Abstract
Mycobacterium (M.) bovis, a bacterium in the M. tuberculosis complex, is a causative agent of bovine tuberculosis, a contagious disease of animals. Mycobacterial culture is the gold standard for diagnosing bovine tuberculosis, but this technique is laborious and time-consuming. In the present study, performance of the SD Bioline TB Ag MPT4 Rapid test, an immunochromatographic assay, was evaluated using reference bacterial strains and M. bovis field isolates collected from animals. The SD MPT64 Rapid test produced positive results for 95.5% (63/66) of the M. bovis isolates from cattle and 97.9% (46/47) of the isolates from deer. Additionally, the test had a sensitivity of 96.5% (95% CI, 91.2-99.0), specificity of 100% (95% CI, 96.7-100.0), positive predictive value of 100% (95% CI, 96.7-100.0), and negative predictive value of 92.9% (95% CI, 82.7-98.0) for M. bovis isolates. In conclusion, the SD MPT64 Rapid test is simple to use and may be useful for quickly confirming the presence of M. bovis in animals.
Keywords: animals, Mycobacterium bovis, SD MPT64 Rapid test
Introduction
Bovine tuberculosis is a contagious disease of animals caused by Mycobacterium (M.) bovis, which is part of the M. tuberculosis complex [2,21]. Bovine tuberculosis is responsible for major agricultural economic loss and is a public health concern [7,18]. In most countries, control and eradication of bovine tuberculosis involves testing and slaughter [17].
The intradermal tuberculin skin test is widely used to identify infected animals and has contributed to the control of bovine tuberculosis [8]. In Korea, the incidence of bovine tuberculosis was approximately 15% in the 1940s; this was reduced to 0.15% in 2005 by a bovine tuberculosis eradication program involving the intradermal tuberculin skin test [6]. However, the current incidence has increased to 0.25% (approximately 1,000 cattle per year) [24]. An efficient and effective diagnostic system or strategy is urgently needed to eradicate bovine tuberculosis. Serological tests using M. bovis-specific antigens and the interferon-gamma (IFN-γ) assay are currently being considered or evaluated in Korea. These assays use mycobacterial cultures as the gold standard for diagnosing bovine tuberculosis. Culturing takes 3 to 6 weeks while the presence of M. bovis can be confirmed using acid-fast bacilli staining and biochemical tests [22]. The Ziehl-Neelsen stain is simple and rapid, but cannot differentiate M. bovis from nontuberculous mycobacteria. Furthermore, conventional biochemical tests are laborious and time-consuming.
The SD Bioline TB Ag MPT64 Ag MPT64 Rapid test (SD MPT64 Rapid test), a simple immunochromatographic test (ICT) for the M. tuberculosis complex, has been developed and uses monoclonal antibodies to detect MPT64 protein [1,9]. MPT64 is a protein specifically secreted by members of the M. tuberculosis complex including M. tuberculosis, M. bovis, and M. africanum as well as some strains of M. bovis bacilli Calmette-Guérin (BCG) [12]. The SD MPT64 Rapid Test is used widely to confirm the identity of M. tuberculosis isolates from humans. Furthermore, the test is highly sensitive and specific. The SD MPT64 Rapid test is potentially useful for identifying M. bovis, a member of the M. tuberculosis complex, because the MPT64 antigen is expressed by all members of the M. tuberculosis complex [10]. However, few reports have evaluated the performance of this test for analyzing M. bovis isolates from animals. We therefore assessed the ability of the SD MPT64 Rapid test to identify M. bovis isolates from cattle and deer.
Materials and Methods
Bacterial strains
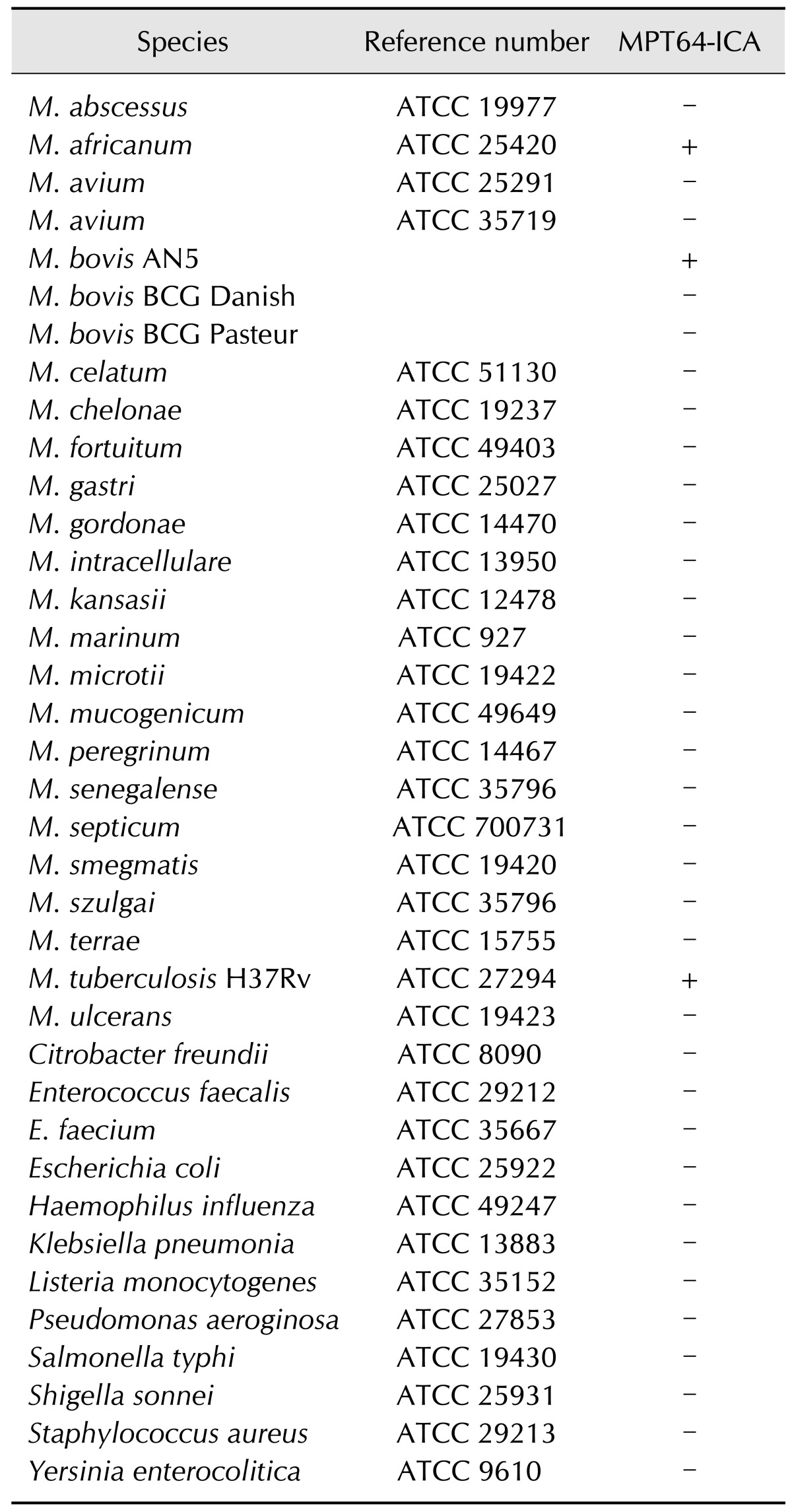
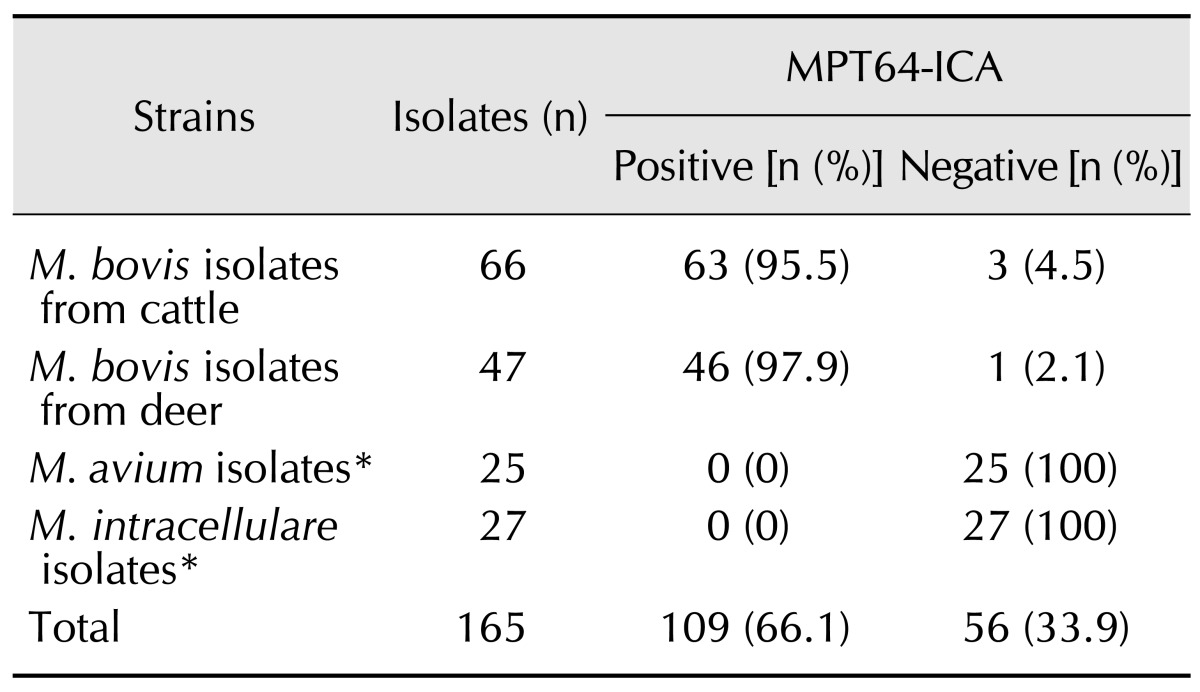
Reference bacterial strains consisting of 25 mycobacteria and 12 other bacteria were obtained from American Type Culture Collection (ATCC, USA) and used for the present investigation (Table 1). M. bovis AN5 was grown in Middlebrook 7H9 broth media (Difco, USA) supplemented with 10% Middlebrook OADC enrichment medium (BBL, USA) to determine the detection limit of the test. Mycobacterium species grown on Löwenstein-Jensen medium (UNION LAB, Korea) and other bacterial strains grown on Luria-Bertani agar plates (Difco) were used for the SD MPT64 Rapid test. This study evaluated 113 M. bovis field strains isolated from cattle and deer in Korea along with 25 M. avium and 27 M. intracellulare clinical strains isolated from humans (Table 2). All mycobacterial isolates were identified by Ziehl-Neelsen staining and biochemical tests as previously described [22]. AccuProbe (Gen-Probe, USA), REBA Myco-ID (M&D, Korea), and multiplex PCR were performed to identify Mycobacterium species and distinguish M. bovis from other members of the M. tuberculosis complex [14,15,20].
Table 1. Reference bacteria strains used in this study and results of the SD MPT64 Rapid test.
M.: Mycobacterium, ATCC: American Type Culture Collection.
Table 2. Results of the SD MPT64 Rapid test for M. bovis isolates from animals.
*Strains isolated from humans.
SD MPT64 Rapid test
SD MPT64 Rapid test was performed according to the manufacturer's guidelines. Three or four colonies of mycobacterial strains grown in Löwenstein-Jensen media and other bacterial strains grown on Luria-Bertani agar plates were emulsified in 200 µL of extraction buffer. Next, 100 µL were placed in sample wells of the test and the results were visually assessed based on color development after incubating at room temperature for 15 min. Non-recactive M. bovis isolates in the test was confirmed by sequencing of the mpt64 gene.
To determine the detection limit of the SD MPT64 Rapid test, a series of diluted M. bovis AN5 suspension from 2.1 × 103 to 2.0 × 106 cultured in Middlebrook 7H9 medium with 10% enrichment medium were analyzed with the SD MPT64 Rapid test and colony-forming unit (CFU) counted using Middlebrook 7H10 (Difco) plates with 10% enrichment medium.
Statistical analysis
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were determined using GraphPad Prism software (ver. 4.0; GraphPad Software, USA). Agreement of test results was estimated based on kappa values using the SPSS 13.0 (SPSS, USA).
Results
SD MPT64 Rapid test results for the reference strains
The SD MPT64 Rapid test was performed for 25 reference strains of mycobacteria and 12 other bacteria to identify members of the M. tuberculosis complex. The SD MPT64 Rapid test produced strong results for M. bovis, M. africanum, and M. tuberculosis, but not M. bovis BCG Pasteur or M. bovis BCG Danish (Table 1). No positive signal was observed for non-tuberculous mycobacteria (NTM) bacilli or any other bacteria tested. These results imply that the SD MPT64 Rapid test can identify members of the M. tuberculosis complex, including M. bovis, with a high degree of specificity.
Detection limit of the SD MPT64 Rapid test for M. bovis
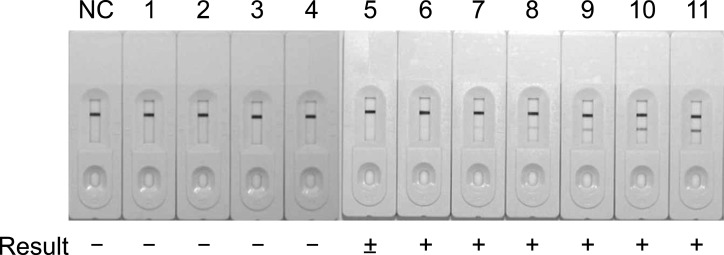
Ten-fold dilutions of M. bovis AN5 were used to measure the detection limit of the SD MPT64 Rapid test. As shown in Fig. 1, the detection limit of the test for M. bovis was estimated to be 5.5 × 104 CFU/mL. The test produced a strong positive signal (purple line) for samples containing more than 5.4 × 105 CFU/mL of M. bovis and a clear positive signal for samples containing 1.3 × 105 CFU/mL or 2.7 × 105 CFU/mL of M. bovis. The test signal was weakly positive for the sample containing 6.8 × 104 CFU/mL of M. bovis, and weak but determinable as positive with careful examination for the sample containing 3.4 × 104 CFU/mL of M. bovis.
Fig. 1. Detection limit of the SD MPT64 Rapid test for Mycobacterium (M.) bovis. The test was used to analyze a series of diluted suspensions of M. bovis AN5 cultured in Middlebrook 7H9 broth with enrichment. A representative image of three tests is shown. The results were interpreted as positive (+), negative (-), or weak (±). NC, negative control; 1, 2.1 × 103 CFU/mL of M. bovis; 2, 4.2 × 103 CFU/mL of M. bovis; 3, 8.5 × 103 CFU/mL of M. bovis; 4, 1.7 × 104 CFU/mL of M. bovis; 5, 3.4 × 104 CFU/mL of M. bovis; 6, 6.8 × 104 CFU/mL of M. bovis; 7, 1.3 × 105 CFU/mL of M. bovis; 8, 2.7 × 105 CFU/mL of M. bovis; 9, 5.4 × 105 CFU/mL of M. bovis; 10, 1.0 × 106 CFU/mL of M. bovis; 11, 2.0 × 106 CFU/mL of M. bovis.
MPT64 Rapid test results for M. bovis isolates from animals
In total, 113 M. bovis isolates from animals and 52 NTM isolates were used to evaluate ability of the SD MPT64 Rapid test to identify M. bovis isolates from animals (Table 2). Among the M. bovis isolates, 109 (96.5%) had a positive SD MPT64 Rapid test result; all M. avium and M. intracellulare isolates produced negative results. Positive results were obtained for 95.5% (63/66) of the M. bovis isolates from cattle and 97.9% (46/47) of the isolates from deer. Sensitivity was 96.5% (95% confidence interval [CI], 91.2-99.0), specificity was 100% (95% CI, 96.7-100.0), the positive predictive value was 100.0% (95% CI, 96.7-100.0), the negative predictive value was 92.9% (95% CI, 82.7-98.0), and the kappa value was 0.945.
Discussion
The purpose of this study was to evaluate the SD MPT64 Rapid test, an ICT for M. tuberculosis. We assessed the ability of the test to confirm the identity of M. bovis isolates from animals. ICT is widely used to diagnose infectious diseases because of its simple and rapid nature. The SD MPT64 Rapid test has a high level of sensitivity and specificity for detecting M. tuberculosis in humans [1,9,19]. A few groups included M. bovis isolates in their evaluation of the SD MPT64 Rapid test and found that some M. bovis isolates did not produce a reaction [5,11,16]. The present study evaluated the ability of SD MPT64 Rapid test to detect M. bovis isolates from animals using a considerable numbers of specimens.
The sensitivity (96.5%) and specificity (100.0%) of the SD MPT64 Rapid test for M. bovis isolates observed in the present investigation were comparable to those of previous reports (sensitivity of 92~99% and specificity of 97~100%) for M. tuberculosis [3,4,19]. These results indicate that the SD MPT64 Rapid test is as specific and sensitive for M. bovis as it is for M. tuberculosis. The detection limit for M. bovis was similar to that reported in studies that measured the detection limit for M. tuberculosis [1,19].
In this investigation, four out of the 113 M. bovis isolates (3.5%) were nonreactive in the SD MPT64 Rapid test. This finding is comparable to data from other studies in which the MPT64 Rapid test was used to detect M. tuberculosis isolates from humans. The false-negative rate of the MPT64 Rapid test is 3.1% (12 out of 384) according to Hirano et al. [13] and 1.4% (2 out of 146) according to Wang et al. [23]. These reports indicate that the performance of the SD MPT64 Rapid test is similar for M. tuberculosis isolates from humans and M. bovis from animals. For three out of the four nonreactive M. bovis isolates, two contained a deletion between 512 and 688 base pairs (bp) in the mpt64 gene, and the remaining one had a point mutation at position 402 (G to A) that created a stop codon. These findings were similar to ones in a report by Hirano et al. [13] and imply that these changes might have produced false-negative results in the SD MPT64 Rapid test.
A mutation was identified by sequencing the mpt64 gene in three of the four nonreactive M. bovis isolates (data not shown). This might have resulted in the false-negatives obtained from the SD MPT64 Rapid test as also reported by Hirano et al. [13]. Because incorrect identification of M. bovis can have serious consequences, conventional or molecular tests to confirm the presence of this microorganism should be performed when microbiological findings are suggestive of M. bovis but the SD MPT64 Rapid test result is negative.
Results of this study suggest that the SD MPT64 Rapid test might be a useful method for quick identification of M. bovis isolates from animals. The test could possibly replace conventional confirmation assays. The greater simplicity and lower cost of the SD MPT64 Rapid test compared to other methods make this technique a good choice for confirming M. bovis isolates. Using this test for identifying M. bovis isolates from animals might contribute to the establishment of an eradication strategy for bovine tuberculosis. In conclusion, the SD MPT64 Rapid test is a simple and reliable method that may serve as a diagnostic tool for confirming the presence of M. bovis in animals.
Acknowledgments
This research was supported by a grant from the Bio-industry Technology Development Program, the Ministry of Agriculture, Food and Rural Affairs, Korea (grant no. 314025-03) and in part by a grant from the Animal and Plant Quarantine Agency (Z-AD13-2010-11-0502).
Footnotes
There is no conflict of interest.
References
- 1.Abe C, Hirano K, Tomiyama T. Simple and rapid identification of the Mycobacterium tuberculosis complex by immunochromatographic assay using anti-MPB64 monoclonal antibodies. J Clin Microbiol. 1999;37:3693–3697. doi: 10.1128/jcm.37.11.3693-3697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanfu W. The situation of tuberculosis and tuberculosis control in animals of economic interest. Tuberculosis (Edinb) 2006;86:330–335. doi: 10.1016/j.tube.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Ang CF, Cajucom MAM, Kim Y, Bang H, Lee H, Cho SN, Montalban CS. Evaluation of a rapid assay for identification of Mycobacterium tuberculosis grown in solid and liquid media. Int J Tuberc Lung Dis. 2011;15:1475–1477. doi: 10.5588/ijtld.10.0709. [DOI] [PubMed] [Google Scholar]
- 4.Brent AJ, Mugo D, Musyimi R, Mutiso A, Morpeth S, Levin M, Scott JA. Performance of the MGIT TBc identification test and meta-analysis of MPT64 assays for identification of the Mycobacterium tuberculosis complex in liquid culture. J Clin Microbiol. 2011;49:4343–4346. doi: 10.1128/JCM.05995-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chikamatsu K, Aono A, Yamada H, Sugamoto T, Kato T, Kazumi Y, Tamai K, Yanagisawa H, Mitarai S. Comparative evaluation of three immunochromatographic identification tests for culture confirmation of Mycobacterium tuberculosis complex. BMC Infect Dis. 2014;14:54. doi: 10.1186/1471-2334-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YS. Outbreak and research trends of bovine tuberculosis in Republic of Korea. Korean J Vet Public Health. 2007;31:61–67. [Google Scholar]
- 7.de la Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb) 2006;86:77–109. doi: 10.1016/j.tube.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res Vet Sci. 2006;81:190–210. doi: 10.1016/j.rvsc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Gaillard T, Fabre M, Martinaud C, Vong R, Brisou P, Soler C. Assessment of the SD Bioline Ag MPT64 RapidTM and the MGITTM TBc identification tests for the diagnosis of tuberculosis. Diagn Microbiol Infect Dis. 2011;70:154–156. doi: 10.1016/j.diagmicrobio.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Harboe M, Nagai S, Patarroyo ME, Torres ML, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa N, Miura T, Ishii K, Yamaguchi K, Lindner TH, Merritt S, Matthews JD, Siddiqi SH. New simple and rapid test for culture confirmation of Mycobacterium tuberculosis complex: a multicenter study. J Clin Microbiol. 2002;40:908–912. doi: 10.1128/JCM.40.3.908-912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillemann D, Rüsch-Gerdes S, Richter E. Application of the Capilia TB assay for culture confirmation of Mycobacterium tuberculosis complex isolates. Int J Tuberc Lung Dis. 2005;9:1409–1411. [PubMed] [Google Scholar]
- 13.Hirano K, Aono A, Takahashi M, Abe C. Mutations including IS6110 insertion in the genome encoding the MPB64 protein of Capilia TB-negative Mycobacterium tuberculosis isolates. J Clin Microbiol. 2004;42:390–392. doi: 10.1128/JCM.42.1.390-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Choi Y, Jeon BY, Jin H, Cho SN, Lee H. A simple and efficient multiplex PCR assay for the identification of Mycobacterium genus and Mycobacterium tuberculosis complex to the species level. Yonsei Med J. 2013;54:1220–1226. doi: 10.3349/ymj.2013.54.5.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim J, Lyu J, Choi CM, Oh YM, Lee SD, Kim WS, Kim DS, Lee H, Shim TS. Non-tuberculous mycobacterial diseases presenting as solitary pulmonary nodules. Int J Tuberc Lung Dis. 2010;14:1635–1640. [PubMed] [Google Scholar]
- 16.Marzouk M, Kahla IB, Hannachi N, Ferjeni A, Salma WB, Ghezal S, Boukadida J. Evaluation of an immunochromatographic assay for rapid identification of Mycobacterium tuberculosis complex in clinical isolates. Diagn Microbiol Infect Dis. 2011;69:396–399. doi: 10.1016/j.diagmicrobio.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Morris RS, Pfeiffer DU. Directions and issues in bovine tuberculosis epidemiology and control in New Zealand. N Z Vet J. 1995;43:256–265. doi: 10.1080/00480169./1995.35904. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995;76(Suppl 1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 19.Park MY, Kim YJ, Hwang SH, Kim HH, Lee EY, Jeong SH, Chang CL. Evaluation of an immunochromatographic assay kit for rapid identification of Mycobacterium tuberculosis complex in clinical isolates. J Clin Microbiol. 2009;47:481–484. doi: 10.1128/JCM.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts SA, Lowe O, Pandey S, Williamson DA, Newton S, Vaughan R. Comparison of the MGIT TBc immunochromatographic assay with the Accuprobe Gen-Probe TB assay for identification of Mycobacterium tuberculosis complex: results from a low-burden tuberculosis setting. Diagn Microbiol Infect Dis. 2012;74:415–416. doi: 10.1016/j.diagmicrobio.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Thoen CO, LoBue PA. Mycobacterium bovis tuberculosis: forgotten, but not gone. Lancet. 2007;369:1236–1238. doi: 10.1016/S0140-6736(07)60572-8. [DOI] [PubMed] [Google Scholar]
- 22.Vincent V, Gutiérrez MC. Mycobacterium: laboratory characteristics of slowly growing mycobacteria. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. 9th ed. Washington D.C.: ASM Press; 2007. pp. 573–588. [Google Scholar]
- 23.Wang JY, Lee LN, Lai HC, Hsu HL, Jan IS, Yu CJ, Hsueh PR, Yang PC. Performance assessment of the Capilia TB assay and the BD ProbeTec ET system for rapid culture confirmation of Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2009;59:395–399. doi: 10.1016/j.diagmicrobio.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Wee SH, Kim CH, More SJ, Nam HM. Mycobacterium bovis in Korea: an update. Vet J. 2010;185:347–350. doi: 10.1016/j.tvjl.2009.07.002. [DOI] [PubMed] [Google Scholar]