopsy punch (HB926; HEBUmedical, Germany) was used to obtain skin tissue samples from the irradiated sites at weeks 1, 2, 3, 4, 6, 8, 10, 14, and 18. Non-irradiated skin biopsy samples were obtained from the sham pigs at the same time points. The tissue samples were processed by embedding in paraffin wax after fixation in 10% buffered formalin. The paraffin-embedded tissues were then cut into 4-µm coronal sections (SM2010R; Leica, Germany). The sections were stained with hematoxylin and eosin (Harris's hematoxylin, Eosin Y; Thermo Fisher Scientific, USA). The longest rete ridge in each slide was measured from the bottom of the basal layer to the bottom of the stratum corneum while avoiding areas where the inclusion seemed to be oblique. Mean values were calculated using the measurements for each section on each slide. Cell density in the basal layer was determined by counting the cells along a length of basement membrane at least 5-mm long. The results are expressed as the number of cells per mm of basement membrane.
Immunohistochemical staining and scoring
The sections were incubated in blocking nonspecific binding serum (Cap-Plus detection kit; Zymed Laboratories, USA) for 20 min at room temperature (RT) prepared according to the manufacturer's instructions. Next, the sections were incubated with mouse anti-NF-κB antibody (sc-109, 1:200 dilution; Santa Cruz Biotechnology, USA) or mouse anti-COX-2 antibody (18-7379, 1:200 dilution; Zymed Laboratories) for 60 min at RT. The sections were then washed three times (5 min each) with PBS that contained 0.05% Triton X-100, and incubated with biotinylated broad-spectrum secondary antibody (Cap-Plus detection kit; Zymed Laboratories) for 25 min at RT prepared according to the manufacturer's instructions. Immunoreactivity was monitored using streptavidin complex labeled with horseradish peroxidase (Elite ABC kit; Vector Labs, USA). The peroxidase reaction was developed using a diaminobenzidine substrate (Vector Labs). The sections finally were counterstained with hematoxylin before being mounted (Consul-Mount; Thermo Fisher Scientific). Images of the sections were taken using a digital camera mounted on a Nikon ECLIPSE 80i microscope (Nikon Instruments, Japan).
NF-κB accumulation was scored as 0 (indicating absence of staining), 1 (cytoplasmic staining with limited nuclear staining), 2 (10~50% of the maximal level of nuclear staining and cytoplasm staining), or 3 (more than 50% of the maximal levels of nuclear and cytoplasm staining). COX-2 expression was scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining) based on cytoplasmic staining. All measurements were performed by the same individual who was blinded to the experimental conditions.
Statistical analysis
Data are reported as the mean ± standard error of the mean (SEM). The results were evaluated using a one-way analysis of variance (ANOVA) followed by a Student- Newman-Keuls post hoc test for multiple comparisons. P values < 0.05 were considered significant.
Results
Physical examination findings
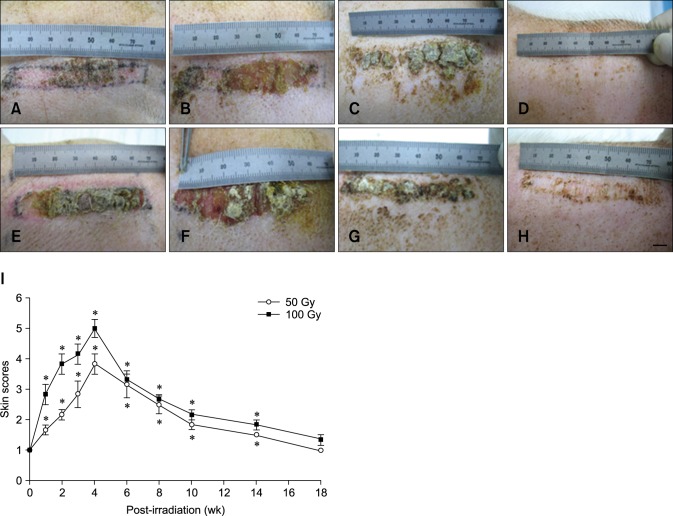
Effects of the duration and intensity of radiation exposure on the gross morphology of the skin are summarized in Fig. 2. Irradiation with 166Ho at either of the two doses tested (50 and 100 Gy) damaged the epidermis and induced gross skin morphology changes in a dose-dependent manner. Acute changes to the skin began with erythema. Pigmentation, epilation, and desquamation also appeared after radiation exposure. The skin usually developed mild bright red erythema 1 week after β-irradiation with 50 or 100 Gy. This reaction worsened until the fourth week as evidenced by dusky red or mauve erythema. Moist desquamation was evident by the fourth week after irradiation with 50 Gy although no ulceration was detected beneath the epidermis.
Fig. 2. Time-dependent changes in skin gross morphology of a mini-pig after treatment with either 50 or 100 Gy of β-irradiation delivered by a 166Ho skin patch. (A) Two weeks after β-irradiation (50 Gy). (B) Four weeks after irradiation (50 Gy). (C) Eight weeks after irradiation (50 Gy). (D) Fourteen weeks after irradiation (50 Gy). (E) Two weeks after irradiation (100 Gy). (F) Four weeks after irradiation (100 Gy). (G) Eight weeks after irradiation (100 Gy). (H) Fourteen weeks after irradiation (100 Gy). (I) The dose- and time-dependent gross morphological changes of pig skin after application of a 166Ho skin patch (50 or 100 Gy). Data are expressed as the mean ± SEM. *p < 0.05 vs. the sham controls. Scale bar = 1 cm.
Gross skin morphology changes appeared earlier and were more severe in the samples irradiated with 100 Gy compared to ones exposed to 50 Gy (panel I in Fig. 2). After exposure to 100 Gy, moist desquamation and thick crust formation were apparent from the second week after irradiation and further developed until approximately 4 weeks after radiation treatment. All of the fields irradiated with 100 Gy developed moist desquamation approximately 4 weeks after 166Ho exposure while moist desquamation was observed in only one pig from the 50-Gy group. Some of the fields treated with 100 Gy developed ulceration that penetrated the dermis; this was followed by infection. To compare the dose- and time-response curves for skin changes after β-irradiation, the damage scores were plotted for the different doses (panel I in Fig. 2). After treatment with 50 or 100 Gy, clinical symptoms gradually disappeared after 4 weeks, and the skin returned to a state similar to that of the sham group 18 weeks after irradiation. Although the resulting scar did not show a second wave of reactions, the irradiated fields acquired wrinkles and rough skin.
Histological examination results
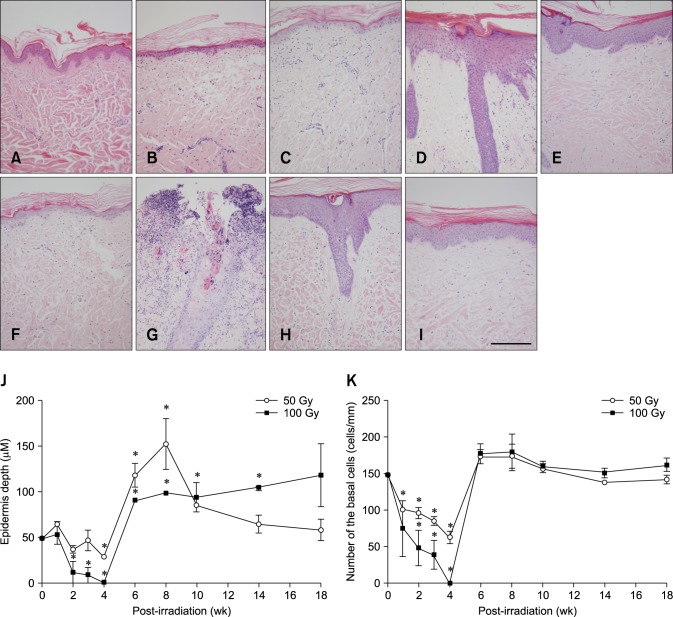
Two histological changes that correlated with gross skin morphology alterations were decreases in basal cell density and epidermal thickness (Fig. 3). Although severity of the damage caused by β-irradiation was dose-dependent, skin samples treated with 50 and 100 Gy were observed to have similar time-dependent changes. Epidermal thicknesses of the sham and irradiated groups are shown in panel J in Fig. 3. Thickness of the epidermis decreased markedly between 2 and 4 weeks after exposure to 50 and 100 Gy (n = 3, p < 0.05 vs. the control; panel J in Fig. 3), increased dramatically between 6 and 8 weeks after irradiation (n = 3, p < 0.05 vs. the control; panel J in Fig. 3), and gradually returned to a level near that of the control. Between 6 and 8 weeks after irradiation, finger-like projections of the rete ridges from the epidermis were observed in addition to epidermal hyperplasia (panels D and H in Fig. 3). Epidermal thickness in the irradiated field had not significantly decrease to a normal level by 18 weeks after irradiation.
Fig. 3. Histological changes in mini-pig skin after 50 or 100 Gy of β-irradiation delivered by a 166Ho skin patch. (A) Before exposure to irradiation. (B) Two weeks after β-irradiation (50 Gy). (C) Four weeks after irradiation (50 Gy). (D) Eight weeks after irradiation (50 Gy). (E) Fourteen weeks after irradiation (50 Gy). (F) Two weeks after β-irradiation (100 Gy). (G) Four weeks after irradiation (100 Gy). (H) Eight weeks after irradiation (100 Gy). (I) Fourteen weeks after irradiation (100 Gy). (J) Dose- and time-dependent changes in epidermal thickness a mini-pig following β-irradiation (50 or 100 Gy). (K) Dose- and time-dependent changes in basal cell density in mini-pig skin following β-irradiation (50 or 100 Gy). Data are presented as the mean ± SEM. *p < 0.05 vs. the sham controls. (A~I) Hematoxylin and eosin staining; 200× magnification. Scale bar = 100 µm.
Basal cell density in the epidermis of the sham and β-irradiated skin is shown in panel K in Fig. 3. Density of the basal cells in the epidermis gradually decreased from the first to fourth week after β-irradiation (n = 3, p < 0.05 vs. the control; panel K in Fig. 3), and then sharply increased to the sham control level from the sixth to eighteenth week after radiation exposure (panel K in Fig. 3). Both the epidermal thickness and basal cell density decreased until 4 weeks after β-irradiation. Thereafter, both increased significantly and caused epithelial hyperplasia that potentially contributed to recovery.
NF-κB expression in β-irradiated mini-pig skin
Minimal NF-κB expression was found in the normal mini-pig skin with weak cytoplasmic staining in some parts of the sebaceous glands, hair follicles, and epidermis. No nuclear NF-κB expression was seen in the epidermal layers of the non-irradiated controls throughout the experiment (panel A in Fig. 4). β-irradiation increased NF-κB expression in a time-dependent manner (Fig. 4). The fields contained increased levels of cytoplasmic NF-κB 1~2 weeks after β-irradiation (panels B and G in Fig. 4). Diffuse but strong cytoplasmic staining was identified throughout the epidermal layers 4~12 weeks after irradiation. Nuclear NF-κB was detected between the sixth and twelfth week after β-irradiation.
Fig. 4. Time-dependent changes in NF-κB expression after β-irradiation of mini-pig skin (50 or 100 Gy) with a 166Ho patch. (A) No NF-κB expression was observed in the skin before exposure to irradiation. (B) Two weeks after β-irradiation (50 Gy). (C) Intense cytoplasmic NF-κB expression with occasional nuclear staining 4 weeks after irradiation (50 Gy). (D) Strong nuclear expression of NF-κB throughout the hyperplastic epidermis 6 weeks after irradiation (50 Gy). (E) Persistent and strong nuclear expression of NF-κB in the full layers of the hyperplastic epidermis 8 weeks after β-irradiation (50 Gy). (F) Loss of nuclear NF-κB expression 14 weeks after irradiation (50 Gy). (G) NF-κB expression 2 weeks after β-irradiation (100 Gy). (H) Epidermal loss 4 weeks after irradiation (100 Gy). (I) Strong nuclear expression of NF-κB in the full layers of the hyperplastic epidermis 6 weeks after irradiation (100 Gy). (J) Persistent and strong nuclear expression of NF-κB 8 weeks after irradiation (100 Gy). (K) Striking decrease in the expression of nuclear NF-κB scattered in a few cells 14 weeks after irradiation (100 Gy). (L) Dose- and time-dependent changes in NF-κB expression in the epidermis following β-irradiation (50 or 100 Gy). Data are expressed as the mean ± SEM. *p < 0.05 vs. the sham controls. (A~K) Immunohistochemical counterstaining with hematoxylin; 400× magnification. Scale bar = 50 µm.
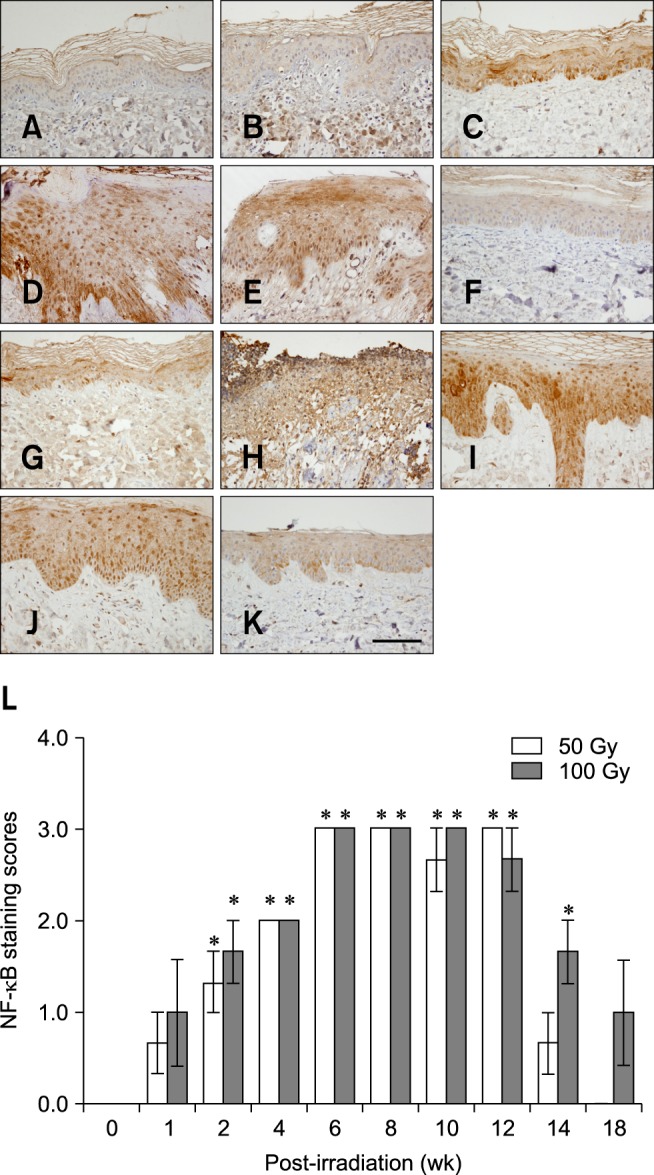
All of the skin samples from the 100-Gy irradiated group shed epidermal cells 4 weeks after radiation treatment; however, high levels of NF-κB were still detected in the epithelial cells of hair follicles of the dermal layers. NF-κB expression was particularly prominent in the basal layer beneath areas characterized by epidermal hyperplasia (panels D and I in Fig. 4). By 18 weeks, NF-κB levels in the group irradiated with 50 Gy decreased to that observed in the sham samples. Nucleocytoplasmic staining was also observed in the sweat glands, sebaceous glands, hair follicles, and vascular endothelial cells of the irradiated skin. As shown in panel L in Fig. 4, the group treated with 100 Gy had higher NF-κB expression scores than the 50-Gy irradiated group. These findings indicate the dose-dependent effects of β-irradiation on NF-κB expression in mini-pig skin. However, changes in NF-κB expression over time were essentially indistinguishable regardless of the radiation dose.
COX-2 expression in β-irradiated mini-pig skin
No COX-2 staining was visible in the epidermis of non-irradiated skin (Fig. 5) while the sebaceous glands and dermis showed minimal staining. Cytoplasmic COX-2 was detected in the epidermis of irradiated skin. Semi- quantitative scoring of cytoplasmic COX-2 expression for each layer of the epidermis is presented in Table 1. One week after irradiation with 50 Gy, some scattered epidermal COX-2 staining was limited to the basal layer. Between 2 and 3 weeks after irradiation with 50 Gy, COX-2 expression was apparent in the basal and spinous layers (panel B in Fig. 5). Four weeks after exposure to 50 Gy, COX-2 staining was observed throughout all layers of the epidermis (panel C in Fig. 5); this persisted until 10 weeks after the time of β-irradiation (Fig. 5 and Table 1). Interestingly, the COX-2 staining intensity seemed to shift from the basal layer to the granular layer over time (Fig. 5 and Table 1). The patterns of COX-2 expression found the groups irradiated with either 50 or 100 Gy were similar. However, density of the COX-2 signals showed that β-irradiation had a dose-dependent effect (Fig. 5 and Table 1).
Fig. 5. Time-dependent changes in COX-2 expression in mini-pig epidermis following β-irradiation (50 or 100 Gy). (A) No COX-2 expression was observed in the skin before β-irradiation. (B) COX-2 expression in basal cells 2 weeks after β-irradiation (50 Gy). (C) COX-2 expression in full layers of the atrophic epidermis 4 weeks after irradiation (50 Gy). (D) Intermediate levels of COX-2 expression in the basal and spinous layer of the hyperplastic epidermis 6 weeks after irradiation (50 Gy). (E) COX-2-positive staining in the superficial granular layer with no expression in the basal layer 8 weeks after β-irradiation (50 Gy). (F) Almost complete loss of COX-2 expression 14 weeks after irradiation (50 Gy). (G) COX-2 expression in the basal and spinous layers of the atrophic epidermis 2 weeks after β-irradiation (100 Gy). (H) Four weeks after irradiation (100 Gy). (I) Intermediate levels of COX-2 expression in the full layers of the hyperplastic epidermis 6 weeks after irradiation (100 Gy). (J) COX-2 expression in the upper layers but not in the basal layer 8 weeks after irradiation (100 Gy). (K) Loss of COX-2 expression in the basal layer with persistent COX-2 expression seen in the upper layers 14 weeks after irradiation (100 Gy). (A~K) Immunohistochemical counterstaining with hematoxylin; 400× magnification. Scale bar = 50 µm.
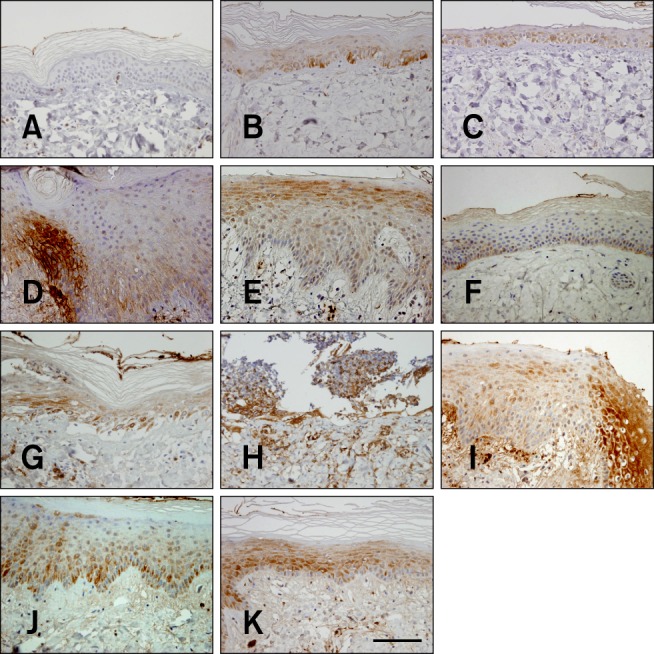
Table 1. Immunohistochemical staining for COX-2 in various epidermal layers of pig skin after treatment with either 50 or 100 Gy of β-irradiation.
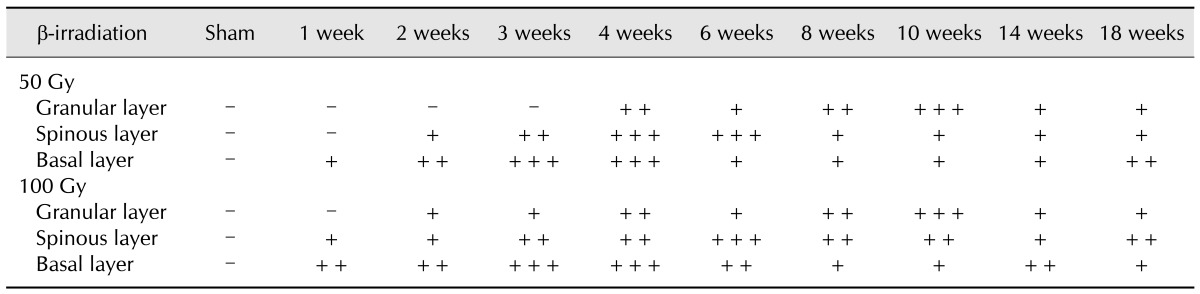
Immunolabeled sections were scored for the density of positive cells per field as follows: -, no immunoreactivity; +, weak; ++, moderate; +++, intense immunoreactivity.
All of the epidermal cells had been shed from the skin of the 100-Gy group by 4 weeks after irradiation. Nevertheless, high levels of COX-2 were still detected in the hair follicles of the dermis at that time. By the sixth week after β-irradiation, increased COX-2 staining was especially prevalent in the spinous and granular layers (panels D and I in Fig. 4). By weeks 6~10, increased expression of the enzyme was observed in all of the epidermal layers (Fig. 5 and Table 1).
Discussion
In the present study, the roles of NF-κB and COX-2 were evaluated as well as the association of these factors with histopathological changes that occurred as a result of β-irradiation of mini-pig skin. The 166Ho skin patches used in the investigation to deliver localized doses of β-radiation have been proposed as a treatment of early skin cancer [15]. The mini-pigs employed in the current study have also been widely used in many fields of biomedical research given their anatomical and physiological similarities with humans [28]. We treated both 50 and 100 Gy in the same animals to examine the sequence of skin changes caused by different doses to avoid individual variation. The use of 50 Gy in this study was based on a report stating that 42~45 Gy of β-irradiation can be used to treat skin cancer in mice and humans [15]. Doses of 100 Gy were also administered to evaluate the dose-dependent effects of β-irradiation on skin. After treatment with 50 Gy, moist desquamation and progressive late dermal fibrosis was found in 100% of mice without any morbidity caused by internal complications [22]. In the present study, however, 50 Gy did not induce noticeable skin damage in in a piglet model. Highly localized irradiation of the skin has gross effects that visibly manifest after delivery of 100 Gy [12]. In the present study, the time courses of tissue changes after irradiation with either 50 Gy or 100 Gy were basically identical. However, the initiation of both clinically relevant symptoms and histological changes appeared to be dose-dependent because pigs exposed to 100 Gy developed more severe clinical and histological changes at an earlier time than animals treated with 50 Gy.
In the present study, erythema (associated with itching) occurred within 1 week after β-irradiation. This progressed to intense reddening, dry desquamation, moist desquamation, and crust formation that peaked 4 weeks after irradiation. The subsequent recovery phase culminated in disappearance of the symptoms 18 weeks after radiation exposure. The gross skin morphology changes closely correlated with histological alterations in the epidermis. The skin changes observed after β-irradiation in this study resembled those reported in previous investigation that used other sources of β particles [13,22] although a slight difference was observed on the nadir day (4~6 weeks after β-irradiation).
In the present study, changes in basal cell density and epidermal depth corresponded to the gross skin morphology scores. Administration of a large single dose of β-, γ-, or X-rays appears to usually trigger a linear loss of basal cells [20]. This decrease reaches a maximum level between 14 and 25 days after irradiation, and is followed by exponential re-epithelialization until control levels and above are reached 28~32 days after irradiation. The dominant erythematous reaction observed indirectly reflects variations in the severity of epidermal basal cell loss. Either a dry or a moist desquamatory response may be seen 3~6 weeks after irradiation [12].
Basal cells in the epidermis are targeted for damage by β-irradiation [11,12]. Proliferating cells are found at the base of the rete pegs in hyperplastic epidermis of pigs after recovery from X-ray irradiation [19]. Cell survival at the bases of rete pegs is likely to be substantial, and three sources for repopulation originating from rete pegs, hair follicles, or the field periphery have been suggested [21]. The low capacity of 166Ho radiation used to penetrate tissues in the present study means that most of the effects were restricted to the epidermis. We observed finger-like projections on the rete ridges during the healing stage 6~8 weeks after β-irradiation. Healing of the epidermal layers appeared to be associated with repopulation by hair follicle epithelial cells as previously reported [21].
Given that NF-κB and COX-2 play important roles in inflammation and programmed cell death, changes in expression of the factors might contribute to acute and chronic changes observed in skin after irradiation [6,24,30]. Ionizing radiation is a direct and indirect activator of NF-κB [6,30,31]. In turn, NF-κB up-regulates the transcription of the gene that encodes COX-2, a stress- response protein [24,30,31]. In the current study, increased levels of NF-κB and COX-2 in β-irradiated epidermal cells were found. It is likely that normal tissues differ in their sensitivity to radiation-induced activation of NF-κB and COX-2 expression, and that these differences correlate with susceptibility to radiation-associated damage [6]. However, the mechanisms underlying differences in the effects of ionizing radiation on NF-κB and COX-2 expression remain unclear. To the best of our knowledge, the current report is the first to describe the effects of β-irradiated skin injury on NF-κB and COX-2 production.
Following exposure to the high doses of β-irradiation administered in our study, expression of both NF-κB and COX-2 first became apparent in the basal cell layer and then gradually increased along with the appearance of epidermal hyperplasia during the healing stage. NF-κB and COX-2 expression seemed to have a similar pattern in this study with increased levels being maintained throughout the healing process. Recovery of the epithelial layer is very important for injured skin repair. The purpose of wound healing that begins immediately after injury to the skin is to provide a protective barrier against further potential damage [7]. Wound healing proceeds through phases of inflammation, tissue formation, re-epithelialization, and tissue remodeling [16]. Given that β-irradiation cannot penetrate to the deep dermis and does not provoke a severe inflammatory reaction in the dermis, maintenance of the epidermal layer seems important for preventing a subsequent inflammatory reaction in the dermis. In this regard, our results suggest that NF-κB and COX-2 are important factors for recovery from epidermal injury caused by β-irradiation. Our experimental data indicate that NF-κB and COX-2 promote skin regeneration after radiation exposure. Although no other investigation on the roles of NF-κB and COX-2 in β-irradiated skin injury has been published, study of UVB-induced skin damage [3] support our hypothesis that NF-κB and COX-2 induce epidermal regeneration.
In conclusion, we documented the sequence of gross mini-pig skin morphology changes following β-irradiation. Additionally, possible roles of NF-κB and COX-2 in epidermal regeneration were proposed. We believe that the mini-pig is a useful experimental model for studying skin damage caused by β-irradiation, and evaluating the efficacy of therapeutic modalities to reduce skin damage and accelerate skin repair after radiation exposure. More work is clearly required to clarify the precise effects of NF-κB and COX-2 accumulation in the epithelial layer after irradiation.
Acknowledgments
This study was supported by a project titled 'Development of therapeutic improvement on acute radiation syndrome (50581-2013)' of the Ministry of Science, ICT and Future Planning (MSIP) of the Korean government.
Footnotes
There is no conflict of interest.
References
- 1.Abd-El-Aleem SA, Ferguson MWJ, Appleton I, Bhowmick A, McCollum CN, Ireland GW. Expression of cyclooxygenase isoforms in normal human skin and chronic venous ulcers. J Pathol. 2001;195:616–623. doi: 10.1002/path.992. [DOI] [PubMed] [Google Scholar]
- 2.Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171–1185. doi: 10.1016/0360-3016(94)00423-I. [DOI] [PubMed] [Google Scholar]
- 3.Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, Epstein EH, Jr, Bickers DR. Ultraviolet B (UVB)-induced COX-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001;280:1042–1047. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barabanova AV. Significance of beta-radiation skin burns in Chernobyl patients for the theory and practice of radiopathology. Vojnosanit Pregl. 2006;63:477–480. doi: 10.2298/vsp0605477b. [DOI] [PubMed] [Google Scholar]
- 6.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor κB. J Clin Invest. 1991;88:691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark RAF, Ashcroft GS, Spencer MJ, Larjava H, Ferguson MWJ. Re-epithelialization of normal human excisional wounds is associated with a switch from αvβ5 to αvβ6 integrins. Br J Dermatol. 1996;135:46–51. [PubMed] [Google Scholar]
- 8.Egan LJ, Eckmann L, Greten FR, Chae S, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci U S A. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hei TK. Cyclooxygenase-2 as a signaling molecule in radiation-induced bystander effect. Mol Carcinog. 2006;45:455–460. doi: 10.1002/mc.20219. [DOI] [PubMed] [Google Scholar]
- 10.Hoashi T, Okochi H, Kadono T, Tamaki K, Nishida M, Futami S, Maekawa K. A case of acute radiation syndrome from the dermatological aspect. Br J Dermatol. 2008;158:597–602. doi: 10.1111/j.1365-2133.2008.08475.x. [DOI] [PubMed] [Google Scholar]
- 11.Hopewell JW. Mechanisms of the action of radiation on skin and underlying tissues. Br J Radiol Suppl. 1986;19:39–47. [PubMed] [Google Scholar]
- 12.Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990;57:751–773. doi: 10.1080/09553009014550911. [DOI] [PubMed] [Google Scholar]
- 13.Hopewell JW, Sieber VK, Heryet JC, Wells J, Charles MW. Dose- and source-size-related changes in the late response of pig skin to irradiation with single doses of beta radiation from sources of differing energy. Radiat Res. 1993;133:303–311. [PubMed] [Google Scholar]
- 14.Kumar S, Kolozsvary A, Kohl R, Lu M, Brown S, Kim JH. Radiation-induced skin injury in the animal model of scleroderma: implications for post-radiotherapy fibrosis. Radiat Oncol. 2008;3:40. doi: 10.1186/1748-717X-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JD, Park KK, Lee MG, Kim EH, Rhim KJ, Lee JT, Yoo HS, Kim YM, Park KB, Kim JR. Radionuclide therapy of skin cancers and Bowen's disease using a specially designed skin patch. J Nucl Med. 1997;38:697–702. [PubMed] [Google Scholar]
- 16.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 17.Meng A, Yu T, Chen G, Brown SA, Wang Y, Thompson JS, Zhou D. Cellular origin of ionizing radiation-induced NF-κB activation in vivo and role of NF-κB in ionizing radiation-induced lymphocyte apoptosis. Int J Radiat Biol. 2003;79:849–861. doi: 10.1080/09553000310001622814. [DOI] [PubMed] [Google Scholar]
- 18.Mettler FA, Jr, Gus'kova AK, Gusev I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 2007;93:462–469. doi: 10.1097/01.HP.0000278843.27969.74. [DOI] [PubMed] [Google Scholar]
- 19.Morris GM, Hamlet R, Hopewell JW. The cell kinetics of the epidermis and follicular epithelium of the rat: variations with age and body site. Cell Tissue Kinet. 1989;22:213–222. doi: 10.1111/j.1365-2184.1989.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 20.Morris GM, Hopewell JW. Cell kinetic changes in the follicular epithelium of pig skin after irradiation with single and fractionated doses of X rays. Br J Radiol. 1989;62:41–47. doi: 10.1259/0007-1285-62-733-41. [DOI] [PubMed] [Google Scholar]
- 21.Peel DM, Hopewell JW, Wells J, Charles MW. Nonstochastic effects of different energy β emitters on pig skin. Radiat Res. 1984;99:372–382. [PubMed] [Google Scholar]
- 22.Randall K, Coggle JE. Long-term expression of transforming growth factor TGF β1 in mouse skin after localized β-irradiation. Int J Radiat Biol. 1996;70:351–360. doi: 10.1080/095530096145085. [DOI] [PubMed] [Google Scholar]
- 23.Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008;84:322–329. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 25.Scholz K, Fürstenberger G, Müller-Decker K, Marks F. Differential expression of prostaglandin-H synthase isoenzymes in normal and activated keratinocytes in vivo and in vitro. Biochem J. 1995;309:263–269. doi: 10.1042/bj3090263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon GA, Maibach HI. The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations-an overview. Skin Pharmacol Appl Skin Physiol. 2000;13:229–234. doi: 10.1159/000029928. [DOI] [PubMed] [Google Scholar]
- 27.Sonis ST, O'Donnell KE, Popat R, Bragdon C, Phelan S, Cocks D, Epstein JB. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004;40:170–176. doi: 10.1016/s1368-8375(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang XJ, Lin S, Kang HF, Dai ZJ, Bai MH, Ma XL, Ma XB, Liu MJ, Liu XX, Wang BF. The effect of RHIZOMA COPTIDIS and COPTIS CHINESIS aqueous extract on radiation-induced skin injury in a rat model. BMC Complement Altern Med. 2013;13:105. doi: 10.1186/1472-6882-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeoh ASJ, Bowen JM, Gibson RJ, Keefe DMK. Nuclear factor κB (NFκB) and cyclooxygenase-2 (Cox-2) expression in the irradiated colorectum is associated with subsequent histopathological changes. Int J Radiat Oncol Biol Phys. 2005;63:1295–1303. doi: 10.1016/j.ijrobp.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 31.Yeoh ASJ, Gibson RJ, Yeoh EEK, Bowen JM, Stringer AM, Giam KA, Keefe DMK. A novel animal model to investigate fractionated radiotherapy-induced alimentary mucositis: the role of apoptosis, p53, nuclear factor-κB, COX-1, and COX-2. Mol Cancer Ther. 2007;6:2319–2327. doi: 10.1158/1535-7163.MCT-07-0113. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. Mitochondrial function and nuclear factor-κB-mediated signaling in radiation-induced bystander effects. Cancer Res. 2008;68:2233–2240. doi: 10.1158/0008-5472.CAN-07-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]