Abstract
Type I IFN production is an important host immune response against viral and bacterial infections. However, little is known about the ligands and corresponding host receptors that trigger type I IFN production during bacterial infections. We used a model intracellular pathogen, Francisella novicida, to begin characterizing the type I IFN response to bacterial pathogens. F. novicida replicates in the cytosol of host cells and elicits a robust type I IFN response that is largely TLR independent, but is dependent on the adapter molecule STING, suggesting that the type I IFN stimulus during F. novicida infection is cytosolic. In this study, we report that the cytosolic DNA sensors, cyclic GMP-AMP synthase (cGAS) and Ifi204, are both required for the STING-dependent type I IFN response to F. novicida infection in both primary and immortalized murine macrophages. We created cGAS, Ifi204, and Sting functional knockouts in RAW264.7 macrophages and demonstrated that cGAS and Ifi204 cooperate to sense dsDNA and activate the STING-dependent type I IFN pathway. In addition, we show that dsDNA from F. novicida is an important type I IFN stimulating ligand. One outcome of cGAS–STING signaling is the activation of the absent in melanoma 2 inflammasome in response to F. novicida infection. Whereas the absent in melanoma 2 inflammasome is beneficial to the host during F. novicida infection, type I IFN signaling by STING and IFN regulatory factor 3 is detrimental to the host during F. novicida infection. Collectively, our studies indicate that cGAS and Ifi204 cooperate to sense cytosolic dsDNA and F. novicida infection to produce a strong type I IFN response.
Introduction
The innate immune system plays a key role in the early recognition and elimination of invading pathogens. Many recognition systems are in place to detect conserved pathogen-associated molecular patterns (PAMPs), such as nucleic acids and cell-wall components (1). Upon PAMP recognition, immune cells initiate signal transduction cascades that trigger a type I IFN transcriptional response, which can prompt a broad range of additional responses to infection, including caspase-1–mediated cell death and proinflammatory cytokine release (2, 3). Although it is appreciated that many bacterial species, including Francisella tularensis, Listeria monocytogenes, Salmonella typhimurium, Mycobacterium tuberculosis, and Chlamydia trachomatis, can initiate a type I IFN response, the mechanism of host recognition remains largely undetermined (4, 5).
Microbes can stimulate the type I IFN response either by activating members of the TLR family that signal through the endosomal adapter TRIF or by activating cytosolic receptors that activate a type I IFN transcriptional response (6, 7). One important class of cytosolic receptors includes DNA sensors that activate the endoplasmic reticulum membrane protein stimulator of IFN genes (STING, also known as MITA, MPSY, ERIS, and TMEM173). STING activation leads to the recruitment of the kinase TBK1, which phosphorylates IFN regulatory factor 3 (IRF3), a transcription factor required for the induction of IFN-β1 (8, 9). Recent studies have demonstrated that the host-derived second-messenger cyclic GMP-AMP (cGAMP) synthesized from the DNA sensor cyclic GMP-AMP synthase (cGAS) or bacterial-derived cyclic dinucleotides can directly activate STING (8, 10, 11). In addition to cGAS, a number of cytosolic sensors were identified to bind DNA and trigger the type I IFN response. They include RNA polymerase III, DNA-dependent activator of IFN-regulatory factors, Lrrfip1, Ifi204 (human: IFI16), Mre11, DNA-dependent protein kinase, and Ddx41 (7, 12–17). Although many cytosolic DNA sensors have been identified, the role of these sensors during bacterial infections remains unclear.
Francisella novicida is a model organism used to study the cytosolic responses of immune cells to intracellular bacteria (18). Upon phagocytosis by host macrophages, F. novicida rapidly escapes the Francisella-containing vacuole and replicates in the cytosol (19). Cytosolic F. novicida trigger a proinflammatory response characterized by the production of type I IFNs followed by pyroptotic cell death (20, 21). The type I IFN response to F. novicida infection is largely TLR independent, but STING dependent, making F. novicida an ideal organism to study the cytosolic responses in macrophages to an intracellular bacterial pathogen (20, 22, 23). To date, the Francisella ligand(s) and corresponding host sensor(s) have not been identified. Ultimately, production of type I IFNs increases protein levels of the DNA sensor, absent in melanoma 2 (AIM2), a protein that binds cytosolic DNA and engages the adaptor protein ASC to form a caspase-1 inflammasome complex (24–27). An active AIM2 inflammasome leads to the secretion of proinflammatory cytokines (IL-18 and IL-1β) and caspase-1–dependent cell death (20), which are required for a protective innate immune response in mice (23).
In this study, we identified cGAS and Ifi204 as two important host factors involved in type I IFN signaling in response to F. novicida infection in both bone marrow–derived macrophages (BMMs) and RAW264.7 macrophages. Using targeted knockouts (KOs) and complementation vectors, we demonstrated that cGAS and Ifi204 both contribute to STING-dependent type I IFN response to high concentrations of cytosolic dsDNA. In addition, we showed that dsDNA is the primary molecule found in F. novicida lysates that stimulate cGAS- and Ifi204-dependent type I IFN production. Taken together, our results suggest that cGAS and Ifi204 sense dsDNA during a F. novicida infection to elicit STING activation and the type I IFN response.
Materials and Methods
Bacteria, plasmids, primers, and generation of RAW264.7 KO cell lines
Bacterial strains used in this study include wild-type (WT) F. novicida U112, ΔFPI (28), and ΔfopA (29) and WT L. monocytogenes strain 10403S. The cDNA from cGAS (Clone ID: 40130956; Thermo Scientific) and Ifi204 (Clone ID: 4018506; Thermo Scientific) were amplified with primers listed in Supplemental Table I and cloned into MSCV2.2 retroviral expression construct upstream of an internal ribosome entry site-GFP. MSCV2.2 Sting (30) and MSCV2.2 Sting R231A (31) were kindly provided by R. Vance (University of California, Berkeley, Berkeley, CA). pCherry and hCas9 (32) were kindly provided by J. Carette (Stanford University). pCherry was created by replacing DsRed with mCherry RFP (PubMed ID 15558047) in pDsRed C1 (Clontech). cGAS, Ifi204, and Sting KOs were generated in RAW264.7 cells using the CRISPR/Cas9 system (32). Target guide RNA expression constructs were generated from 455-bp gene blocks (IDT) containing target RNA sequences listed in Supplemental Table II cloned into pCR-Blunt cloning vector (Invitrogen). A total of 1 × 106 RAW264.7 macrophages was transfected with 2.5 μg hCas9, 2.5 μg Target guide RNA, and 0.5 μg pCherry in a six-well tissue culture–treated plate with Targefect-RAW (Targeting Systems). Two days posttransfection, macrophages were single-cell sorted into 96-well tissue culture–treated plates and allowed to grow up ∼2 wk. Genomic DNA from macrophage colonies was extracted using QIAamp DNA mini kit (Qiagen). The targeted DNA sequence was amplified with primers (Supplemental Table I) flanking the mutant target site and sequenced (Elim Biopharm). Quantitative RT-PCR primers are listed in Supplemental Table I.
Cell culture, infections, and immunofluorescence
BMMs were isolated, differentiated, and cultured as previously described (33). Aim2−/− and ASC−/− BMM femurs from C57BL/6-Tmem173gt (Stinggt) (30) and C57BL/6-cGAS−/− (34) were kindly provided by R. Vance (University of California, Berkeley) and H. Virgin (Washington University, St. Louis, MO), respectively. Infections with F. novicida were performed as previously described (20). For infections with L. monocytogenes, log-phase bacteria grown in Brain-Heart Infusion broth at 37°C, shaking, were washed twice with PBS and infected similarly to F. novicida.
RAW264.7 macrophages and constructed KOs were cultured in DMEM 10% FBS. RAW264.7 macrophages were seeded at a density of 1 × 105 macrophages per well of a 96-well tissue culture–treated plate or 2.5 × 105 macrophages per well of a 24-well tissue culture–treated plate and allowed to adhere overnight at 37°C, 5% CO2. Infections were conducted as described for BMMs (20). Immunofluorescence microscopy and Western blots were performed as previously described (23, 35). A minimum of 50 bacterial cells was quantified for LAMP-1 colocalization. Abs used include caspase-1 p10 (sc514; Santa Cruz Biotech), β-actin (M-2; Santa Cruz Biotech), anti–F. novicida (Monack laboratory), and anti–LAMP-1 (1D4B; Abcam).
Retroviral transductions and small interfering RNA knockdown
Retroviral constructs were transduced into RAW264.7 macrophages using vesicular stomatitis pseudotyped virus packaged in 293FT cells and sorted by FACS to select for GFP+ macrophages. Gene expression in BMMs was knocked down using small interfering RNA (siRNA) and TransIT-siQUEST transfection reagent according to manufacturer’s recommendations (Mirus). siRNAs used were nontargeting (NT) D-001206-13, cGAS D-0555608-01, Ddx41 m-052130-00, lrrfip1 m-047145-01, Ifi204 m-044641-01, and RIG-I m-0655328-01 (Dharmacon, GE Healthcare).
Cytotoxicity, cytokine measurement, and cell stimulations
Secreted type I IFNs were measured using the ISRE-L929 reporter cells as previously described (29, 36). IL-1β was measured by ELISA (R&D Systems). Cytotoxicity was measured via lactate dehydrogenase release using CytoTox 96 (Non-Radioactive Cytotoxicity Assay; Promega). When specified, RAW264.7 macrophages were stimulated with 100 ng/ml LPS. dsDNA (pCherry) and polyinosinic-polycytidylic acid (Invivogen) were transfected into RAW264.7 macrophages using Targefect-Raw (Targeting Systems). c-di-GMP (Invivogen) was transfected into RAW264.7 macrophages using Lipofectamine 2000 (Life Technologies) as previously described (22).
F. novicida lysates were prepared similarly to previously described protocols (37). In brief, a 3-ml overnight culture was pelleted and resuspended in 1 ml PBS supplemented with 1 mg/ml lysozyme. The cells were lysed using multiple freeze–thaw cycles. Remaining debris was removed by centrifugation, and cleared extracts were adjusted to contain 2 mM MgCl2, 50 mM KCl, and 20 mM Tris, pH 8.0. A total of 100 μl aliquots was treated with 100 U/ml DNase I (Invitrogen) or 100 μg/ml RNase A (Qiagen) for 45 min at 37°C. EDTA was added to 2.5 mM, and samples were heated to 70°C for 10 min. DNase I was heat inactivated before treating the lysate by heating the enzyme to 70°C for 20 min. Extracts were cleared by centrifugation, and 5 μl lysate was complexed with 1 μl Targefect-Raw and transfected into RAW264.7 macrophages.
Mouse infections
Mice between 7 and 9 wk were used for in vivo experiments comparing C57BL/6J mice and C57BL/6J-Tmem173gt/J (Stinggt/gt) (The Jackson Laboratory). D. Schneider (Stanford University), with permission from T. Taniguchi (University of Tokyo, Tokyo, Japan), generously provided 9 C57BL/6 and 10 IRF3−/− mice (38, 39) 8–16 wk old. Mice were infected by s.c. injection with a target dose of 105 CFU F. novicida strain U112. After 72 h, spleens and livers were harvested, weighed, and ground in PBS for CFU determination. For survival experiments, mice were monitored twice daily for 15 d for survival.
Results
F. novicida induces a type I IFN response in macrophages that is dependent on cGAS, Ifi204, and STING
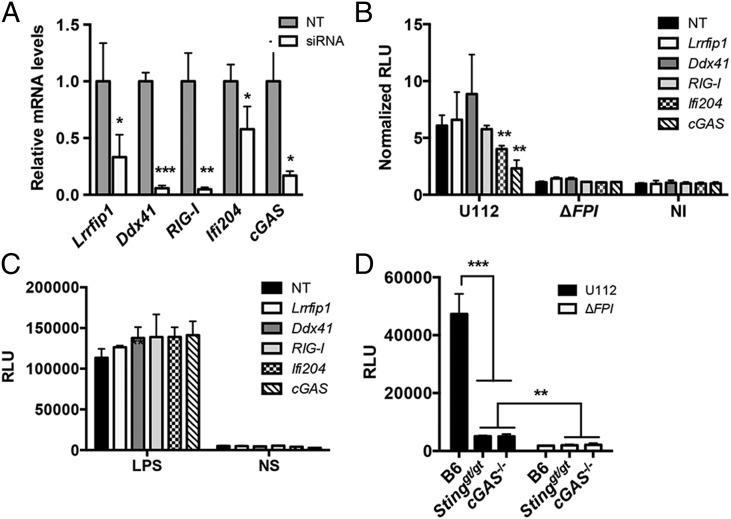
Previous studies showed that F. novicida releases dsDNA into the macrophage cytosol during infection and elicits a type I IFN response that is dependent on STING (22, 23, 29). We hypothesized that a DNA sensor is necessary to mediate the STING-dependent type I IFN response. To test this notion, we knocked down the expression of known DNA sensors that are important for triggering a type I IFN response (10, 13, 14, 17, 40). siRNAs were generated for Lrrfip1, RIG-I, Ddx41, Ifi204, and cGAS and transfected into BMMs. Gene expression levels for each siRNA-targeted gene were reduced compared with the NT control after 36 h (Fig. 1A). Upon F. novicida infection, siRNA knockdown of cGAS and Ifi204 resulted in reduced type I IFN production, whereas siRNA knockdown of Lrrfip1, RIG-I, and Ddx41 did not influence the type I IFN response (Fig. 1B). The type I IFN response was measured using the L929-ISRE reporter cell line (29, 36). A representative standard curve for purified IFN-β and relative luciferase units (RLUs) is shown (Supplemental Fig. 1A). To confirm that these siRNAs were not indirectly affecting other type I IFN pathways, we stimulated the siRNA knockdowns with LPS and observed similar type I IFN responses across all knockdowns (Fig. 1C). In addition, we isolated BMMs from the recently described cGAS−/− (34) mice and examined the type I IFN response to F. novicida infection. As expected, the type I IFN response to F. novicida infection was dampened in Sting-deficient (Stinggt/gt) and cGAS−/− BMMs compared with WT B6 BMMs (Fig. 1D and Supplemental Fig. 1C). Collectively, these results indicate that cGAS and Ifi204 contribute to the type I IFN response to cytosolic F. novicida in BMMs.
FIGURE 1.
cGAS and Ifi204 are required for type I IFN production in response to cytosolic F. novicida in BMMs. siRNA targeting known cytosolic sensor genes or an NT control was transfected into BMMs for 36 h. (A) Quantitative RT-PCR measured mRNA levels for each targeted gene in uninfected BMMs. Gene expression was normalized to GAPDH and the NT control. Type I IFN levels were measured (B) 9 h postinfection with the indicated F. novicida strain at an MOI of 10 or (C) 4 h poststimulation with LPS. Results are presented as RLUs. Data presented as normalized RLUs are type I IFN levels normalized to the uninfected, NT control. (D) Type I IFN levels were measured from unstimulated primary C57BL/6 (WT), Stinggt/gt, and cGAS−/− BMMs infected with F. novicida strains at an MOI of 10 for 12 h. Graphs show the mean ± SD of triplicate wells and are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
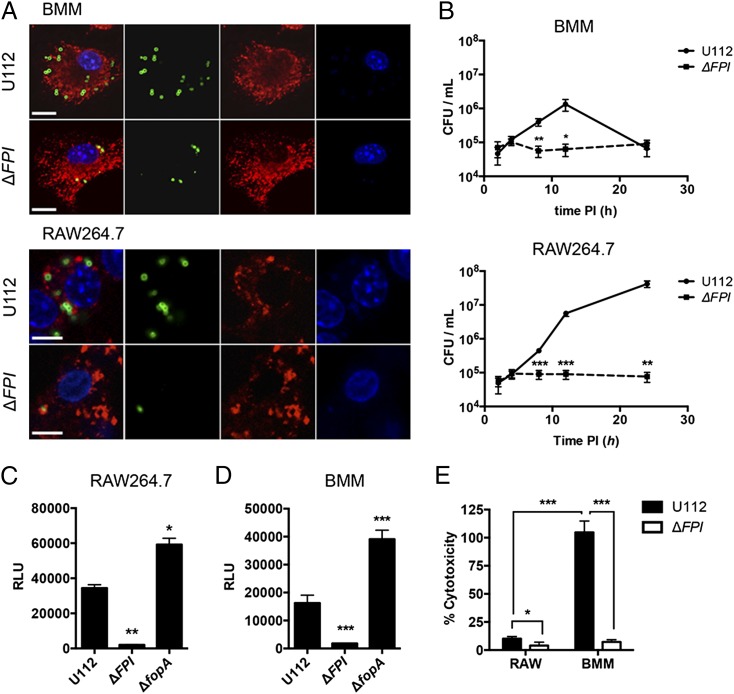
To our knowledge, Ifi204-deficient mice are not available. In addition, the siRNA knockdown efficiency of Ifi204 was only moderately effective, reducing Ifi204 mRNA levels ∼50% (Fig. 1A). Despite this modest siRNA knockdown, type I IFN production was significantly reduced upon infection with F. novicida compared with the NT control (Fig. 1B). To further dissect the roles of Ifi204 and cGAS in the generation of type I IFNs during F. novicida infection of macrophages, we created gene KOs in immortalized RAW264.7 murine macrophages using the newly described CRISPR/Cas9 system (32). However, we first needed to determine whether F. novicida infection in RAW264.7 macrophages induces type I IFN responses that are similar to infections of BMMs. It has been previously published that Francisella escapes the initial phagosome and replicates in the cytosol of BMMs (41). We confirmed that WT F. novicida quickly and efficiently escaped the Francisella-containing vacuole and entered the cytosol of both C57BL/6 BMMs and RAW264.7 macrophages. At 8 h postinfection only 15.2% of WT F. novicida in BMMs and 14% of WT F. novicida in RAW264.7 macrophages colocalized with the lysosomal marker LAMP-1 (Fig. 2A). These levels are similar to previous studies (41). Moreover, F. novicida replicated to high levels in the cytosol of RAW264.7 macrophages, similar to the first 12 h of infected BMMs (Fig. 2B). In contrast, a F. novicida strain lacking the Francisella pathogenicity island (FPI), a predicted type 6 secretion system that is important for escaping the phagosome, colocalized with LAMP-1 at a higher percentage (78% in BMMs and 61% in RAW264.7 macrophages) and did not replicate (Fig. 2A, 2B). The FPI mutant also failed to stimulate type I IFNs in BMMs (Fig. 2D) and dramatically reduced type I IFN stimulation in RAW264.7 macrophages compared with the parental strain (Fig 2C). In addition, a F. novicida strain lacking FopA, a membrane protein important for maintaining cell-wall stability, elicited a higher type I IFN response compared with WT F. novicida in both BMMs and RAW264.7 macrophages (Fig. 2C, 2D) (29). One notable difference was that the type I IFN response pattern in RAW264.7 macrophages was delayed compared with BMMs (Supplemental Fig. 1B). We measured type I IFN levels at 24 h postinfection of RAW264.7 macrophages and 12 h postinfection of BMMs (Fig. 2C, 2D). We, and others, have shown that. F. novicida infection of BMMs results in cell death, which requires AIM2, ASC, and caspase-1 (23, 24). In contrast, F. novicida infection of RAW264.7 macrophages did not result in high levels of cell death (Fig. 2E), which is consistent with the observation that this cell line does not express ASC (42, 43). Moreover, the induction of cell death in BMMs mirrored a reduction in bacterial CFUs in our intracellular replication assays likely due to bacterial exposure to the extracellular antibiotic gentamicin (Fig. 2B). These results indicate that RAW264.7 macrophages are suitable to study mechanisms of type I IFN production during F. novicida infections.
FIGURE 2.
The type I IFN response to F. novicida is similar in BMMs and RAW264.7 macrophages. BMMs and RAW264.7 macrophages were infected with the indicated F. novicida strains at an MOI of 10. (A) Immunofluorescence microscopy of BMMs and RAW264.7 macrophages stained for F. novicida (green), LAMP-1 (red), and DAPI (blue) 8 h postinfection. Scale bars, 10 μm. (B) Intracellular survival was assessed by CFU plating. Type I IFN levels from (C) RAW264.7 macrophages or (D) C57BL/6 BMMs were measured 24 h and 12 h postinfection, respectively. (E) Cytotoxicity was determined by measuring LDH release 24 h postinfection. Graphs show the mean ± SD of triplicate wells and are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
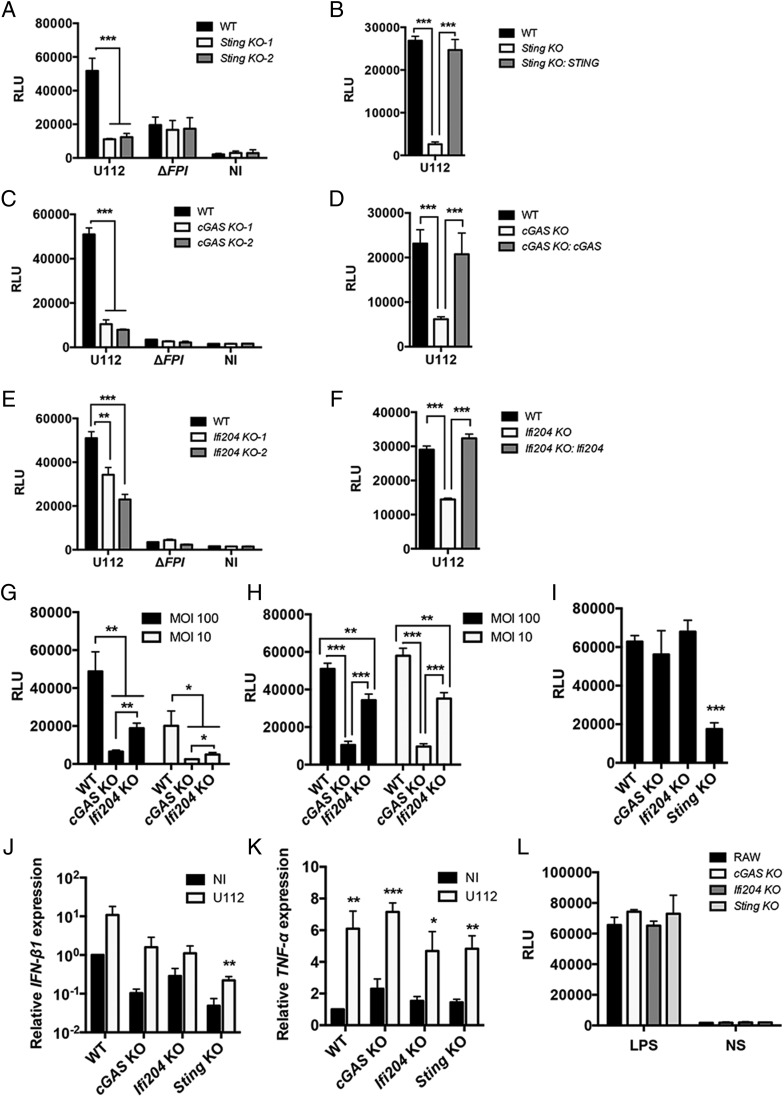
To determine whether cGAS, Ifi204, and STING are important factors in mediating the type I IFN response to F. novicida in RAW264.7 macrophages, we created out-of-frame biallelic indel mutations in each gene using the CRISPR/Cas9 genome editing system, resulting in functional KOs (32). KOs were verified by DNA sequencing (Supplemental Table II), and two clones were chosen for each gene for further study. As a proof of principle, F. novicida infection of Sting KO RAW264.7 macrophages did not produce type I IFNs (Fig. 3A and Supplemental Fig. 1D), similar to infections with Sting-deficient BMMs (Fig. 1D) (22, 23). To verify that the altered type I IFN response was not due to off-target effects of the CRISPR/Cas9 system, we stably expressed STING in Sting KO cells and complemented the type I IFN response to F. novicida infection (Fig. 3B). cGAS KO macrophages and Ifi204 KO macrophages infected with WT F. novicida strain U112 produced significantly lower amounts of type I IFN compared with the RAW264.7 parental genotype (Fig. 3C, 3E, and Supplemental Fig. 1D). Stable expression of cGAS in cGAS KO macrophages complemented the type I IFN response to F. novicida infection, as did stable expression of Ifi204 in Ifi204 KO macrophages (Fig. 3D and 3F, respectively). To further evaluate the contribution of cGAS and Ifi204 to the type I IFN response during a F. novicida infection, we infected the KO cell lines with low and high multiplicity of infection (MOIs) and examined two time points: 12 h postinfection, an early time point, and 24 h postinfection. Similar to the siRNA knockdown experiments (Fig. 1B), cGAS deficiency resulted in a more pronounced reduction in type I IFN response to F. novicida infection compared with Ifi204 KO cells (Fig. 3G, 3H).
FIGURE 3.
The type I IFN response to cytosolic F. novicida requires cGAS, Ifi204, and STING in RAW264.7 macrophages. Type I IFN levels were measured from RAW264.7 (WT) macrophages and the indicated genotypes (A, C, and E) infected with F. novicida strains at an MOI of 10 for 24 h or not infected (NI). Type I IFN levels were measured from deficient macrophages stably expressing Sting (B), cGAS (D), or Ifi204 (F) infected with U112 at an MOI of 10 for 24 h. Type I IFN levels were measured from RAW264.7 (WT) macrophages and indicated genotypes infected with F. novicida for 12 (G) and 24 h (H) or infected with WT L. monocytogenes for 8 h at an MOI of 20 (I). mRNA expression levels of IFN-β1 (J) and TNF-α (K) from uninfected and U112-infected macrophage cell lines at an MOI of 10 for 8 h. mRNA levels were normalized to GAPDH and the WT RAW264.7 macrophages. (L) Type I IFN levels were measured from macrophages stimulated with LPS for 8 h. Graphs show the mean ± SD of triplicate wells and are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001. NS, not stimulated.
To corroborate our type I IFN data as measured by the L929-ISRE assay, we also measured IFN-β1 gene expression in each RAW264.7 macrophage cell line 8 h after F. novicida infection. IFN-β1 gene expression increased, albeit to different levels, upon F. novicida infection in all macrophage cell lines, but was reduced in expression compared with the RAW264.7 parental genotype in both uninfected and infected conditions (Fig. 3J). To confirm that these cell lines are not altered in type I IFN–independent responses, we measured mRNA expression of TNF-α. Each macrophage cell line expressed similar levels of TNF-α before infection, and there were no differences in the levels of TNF-α mRNA between the cell lines postinfection (Fig. 3K). We also stimulated type I IFN production through STING-independent mechanisms (44, 45). TLR4-dependent type I IFN production via LPS stimulation resulted in comparable type I IFN levels for all macrophage cell lines (Fig. 3L). Thus, cGAS, Ifi204, and STING specifically influence type I IFNs in response to F. novicida infection.
We next tested whether cGAS and Ifi204 are important for type I IFN signaling during L. monocytogenes infection, which is another cytosolic bacterial pathogen known to trigger a robust STING-dependent type I IFN response (30, 46). Although L. monocytogenes can directly activate STING through the secretion of c-di-AMP (11), it is unknown whether L. monocytogenes DNA is an important ligand during infection and whether cGAS and Ifi204 contribute to the type I IFN response. We infected WT, cGAS, Ifi204, and Sting KO macrophages with L. monocytogenes and found that only the Sting KO macrophages had reduced type I IFN levels (Fig. 3I). These results suggest that cGAS and Ifi204 are important for triggering the type I IFN response to F. novicida, but not L. monocytogenes, in RAW264.7 macrophages.
cGAS regulates Ifi204 expression
In addition to viruses and bacteria releasing nucleic acids in the cytosol of infected cells, endogenous nucleic acids, such as retroelements, can also trigger a robust type I IFN response (47, 48). Not surprisingly, we found that reducing either cGAS or Ifi204 expression levels resulted in lower endogenous IFN-β1 gene expression and other IFN-stimulated genes (ISGs), including RIG-I (Supplemental Fig. 2A, 2B). Ifi204 is an ISG (49), and reduction of cGAS gene expression also resulted in lower Ifi204 gene expression (Supplemental Fig. 2A, 2B). In contrast, reduction of Ifi204 did not alter cGAS gene expression, and reduced cGAS or Ifi204 levels did not alter Sting or Ddx41 gene expression. We also examined gene expression changes during an infection. Ifi204 gene expression increased in WT and cGAS KO RAW264.7 macrophages, but not Sting KO RAW264.7 macrophages (Supplemental Fig. 2C), whereas cGAS expression levels were unchanged (Supplemental Fig. 2E). Interestingly, Sting expression levels were lower in all macrophages during F. novicida infection (Supplemental Fig. 2D). These data demonstrate that Ifi204 and cGAS are important in regulating type I IFN levels in the absence of infection and indicate that a cGAS KO may, in effect, function as a cGAS and Ifi204 double KO.
Signaling through both cGAS and Ifi204 is required for the full type I IFN response to F. novicida infection
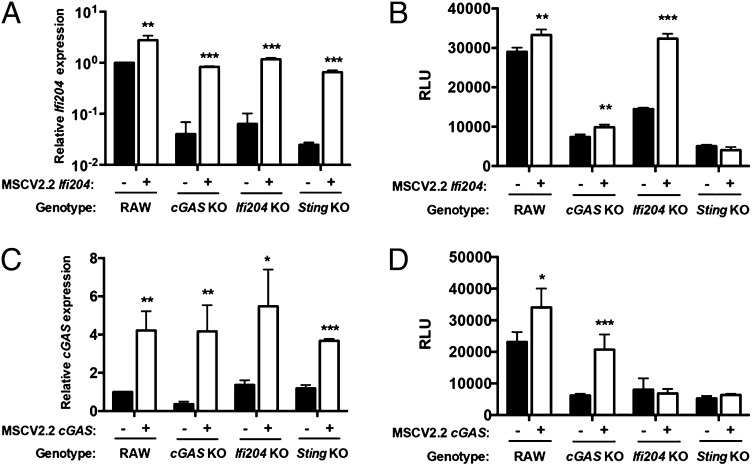
To determine whether the reduced type I IFN response to F. novicida in cGAS KO macrophages was due to lower expression of the Ifi204 gene, we stably expressed Ifi204 in WT, cGAS KO, Ifi204 KO, and Sting KO RAW264.7 macrophages. Ectopic expression of Ifi204 led to increased Ifi204 mRNA expression in all cells (Fig. 4A). As expected, expression of Ifi204 in WT and Ifi204 KO macrophages complemented the type I IFN phenotype in response to F. novicida infection, but only modestly increased the response in cGAS KO (Fig. 4B). From these data, we hypothesized that cGAS was epistatic to Ifi204. To test this, we ectopically expressed cGAS in an Ifi204 KO and measured type I IFN levels after F. novicida infection. Surprisingly, ectopic expression of cGAS in Ifi204 KO macrophages did not rescue the ability of Ifi204 KO macrophages to secrete type I IFN (Fig. 4D). Importantly, ectopic expression of cGAS increased cGAS mRNA levels in all macrophages (Fig. 4C) and type I IFN production in WT and cGAS KO macrophages (Fig. 4D). Ectopic expression of cGAS or Ifi204 in Sting KO macrophages infected with F. novicida did not restore type I IFN production (Fig. 4B, 4D). These data are consistent with previous studies showing cGAS and Ifi204 signal through STING to facilitate the type I IFN response (10, 13). Collectively, our results suggest that Ifi204 and cGAS are both necessary to fully engage STING-dependent type I IFN responses upon F. novicida infection.
FIGURE 4.
Both cGAS and Ifi204 are required for the full type I IFN response to F. novicida infection. mRNA expression levels were measured in uninfected RAW264.7 (WT) cell lines stably expressing Ifi204 (A) or cGAS (C). mRNA expression was normalized to GAPDH and WT RAW264.7 macrophages. Macrophages were infected with WT F. novicida at an MOI of 10 for 24 h and type I IFN levels measured (B and D, respectively). Graphs show the mean ± SD of triplicate wells and are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Type I IFN induction by cytosolic dsDNA and dsDNA from F. novicida lysates is dependent on cGAS and Ifi204
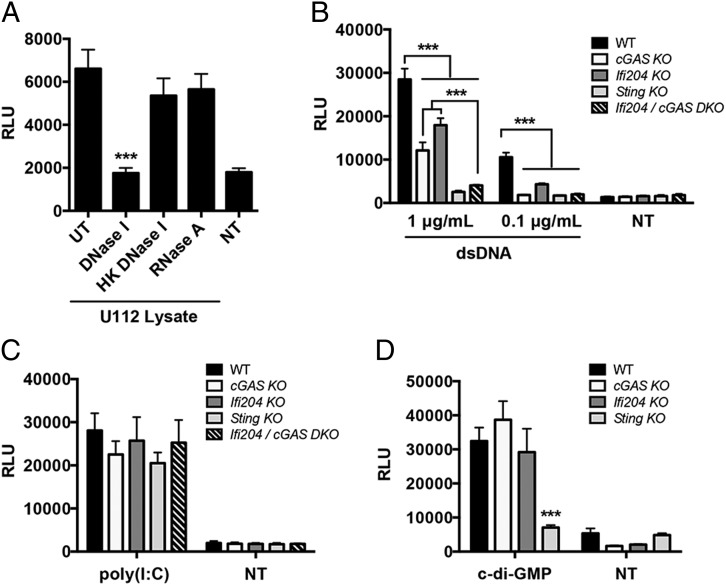
We showed that cGAS and Ifi204 are key factors in the type I IFN response to F. novicida infection (Fig. 3G, 3H). Because cGAS and Ifi204 have both been implicated in cytosolic DNA sensing, we hypothesized that the type I IFN–stimulating PAMP during F. novicida infection is DNA (7). To test this, we transfected RAW264.7 macrophages with untreated F. novicida lysates or with bacterial lysates treated with DNase or RNase. F. novicida lysates stimulated a robust type I IFN response that was abolished when the lysates were treated with DNase I, but not with heat-inactivated DNase I or with RNase A (Fig. 5A). These results suggest that DNA from F. novicida lysates is the primary type I IFN stimulus. We next examined the contribution of cGAS, Ifi204, and STING to type I IFN signaling in response to purified dsDNA. Each macrophage cell line was transfected with two different concentrations of dsDNA. Transfection with 0.1 μg/ml dsDNA resulted in a type I IFN response that was dampened in Ifi204 KO and undetectable in cGAS KO, Sting KO, and cGAS/Ifi204 DKO macrophages (Fig. 5B). Transfecting a higher concentration of dsDNA (1 μg/ml) produced an intermediate type I IFN response for both cGAS KO and Ifi204 KO compared with Sting KO macrophages, which produced a nearly undetectable response (Fig. 5B). Surprisingly, the cGAS/Ifi204 DKO macrophages were equally defective for type I IFN signaling as the Sting KO macrophages (Fig. 5B). These results suggest that cGAS and Ifi204 independently contribute to the cytosolic dsDNA type I IFN response.
FIGURE 5.
cGAS and Ifi204 cooperate to sense cytosolic dsDNA. (A) Type I IFN levels were measured from WT RAW264.7 macrophages transfected for 24 h with U112 lysates either untreated (UT) or treated with DNase I, heat-killed (HK) DNase I or RNase A. Type I IFN levels were measured from RAW264.7 macrophages transfected for 24 h with (B) endo-free plasmid DNA (pCherry), (C) 1 μg/ml polyinosinic-polycytidylic acid [poly(I:C)], or (D) 10 μg/ml c-di-GMP NT, not transfected. Graphs show the mean ± SD of triplicate wells and are representative of three independent experiments. ***p < 0.001.
To determine whether cGAS and Ifi204 are solely involved in the STING-dependent type I IFN signaling pathway, we stimulated type I IFN production through STING-independent mechanisms in the cytosol (44, 45). MDA5-mediated type I IFN production in response to cytosolic dsRNA stimulation also resulted in comparable type I IFN levels (Fig. 5C). In addition, we examined whether cGAS or Ifi204 played a role in responding to bacterial cyclic-dinucleotides. Transfection of c-di-GMP led to similar type I IFN responses in WT, cGAS KO, and Ifi204 KO macrophages (Fig. 5D). In contrast, Sting KO macrophages were defective for signaling in response to c-di-GMP (Fig. 5D), which is consistent with previous studies (31). Our results demonstrate that cGAS and Ifi204 cooperate to signal through the STING-dependent type I IFN pathway in response to cytosolic dsDNA. In addition, these data support dsDNA as the primary F. novicida PAMP that triggers the type I IFN response.
cGAS increases inflammasome activity in BMMs
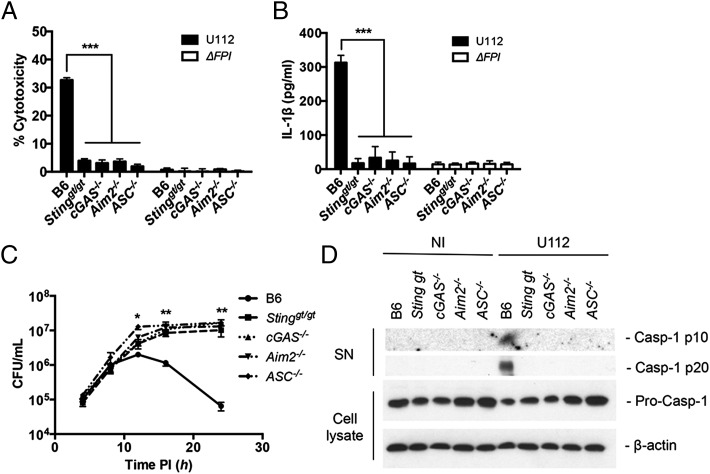
F. novicida infection triggers the activation of the AIM2 inflammasome, a host response pathway that is critical for the defense against F. novicida, resulting in the secretion of proinflammatory cytokines (IL-1β and IL-18) and caspase-1–dependent pyroptotic cell death (23, 24). Previous work demonstrated AIM2 protein levels are regulated by type I IFNs, and in Sting-deficient BMMs, the AIM2 inflammasome is not activated during F. novicida infection (23, 24). Thus, we hypothesized that the AIM2 inflammasome response to F. novicida infection would also be dampened in the absence of either of the DNA sensors that we showed in this study stimulate STING (cGAS or Ifi204). Using the recently described cGAS−/− mice (34), we examined inflammasome activation by measuring cell death, IL-1β secretion, and caspase-1 processing in response to F. novicida infection. As predicted, when WT, Stinggt/gt, cGAS−/−, Aim2−/−, and ASC−/− BMMs were infected with F. novicida, only WT cells showed appreciable levels of cell death (Fig. 6A), secreted IL-1β (Fig. 6B), and processed caspase-1 (Fig. 6D). Moreover, F. novicida replicated to higher levels in Stinggt/gt, cGAS−/−, Aim2−/−, and ASC−/− BMMs compared with WT BMMs, consistent with the increased cell death of WT BMMs limiting the intracellular niche (Fig. 6C). Collectively, these results demonstrate that cGAS is important for activating the AIM2 inflammasome during F. novicida infection.
FIGURE 6.
cGAS increases inflammasome activity in BMMs. (A) Cytotoxicity, (B) IL-1β secretion, and (C) intracellular survival were measured from unstimulated primary C57BL/6 (WT), Stinggt/gt, cGAS−/−, Aim2−/−, and ASC−/− BMMs infected with F. novicida strains at an MOI of 10 for 12 h unless otherwise indicated. (D) Release of processed caspase-1 (casp-1 p10 and p20) into the supernatants (SN) was measured by Western blot from cells either not infected (NI) or infected with WT F. novicida at an MOI of 100 for 12 h. Corresponding cell lysates were probed for procaspase-1 and β-actin. Graphs show the mean ± SD of triplicate wells and are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
STING and IRF3 deficiency enhances host survival during F. novicida infection
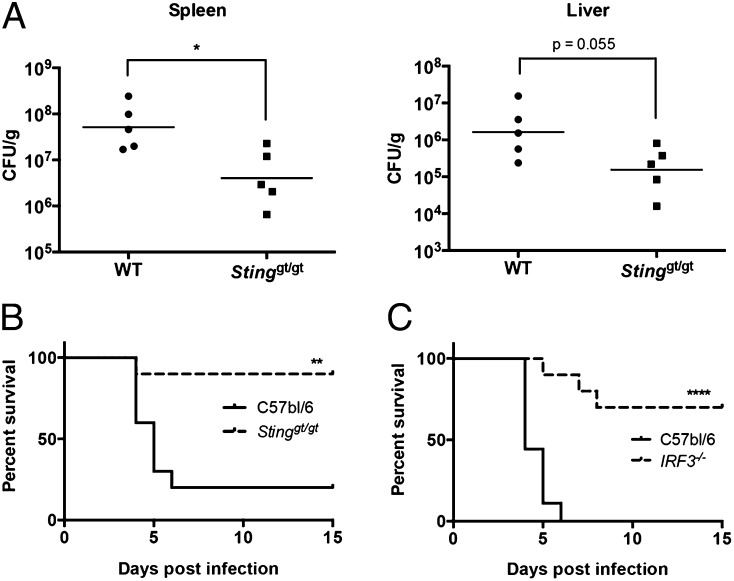
Although type I IFN production during viral infections can lead to increased host survival, there are many examples in which type I IFN production in the context of a bacterial infection is associated with decreased host survival (50–52). Indeed, we have previously shown that mice deficient in the type I IFN signaling receptor, IFNAR, are more resistant to F. novicida infection (20, 52). We have shown in this study that both cGAS and Ifi204, which signal through STING, are required to produce the maximum type I IFN levels in response to F. novicida infection. Thus, to test the role of the cGAS/Ifi204/STING axis in mice, we infected Stinggt/gt mice with F. novicida. Mice were infected s.c. with 105 F. novicida CFU and evaluated for bacterial burdens in the spleen and liver 3 d postinfection. The bacterial loads in the spleen were significantly higher in the WT mice compared with the Stinggt/gt mice and similar trends occurred in the liver (Fig. 7A). Mice were also evaluated for their relative susceptibility to F. novicida as assessed in a survival experiment (Fig. 7B). The median time to death of WT mice was 5 d, and 80% of this group did not survive the F. novicida challenge. In contrast, the median time to death of Stinggt/gt mice was >15 d, and 90% of this group survived the infection. To corroborate our findings that type I IFN–dependent STING signaling was detrimental to the host during F. novicida infection, we also infected IRF3-deficient mice with F. novicida. IRF3 is the transcription factor activated by STING to induce the transcription of IFN-β and other ISGs (9, 53). Similar to Stinggt/gt mice, IRF3−/− mice infected s.c. with 2 × 105 F. novicida CFU were significantly more resistant to F. novicida infection compared with WT mice (Fig. 7C). The median time to death for WT mice and IRF3−/− mice was 4 and >15 d, respectively. These results demonstrate that STING- and IRF3-dependent signaling is detrimental to the host during a F. novicida infection.
FIGURE 7.
STING and IRF3 are detrimental to host survival during F. novicida infection. WT and Stinggt/gt mice were infected s.c. with 105 CFU U112. (A) The spleen and liver from five infected WT and Stinggt/gt mice were harvested and plated for CFU/g 3 d postinfection; geometric mean is shown. (B) Ten WT and 10 Stinggt/gt mice were monitored twice daily over 15 d for survival. (C) Nine WT and 10 IRF3−/− mice were infected s.c. with 2 × 105 CFU U112 and were monitored twice daily over 15 d for survival. *p < 0.05, **p < 0.01, ****p < 0.0001.
Discussion
Recognition of microbial pathogens is essential to initiate an effective immune response. Host cells have developed numerous strategies to identify infection and tissue injury. One mode of detection is surveying the cytosol for the presence of nucleic acids. Although the importance of recognizing and responding to nucleic acids in the cytosol is well appreciated for viral infections, the role of nucleic acids triggering the type I IFN response during bacterial infections is not well understood. To complicate matters, recent studies have identified numerous cytosolic receptors important for type I IFN signaling in response to dsDNA. We were interested in identifying the host sensors important for type I IFN signaling in response to bacterial infections. In this study, we showed that during infection with the cytosolic bacterium, F. novicida, the primary type I IFN response is likely dependent on the presence of F. novicida DNA in the cytosol. Moreover, we demonstrated that two proposed host DNA sensors, cGAS and Ifi204, are required to generate type I IFNs during F. novicida infection and in response to cytosolic dsDNA in murine macrophages.
We observed that during a F. novicida infection, both cGAS and Ifi204 were necessary to mediate a full type I IFN response (Fig. 4). In addition, we show that both cGAS and Ifi204 need to be eliminated (e.g., cGAS/Ifi204 DKO in RAW264.7 cells) to prevent a type I IFN response to high concentrations of transfected DNA in RAW264.7 macrophages. This result was surprising given that cGAS alone was previously demonstrated to bind cytosolic DNA and generate the STING-activating second messenger, cGAMP, resulting in type I IFN production (8, 10, 54, 55). This discrepancy in findings may be attributed to fundamental variances in cell types, including differences in transfection efficiency and/or alterations in the Ifi204 expression signature. In particular, we observe that transfecting RAW264.7 macrophages using standard transfection reagents (i.e., Lipofectamine 2000 and Targefect-Raw) results in a considerably higher percentage of cells being transfected compared with BMMs and much lower cytotoxicity (data not shown). These factors may permit detection of intermediate to low type I IFN responses that are not seen in cGAS−/− BMMs (54). However, further studies in a variety of cell types are needed to assess the role and/or expression of Ifi204 in the absence of cGAS. It also remains to be determined how Ifi204, a member of AIM2-like receptors (ALRs) (49), fits into the cGAS/STING/type I IFN pathway. To date, 13 ALR genes have been identified within the mouse genome (49). They are all encoded within a single continuous locus and contain a Pyrin and/or HIN domain. One possible model for the requirement of Ifi204 in the cGAS/STING/type I IFN pathway is that Ifi204, either due to higher affinity for dsDNA or to appropriate subcellular localization, may initially recognize F. novicida DNA and transport the DNA ligand to cGAS. This would result in the production of cGAMP and subsequent activation of STING to trigger type I IFN production. Evidence for this model is supported by previous studies, which demonstrate that the human ortholog of Ifi204, IFI16, interacts with STING upon DNA stimulation (13). In addition, Ifi204 and STING were found to colocalize in the cytosol when transfected in HeLa cells (49). Future studies are required to resolve the mechanism behind the necessity of Ifi204 and to determine whether other ALR proteins are important in facilitating the cGAS-dependent signaling response to intracellular bacteria and potentially other stimuli.
Access of F. novicida DNA to the host cytosol is a requirement to activate two independent host responses. First, F. novicida DNA is likely recognized by cGAS and Ifi204 to trigger a STING-dependent type I IFN response. Through autocrine and paracrine type I IFN signaling, AIM2 protein levels increase and subsequently associate with F. novicida DNA and ASC to activate caspase-1–mediated cell death and secretion of proinflammatory cytokines (23). Although the mechanism of DNA release by F. novicida into the host cytosol is not fully known, F. novicida mutants that are prone to increased bacterial lysis trigger higher type I IFNs and inflammasome responses compared with the parental strain (29). This observation suggests that one mechanism of facilitating access of the DNA sensors to F. novicida DNA is a low level of bacterial lysis in the cytosol. One notable difference between the two cell types we used in this study is that the FPI mutant elicits a type I IFN response that is higher than the levels produced by uninfected RAW264.7 macrophages (Supplemental Fig. 1D). In contrast, the levels of type I IFN produced by BMMs infected with the FPI mutant are not higher than uninfected BMMs (Supplemental Fig. 1D). These results may be attributed to the higher proportion of FPI mutant bacteria found in the cytosol of RAW264.7 macrophages compared with BMMs. These studies underscore the importance of DNA recognition during a bacterial infection. This is particularly important for a bacterial pathogen like Francisella that is a stealth invader and does not elicit a TLR-mediated type I IFN response (20).
Many intracellular bacterial pathogens elicit a type I IFN host response, and several of these have been demonstrated to require STING, including L. monocytogenes, M. tuberculosis, and C. trachomatis (5, 11, 56). In addition to STING, the type I IFN response in immortalized BMMs to M. tuberculosis infection was also demonstrated to require Ifi204 (56). In this article, we demonstrated that the STING-dependent type I IFN response elicited by L. monocytogenes did not require the DNA sensors, cGAS or Ifi204 (Fig. 3I). These results are consistent with previous findings demonstrating that L. monocytogenes secretes cyclic-di-AMP to directly stimulated STING in murine cells (11). Notably, a recent study showed L. monocytogenes infection in human macrophages induced the type I IFN response that is dependent on cGAS, IFI16 (Ifi204 homolog), and STING (57). Further studies are needed to elucidate the roles of cGAS and Ifi204/IFI16 in recognizing other intracellular bacterial pathogens and potential differences between human and mouse type I IFN signaling pathways.
The role of type I IFNs in controlling bacterial infections is complex. Wild-type mice infected with L. monocytogenes, F. novicida, and M. tuberculosis support increased bacterial burdens compared with mice deficient for the type I IFN receptor (IFNAR1−/−) (50–52). Although these studies were initially surprising, it is now known that type I IFN production leads to the transcription of hundreds of ISGs, which modulate a variety of factors in both innate and adaptive immunity (3). In the case of a F. novicida infection, IFNAR1−/− mice are more resistant to infection largely due to an increased expansion of IL-17A+ γδ T cells and increased splenic neutrophils (52). The role of cGAS and Ifi204 during a F. novicida infection in vivo is unknown. Because cGAS and Ifi204 both require STING for type I IFN signaling, we sought to evaluate Stinggt/gt mice for their susceptibility to F. novicida infection. Previous studies using a different Francisella subspecies, F. tularensis subspecies holarctica live vaccine strain, and different infection route (i.p.) did not identify a difference in splenic bacterial loads between STING (MPYS)-deficient mice and WT mice 48 h postinfection (22). This finding is similar to our previous findings using IFNAR−/− mice, in which there is no difference in bacterial burdens 1 and 2 d postinfection (52). Importantly, we show in this article that Stinggt/gt mice carried lower bacterial burdens in the liver and spleen 3 d postinfection and were significantly more resistant to F. novicida infection compared with WT mice (Fig. 7A). Furthermore, we show that mice deficient for the downstream transcription factor, IRF3, were also significantly more susceptible to F. novicida infection. We postulate that both Stinggt/gt mice and Irf3−/− mice are more resistant to infection due to similar mechanisms described for IFNAR1−/− mice (52). Despite lower bacterial burdens found in Stinggt/gt mice and IFNAR1−/− mice, the type I IFN response is important in activating specific host responses important for bacterial control, including the AIM2 inflammasome during F. novicida infections (23).
Our results begin to illuminate the mechanisms involved in type I IFN production during intracellular bacterial infections. Our data strongly support the hypothesis that F. novicida DNA is sensed by cGAS and Ifi204 to trigger the STING-dependent type I IFN response. Future experiments investigating the cross talk between TLR-mediated type I IFN production and cytosolic DNA-mediated type I IFN production may discover unique signaling signatures depending on the host receptor activated. Understanding how type I IFN production is regulated and what factors are involved may aid in our therapeutic efforts to prevent and treat autoinflammatory and infectious diseases.
Supplementary Material
Acknowledgments
We thank Jan Carette and Caleb Marceau for technical support with CRISPR/Cas9 mutagenesis, Bianca Gomez for technical support on the flow cytometer, Russell Vance for Stinggt/gt femurs, Herbert Virgin for cGAS−/− femurs, Damian Trujillo and David Schneider for C57BL/6 mice and IRF3−/− mice, and all members of the Monack laboratory and Jenny Lumb for valuable comments.
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases Grants AI089722 and AI095396 (to D.M.M.) and Grant F32 AI108089 (to K.M.S.).
The online version of this article contains supplemental material.
- AIM2
- absent in melanoma 2
- ALR
- AIM2-like receptor
- BMM
- bone marrow–derived macrophage
- cGAMP
- cyclic GMP-AMP
- cGAS
- cyclic GMP-AMP synthase
- FPI
- Francisella pathogenicity island
- IRF
- IFN regulatory factor
- ISG
- IFN-stimulated gene
- KO
- knockout
- MOI
- multiplicity of infection
- NT
- nontargeting
- PAMP
- pathogen-associated molecular pattern
- RLU
- relative luciferase unit
- siRNA
- small interfering RNA
- STING
- stimulator of IFN genes
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Vance R. E., Isberg R. R., Portnoy D. A. 2009. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6: 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy M. P., Owczarek C. M., Jermiin L. S., Ejdebäck M., Hertzog P. J. 2004. Characterization of the type I interferon locus and identification of novel genes. Genomics 84: 331–345. [DOI] [PubMed] [Google Scholar]
- 3.Stetson D. B., Medzhitov R. 2006. Type I interferons in host defense. Immunity 25: 373–381. [DOI] [PubMed] [Google Scholar]
- 4.Perry A. K., Chen G., Zheng D., Tang H., Cheng G. 2005. The host type I interferon response to viral and bacterial infections. Cell Res. 15: 407–422. [DOI] [PubMed] [Google Scholar]
- 5.Barker J. R., Koestler B. J., Carpenter V. K., Burdette D. L., Waters C. M., Vance R. E., Valdivia R. H. 2013. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. MBio 4: e00018–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchieri G. 2010. Type I interferon: friend or foe? J. Exp. Med. 207: 2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J., Chen Z. J. 2014. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32: 461–488. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Shi H., Wu J., Zhang X., Sun L., Chen C., Chen Z. J. 2013. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51: 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka Y., Chen Z. J. 2012. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 5: ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L., Wu J., Du F., Chen X., Chen Z. J. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodward J. J., Iavarone A. T., Portnoy D. A. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328: 1703–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaoka A., Wang Z., Choi M. K., Yanai H., Negishi H., Ban T., Lu Y., Miyagishi M., Kodama T., Honda K., et al. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448: 501–505. [DOI] [PubMed] [Google Scholar]
- 13.Unterholzner L., Keating S. E., Baran M., Horan K. A., Jensen S. B., Sharma S., Sirois C. M., Jin T., Latz E., Xiao T. S., et al. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang P., An H., Liu X., Wen M., Zheng Y., Rui Y., Cao X. 2010. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat. Immunol. 11: 487–494. [DOI] [PubMed] [Google Scholar]
- 15.Kondo T., Kobayashi J., Saitoh T., Maruyama K., Ishii K. J., Barber G. N., Komatsu K., Akira S., Kawai T. 2013. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc. Natl. Acad. Sci. USA 110: 2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Brann T. W., Zhou M., Yang J., Oguariri R. M., Lidie K. B., Imamichi H., Huang D. W., Lempicki R. A., Baseler M. W., et al. 2011. Cutting edge: Ku70 is a novel cytosolic DNA sensor that induces type III rather than type I IFN. J. Immunol. 186: 4541–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu Y. H., Macmillan J. B., Chen Z. J. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138: 576–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones C. L., Napier B. A., Sampson T. R., Llewellyn A. C., Schroeder M. R., Weiss D. S. 2012. Subversion of host recognition and defense systems by Francisella spp. Microbiol. Mol. Biol. Rev. 76: 383–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong A., Wehrly T. D., Nair V., Fischer E. R., Barker J. R., Klose K. E., Celli J. 2008. The early phagosomal stage of Francisella tularensis determines optimal phagosomal escape and Francisella pathogenicity island protein expression. Infect. Immun. 76: 5488–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry T., Brotcke A., Weiss D. S., Thompson L. J., Monack D. M. 2007. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204: 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariathasan S., Weiss D. S., Dixit V. M., Monack D. M. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin L., Hill K. K., Filak H., Mogan J., Knowles H., Zhang B., Perraud A. L., Cambier J. C., Lenz L. L. 2011. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J. Immunol. 187: 2595–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones J. W., Kayagaki N., Broz P., Henry T., Newton K., O’Rourke K., Chan S., Dong J., Qu Y., Roose-Girma M., et al. 2010. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 107: 9771–9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T., Yu J. W., Datta P., Wu J., Alnemri E. S. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D. R., Latz E., Fitzgerald K. A. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bürckstümmer T., Baumann C., Blüml S., Dixit E., Dürnberger G., Jahn H., Planyavsky M., Bilban M., Colinge J., Bennett K. L., Superti-Furga G. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10: 266–272. [DOI] [PubMed] [Google Scholar]
- 27.Landolfo S., Gariglio M., Gribaudo G., Lembo D. 1998. The Ifi 200 genes: an emerging family of IFN-inducible genes. Biochimie 80: 721–728. [DOI] [PubMed] [Google Scholar]
- 28.Weiss D. S., Brotcke A., Henry T., Margolis J. J., Chan K., Monack D. M. 2007. In vivo negative selection screen identifies genes required for Francisella virulence. Proc. Natl. Acad. Sci. USA 104: 6037–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng K., Broz P., Jones J., Joubert L. M., Monack D. 2011. Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell. Microbiol. 13: 1586–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauer J. D., Sotelo-Troha K., von Moltke J., Monroe K. M., Rae C. S., Brubaker S. W., Hyodo M., Hayakawa Y., Woodward J. J., Portnoy D. A., Vance R. E. 2011. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 79: 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdette D. L., Monroe K. M., Sotelo-Troha K., Iwig J. S., Eckert B., Hyodo M., Hayakawa Y., Vance R. E. 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mali P., Yang L., Esvelt K. M., Aach J., Guell M., DiCarlo J. E., Norville J. E., Church G. M. 2013. RNA-guided human genome engineering via Cas9. Science 339: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broz P., Newton K., Lamkanfi M., Mariathasan S., Dixit V. M., Monack D. M. 2010. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207: 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoggins J. W., MacDuff D. A., Imanaka N., Gainey M. D., Shrestha B., Eitson J. L., Mar K. B., Richardson R. B., Ratushny A. V., Litvak V., et al. 2014. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505: 691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broz P., von Moltke J., Jones J. W., Vance R. E., Monack D. M. 2010. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8: 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Z., Georgel P., Du X., Shamel L., Sovath S., Mudd S., Huber M., Kalis C., Keck S., Galanos C., et al. 2005. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6: 565–570. [DOI] [PubMed] [Google Scholar]
- 37.Stetson D. B., Medzhitov R. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24: 93–103. [DOI] [PubMed] [Google Scholar]
- 38.Sato M., Suemori H., Hata N., Asagiri M., Ogasawara K., Nakao K., Nakaya T., Katsuki M., Noguchi S., Tanaka N., Taniguchi T. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13: 539–548. [DOI] [PubMed] [Google Scholar]
- 39.Daffis S., Suthar M. S., Szretter K. J., Gale M., Jr., Diamond M. S. 2009. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog. 5: e1000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parvatiyar K., Zhang Z., Teles R. M., Ouyang S., Jiang Y., Iyer S. S., Zaver S. A., Schenk M., Zeng S., Zhong W., et al. 2012. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 13: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmerk C. L., Duplantis B. N., Howard P. L., Nano F. E. 2009. A Francisella novicida pdpA mutant exhibits limited intracellular replication and remains associated with the lysosomal marker LAMP-1. Microbiology 155: 1498–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bryan N. B., Dorfleutner A., Kramer S. J., Yun C., Rojanasakul Y., Stehlik C. 2010. Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. J. Inflamm. (Lond.) 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelegrin P., Barroso-Gutierrez C., Surprenant A. 2008. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol. 180: 7147–7157. [DOI] [PubMed] [Google Scholar]
- 44.Uematsu S., Akira S. 2007. Toll-like receptors and Type I interferons. J. Biol. Chem. 282: 15319–15323. [DOI] [PubMed] [Google Scholar]
- 45.Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y. M., Gale M., Jr., Akira S., et al. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175: 2851–2858. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa H., Ma Z., Barber G. N. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461: 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stetson D. B., Ko J. S., Heidmann T., Medzhitov R. 2008. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkman H. E., Stetson D. B. 2014. The enemy within: endogenous retroelements and autoimmune disease. Nat. Immunol. 15: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunette R. L., Young J. M., Whitley D. G., Brodsky I. E., Malik H. S., Stetson D. B. 2012. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J. Exp. Med. 209: 1969–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanley S. A., Johndrow J. E., Manzanillo P., Cox J. S. 2007. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178: 3143–3152. [DOI] [PubMed] [Google Scholar]
- 51.Auerbuch V., Brockstedt D. G., Meyer-Morse N., O’Riordan M., Portnoy D. A. 2004. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200: 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henry T., Kirimanjeswara G. S., Ruby T., Jones J. W., Peng K., Perret M., Ho L., Sauer J. D., Iwakura Y., Metzger D. W., Monack D. M. 2010. Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections. J. Immunol. 184: 3755–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiscott J. 2007. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 282: 15325–15329. [DOI] [PubMed] [Google Scholar]
- 54.Li X. D., Wu J., Gao D., Wang H., Sun L., Chen Z. J. 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341: 1390–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ablasser A., Hemmerling I., Schmid-Burgk J. L., Behrendt R., Roers A., Hornung V. 2014. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J. Immunol. 192: 5993–5997. [DOI] [PubMed] [Google Scholar]
- 56.Manzanillo P. S., Shiloh M. U., Portnoy D. A., Cox J. S. 2012. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11: 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen K., Prabakaran T., Laustsen A., Jørgensen S. E., Rahbæk S. H., Jensen S. B., Nielsen R., Leber J. H., Decker T., Horan K. A., et al. 2014. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 33: 1654–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.