Abstract
The gut-brain axis (GBA) consists of bidirectional communication between the central and the enteric nervous system, linking emotional and cognitive centers of the brain with peripheral intestinal functions. Recent advances in research have described the importance of gut microbiota in influencing these interactions. This interaction between microbiota and GBA appears to be bidirectional, namely through signaling from gut-microbiota to brain and from brain to gut-microbiota by means of neural, endocrine, immune, and humoral links. In this review we summarize the available evidence supporting the existence of these interactions, as well as the possible pathophysiological mechanisms involved. Most of the data have been acquired using technical strategies consisting in germ-free animal models, probiotics, antibiotics, and infection studies. In clinical practice, evidence of microbiota-GBA interactions comes from the association of dysbiosis with central nervous disorders (i.e. autism, anxiety-depressive behaviors) and functional gastrointestinal disorders. In particular, irritable bowel syndrome can be considered an example of the disruption of these complex relationships, and a better understanding of these alterations might provide new targeted therapies.
Keywords: Gut-brain axis, enteric microbiota, central nervous system, enteric nervous system, irritable bowel syndrome
Introduction
Insights into the gut-brain crosstalk have revealed a complex communication system that not only ensures the proper maintenance of gastrointestinal homeostasis, but is likely to have multiple effects on affect, motivation, and higher cognitive functions. The complexity of these interactions is enclosed in the denomination of “gut-brain axis” (GBA) [1]. Its role is to monitor and integrate gut functions as well as to link emotional and cognitive centers of the brain with peripheral intestinal functions and mechanisms such as immune activation, intestinal permeability, enteric reflex, and entero-endocrine signaling. The mechanisms underlying GBA communications involve neuro-immuno-endocrine mediators.
This bidirectional communication network includes the central nervous system (CNS), both brain and spinal cord, the autonomic nervous system (ANS), the enteric nervous system (ENS) and the hypothalamic pituitary adrenal (HPA) axis (Fig. 1). The autonomic system, with the sympathetic and parasympathetic limbs, drives both afferent signals, arising from the lumen and transmitted though enteric, spinal and vagal pathways to CNS, and efferent signals from CNS to the intestinal wall. The HPA axis is considered the core stress efferent axis that coordinates the adaptive responses of the organism to stressors of any kind [2]. It is a part of the limbic system, a crucial zone of the brain predominantly involved in memory and emotional responses. Environmental stress, as well as elevated systemic pro-inflammatory cytokines, activate this system that, through secretion of the corticotropin-releasing factor (CRF) from the hypothalamus, stimulates adrenocorticotropic hormone (ACTH) secretion from pituitary gland that, in turn, leads to cortisol release from the adrenal glands. Cortisol is a major stress hormone that affects many human organs, including the brain. Thus, both neural and hormonal lines of communication combine to allow brain to influence the activities of intestinal functional effector cells, such as immune cells, epithelial cells, enteric neurons, smooth muscle cells, interstitial cells of Cajal and enterochromaffin cells. These same cells, on the other hand, are under the influence of the gut microbiota [3] whose contributing role in brain-gut reciprocal communications has recently been assessed. The concept of a microbiome GBA is now emerging.
Figure 1.
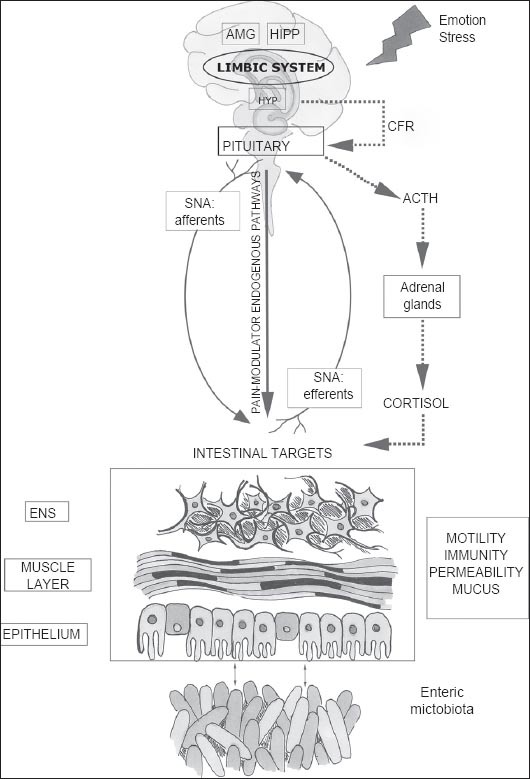
Microbiome gut-brain axis structure
The central nervous system and in particular hypothalamic pituitary adrenal (HPA) axis (in dashed line) can be activated in response to environmental factors, such as emotion or stress. HPA is finalized to cortisol release and is driven by a complex interaction between amygdala (AMG), hippocampus (HIPP), and hypothalamus (HYP), constituting the limbic system. HYP secretion of the corticotropin-releasing factor (CRF) stimulates adrenocorticotropic hormone (ACTH) secretion from pituitary gland that, in turn, leads to cortisol release from the adrenal glands. In parallel, central nervous system communicate along both afferent and efferent autonomic pathways (SNA) with different intestinal targets such as enteric nervous system (ENS), muscle layers and gut mucosa, modulating motility, immunity, permeability and secretion of mucus. The enteric microbiota has a bidirectional communication with these intestinal targets, modulating gastrointestinal functions and being itself modulated by brain-gut interactions
The enteric microbiota is distributed in the human gastrointestinal tract and, although each person’s microbiota profile is distinct, relative abundance and distribution along the intestine of these bacterial phylotypes is similar among healthy individuals. The two more prominent phyla are Firmicutes and Bacteroides accounting for at least ¾ of the microbiome [4]. This microbial community has important metabolic and physiological functions for the host and contributes to its homeostasis during life.
Role of microbiota in GBA
Both clinical and experimental evidence suggest that enteric microbiota has an important impact on GBA, interacting not only locally with intestinal cells and ENS, but also directly with CNS through neuroendocrine and metabolic pathways.
In humans, the most compelling evidence of a gastrointestinal microbe-brain interaction arose more than 20 years ago from the observation of the often dramatic improvement in patients with hepatic encephalopathy, after the administration of oral antibiotics [5]. In the meantime, emerging data support the role of microbiota in influencing anxiety and depressive-like behaviors [6,7] and, more recently, of dysbiosis in autism. In fact, autistic patients present specific microbiota alterations according to the severity of the disease [8,9].
Dysbiosis occurs also in functional gastrointestinal disorders (FGID) that are highly associated with mood disorders and are linked to a disruption of GBA [10-12]. Data have been provided that both brain-gut and gut-brain dysfunctions occur, the former being dominant particularly in irritable bowel syndrome (IBS) [13]. The disruption occurring in the GBA determines changes in intestinal motility and secretion, causes visceral hypersensitivity and leads to cellular alterations of the entero-endocrine and immune system. Microbiota may interplay with multiple of these different pathophysiological IBS targets [14] and its role is supported by varying lines of evidence: the presence in IBS patients of alterations in microbiota composition with defects both in its stability and diversity, the development of post-infectious IBS, the possible coexistence with small intestinal bacterial overgrowth and the efficacious treatment of certain probiotics and non-systemic antibiotics [15-17]. Furthermore, the visceral hypersensitivity phenotype, characteristic of IBS, can be transferred via the microbiota of IBS patients to previously germ-free rats [18]. The concomitant dysregulation of both GBA and gut microbiota in the pathogenesis of IBS has lead to the proposal of considering this FGID as a disorder of the microbioma-GBA [19].
From gut microbiota to brain
In the last years there has been a proliferation of experimental works, conducted mainly on animals, aimed to explore the contribution of the microbiota in modulating GBA. Different technical strategies have been used, consisting in the use of germ-free (GF) animals, probiotics, antibiotics and infection studies [20].
Studies on GF animals have shown that bacterial colonization of the gut is central to development and maturation of both ENS and CNS [21,22]. The absence of microbial colonization is associated to an altered expression and turnover of neurotransmitters in both nervous systems [21,23,24] and also to alterations of gut sensory-motor functions, consisting in delayed gastric emptying and intestinal transit [25,26] reduced migrating motor complex cyclic recurrence and distal propagation [27,28] and enlarged cecal size [29]. Neuromuscular abnormalities resulted associated to a reduction in gene expression of enzymes involved in the synthesis and transport of neurotransmitters, as well as in that of muscular contractile proteins [30]. All these anomalies are restored, after animal colonization in a bacterial species-specific manner.
Studies conduced on GF animals have also demonstrated that microbiota influences stress reactivity and anxiety-like behavior, and regulates the set point for HPA activity. These animals generally show a decreased anxiety [23,24,31-33] and an increased stress response with augmented levels of ACTH and cortisol [31,34]. Microbial colonization of the gut leads to a normalization of the axis in an age-dependent manner, with reversibility of the exaggerated stress response being observed after GF colonization only in very young mice, supporting the existence of a critical period during which the plasticity of neural regulation is sensitive to input from microbiota [34].
In parallel, in GF animals, also memory dysfunction has been reported [35] probably to be ascribed to an altered expression of brain-derived neurotrophic factor (BDNF), one of the most important factors involved in memory. This molecule is a neurotrophic factor, mainly located in the hippocampus and cerebral cortex, which regulates different aspects of brain activities and cognitive functions as well as muscle repair, regeneration, and differentiation [36]. Finally, the presence of the microbiota results also to modulation of the serotoninergic system, since an increase in serotonin turnover and altered levels of related metabolites have been reported in the limbic system of GF animals [24].
The impact of microbiota on GBA has been further supported by studies finalized to the manipulation of gut microbiota through the use of probiotics and/or antibiotics. These studies also confirm that microbiota affects anxiety and HPA system by influencing brain neurochemistry [37]. Chronic treatment with Lactobacillus rhamnosus JB-1 induced region-dependent alterations in GABA mRNA in the brain. In comparison to mice with controlled diet, GABAB1b increased in cortical cingulate and prelimbic regions while concomitantly decreased in the hippocampus, amygdala, and locus coeruleus. In turn GABAAα2 mRNA expression was reduced in the prefrontal cortex and amygdala, but increased in the hippocampus. The probiotics, in parallel, reduced stress-induced release of cortisol, anxiety- and depression-related behavior [38]. Similarly, transient alteration of microbiota composition, obtained by administration of oral antimicrobials (neomycin, bacitracin, and pimaricin) in specific-pathogen-free mice, increased exploratory behavior and hippocampal expression of BDNF [39]. Furthermore, change in microbiota composition with the probiotics association VSL#3 leads to an increase in BDNF expression, attenuation of age-related alterations in the hippocampus [40], and reversion of neonatal maternal separation-induced visceral hypersensitivity in a rat model of IBS [41]. In this latter model of stress, a change in the expression of subsets of genes involved in pain transmission and inflammation has also been described, that was reset by the early life administration of probiotics.
Evidence indicates that microbiota communication with the brain involves the vagus nerve, which transmits information from the luminal environment to CNS. In fact, neurochemical and behavioral effects were not present in vagotomized mice, identifying the vagus as the major modulatory constitutive communication pathway between microbiota and the brain [38]. In a model of chronic colitis associated to anxiety-like behavior, the anxiolytic effect obtained with a treatment with Bifidobacterium longum, was absent in mice that were vagotomized before the induction of colitis [42].
Microbiota may interact with GBA through different mechanisms (Table 1), the principal one likely being modulation of the intestinal barrier, whose perturbation can influence all the underlying compartments. Probiotic species-specific central effects are indeed associated with restoration of tight-junction integrity and the protection of intestinal barrier, as recently reported in an animal model of water avoidance stress [43]. Pre-treatment of animals with probiotic combined formulation of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 restored tight junction barrier integrity and attenuated HPA axis and autonomic nervous system activities, assessed through plasma cortisol and catecholamine measurements. Probiotics also prevented changes in hippocampal neurogenesis and expression in hypothalamic genes involved in synaptic plasticity.
Table 1.
Main principal mechanisms of the bidirectional brain-gut-microbiota axis
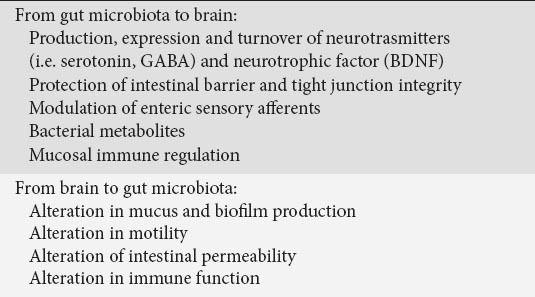
Microbiota can interact with GBA also through the modulation of afferent sensory nerves as reported for Lactobacillus reuteri that, enhancing their excitability by inhibiting calcium-dependent potassium channels opening, modulates gut motility and pain perception [44]. Furthermore, microbiota can influence ENS activity by producing molecules that can act as local neurotransmitters, such as GABA, serotonin, melatonin, histamine and acetylcholine [45] and by generating a biologically active form of catecholamines in the lumen of the gut [46]. Lactobacilli also utilize nitrate and nitrite to generate nitric oxide [47] and to produce hydrogen sulfide that modulates gut motility by interacting with the vanilloid receptor on capsaicin-sensitive nerve fibers [48].
The ENS represents also the target of bacterial metabolites. One of the main product of bacterial metabolism are short-chain fatty acid (SCFAs), such as butyric acid, propionic acid and acetic acid, that are able to stimulate sympathetic nervous system [49], mucosal serotonin release [50] and to influence memory and learning process [51,52]. In this context, it is interesting to report that diet manipulation of microbiota may influence behavior. Mice fed with a diet containing 50% lean ground beef, have a greater diversity of gut bacteria than those receiving standard rodent chow, and presented an increase physical activity, reference memory and less anxiety-like behavior [53].
Given the ability of gut microbiota to alter nutrient availability and the close relationship between nutrient sensing and peptide secretion by enteroendocrine cells, the interaction of microbiota and GBA might also occur through the release of biologically active peptides from enteroendocrine cells that can affect the GBA [54]. For example, galanin stimulates the activity of the central branch of the HPA axis (i.e. the release of CRF and ACTH), thereby enhancing glucocorticoid secretion from the adrenal cortex. Galanin also is able to stimulate directly cortisol secretion from adrenocortical cells, and norepinephrine release from adrenal medulla [55]. Ghrelin too possesses a marked ACTH/cortisol-releasing effect in humans and it is probably involved in the modulation of the HPA response to stress and nutritional/metabolic variations [56].
Last but not least, microbiota affects mucosal immune activation. The enhanced mucosal inflammation induced in mice after treatment with oral antimicrobials, increases substance P expression in ENS, an effect normalized by the administration of Lactobacillus paracasei which also attenuates antibiotic-induced visceral hypersensitivity [57]. The effects of microbiota on immune activation might be in part mediated by proteases. These enzymes are upregulated in intestinal-immune mediated disorders and become the end-stage effectors of mucosal and enteric nervous damage [58-59]. Increased concentration of proteases have been detected in fecal samples of IBS patients associated to specific intestinal bacterial species [60,61]. The current working hypothesis in IBS is that an abnormal microbiota activates mucosal innate immune responses, which increase epithelial permeability, activate nociceptive sensory pathways inducing visceral pain, and dysregulates the enteric nervous system [62,63].
Similar mechanisms may be involved in the effects induced by the gastric mucosa-colonizing microorganism, Helicobacter pylori (H. pylori) on the GBA. The effects induced by this microorganism may arise through both activation of neurogenic inflammatory processes and microelements deficiency secondary to functional and morphological changes in the digestive tract [64]. Nevertheless, unequivocal data concerning the direct and immediate effects of H. pylori infection on the GBA are still lacking, and in clinical practice the relationship between functional dyspepsia and H. pylori infection is not well defined. In fact, the number needed to treat to cure one case of dyspepsia is 14 (95%CI 10-25 [65] suggesting a multifactorial etiology for the increase in H. pylori-related upper FGID.
From brain to gut microbiota
Different types of psychological stressors modulate the composition and total biomass of the enteric microbiota, independently from duration. In fact, also the use of short stressors impact the microbiota, being the exposure to social stressor for only 2 h significantly able to change the community profile and to reduce the relative proportions of the main microbiota phyla [66]. These effects may be mediated, through the parallel neuroendocrine output efferent systems (i.e. autonomic nervous system and HPA), both directly via host-enteric microbiota signaling and indirectly via changes in the intestinal milieu (Table 1). These efferent neural pathways, associated to the pain-modulator endogenous pathways, constitute the so-called “emotional motor system” [1].
The direct influence is mediated by the secretion, under the regulation of brain, of signaling molecules by neurons, immune cells and enterocromaffin cells, which might affect microbiota. Communication between CNS effectors and bacteria relies on the presence of neurotransmitter receptors on bacteria. Several studies have reported that binding sites for enteric neurotransmitters produced by the host are present on bacteria and can influence the function of components of the microbiota, contributing to increase predisposition to inflammatory and infection stimuli [67]. High affinity for GABA system has been reported in Pseudomonas fluorescens with binding properties similar to those of a brain receptor [68]. Escherichia coli O157:H7 possesses a receptor for host-derived epinephrine/norepinephrine that can be blocked specifically by adrenergic antagonists [69].
Besides, brain has a prominent role in the modulation of gut functions, such as motility, secretion of acid, bicarbonates and mucus, intestinal fluid handling and mucosal immune response, all important for the maintenance of the mucus layer and biofilm where individual groups of bacteria grow in a multiplicity of different microhabitats and metabolic niches associated with the mucosa [70]. A dysregulation of GBA can then affect gut microbiota through the perturbation of the normal mucosal habitat.
Stress induces variation in size and quality of mucus secretion [71]. Acoustic stress affects gastric and intestinal postprandial motility in dogs, delaying the recovery of the migrating motor complex pattern and inducing a transient slowing of gastric emptying [72]. Mental stress too increases the frequency of cecocolonic spike-burst activity through the central release of CRF [73]. Regional and global changes in gastrointestinal transit can have profound effects on the delivery of important nutrients, mainly prebiotics and dietary fibers, to the enteric microbiota.
Brain might also affect microbiota composition and function by alteration of intestinal permeability, allowing bacterial antigens to penetrate the epithelium and stimulate an immune response in the mucosa. Acute stress increased colonic paracellular permeability involving overproduction of interferon-g and decrease in mRNA expression of ZO-2 and occluding [74]. Brain, through the ANS, may also modulate immune function. The sympathetic branch modulates number, degranulation and activity of mast cells with consequent imbalance in tryptase and histamine release in stress-related muscle dysfunction [75]. Other mast cell products, such as CRF, in turn, can increase epithelial permeability to bacteria, which facilitates their access to immune cells in the lamina propria [1]. Also corticotropin releasing hormone receptors are involved in colonic barrier dysfunction in response to mild stress in neonatal maternal separation in adult rats that [76] leads to depression and enhanced vulnerability to colitis [77]. Bilateral olfactory bulbectomy induced depression-like behavior associated to elevated central CRF expression and serotonin levels, associated to alterations in colonic motility and intestinal microbial profile in mice [78]. Another possible perturbation in the microbiota habitat induced by stress occurs through the enhancement in secretion of a-defensin, an antimicrobial peptide, from Paneth cells [79].
Finally, it is important to remark that gut alterations associated to stress facilitate the expression of virulent bacteria. Norepinephrine released during surgery induces the expression of Pseudomonas aeruginosa, which might result in gut sepsis [80]. Besides, norepinephrine can also stimulate proliferation of several strains of enteric pathogens and increase the virulent properties of Campylobacter jejuni [81] and might favor overgrowth of non-pathogenic isolates of Escherichia coli, as well as of pathogenic Escherichia coli 0157:H7:3 [82,83].
Concluding remarks
Strong evidence suggests that gut microbiota has an important role in bidirectional interactions between the gut and the nervous system. It interacts with CNS by regulating brain chemistry and influencing neuro-endocrine systems associated with stress response, anxiety and memory function. Many of these effects appear to be strain-specific, suggesting a potential role of certain probiotic strains as novel adjuvant strategy for neurologic disorders. In addition, the effects of CNS on microbiota composition are likely mediated by a perturbation of the normal luminal/mucosal habitat that can also be restored by the use of probiotics and possibly by diet. In clinical practice, an example of this interaction is constituted by FGID, in particular IBS, now considered a microbiome-GBA disorder.
Acknowledgment
The authors kindly thank Dr Laura Carabotti for the artwork of the figures.
Biography
University Sapienza, Rome; S. De Bellis, Castellana Grotte, Bari, Italy
Footnotes
Conflict of Interest: None
References
- 1.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 3.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan MY. The treatment of chronic hepatic encephalopathy. Hepatogastroenterology. 1991;38:377–387. [PubMed] [Google Scholar]
- 6.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 8.Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? Bioessays. 2014;36:933–999. doi: 10.1002/bies.201400075. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simrén M, Barbara G, Flint HJ, et al. Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berrill JW, Gallacher J, Hood K, et al. An observational study of cognitive function in patients with irritable bowel syndrome and inflammatory bowel disease. Neurogastroenterol Motil. 2013;25:918–e704. doi: 10.1111/nmo.12219. [DOI] [PubMed] [Google Scholar]
- 13.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain-gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284–1290. doi: 10.1136/gutjnl-2011-300474. [DOI] [PubMed] [Google Scholar]
- 14.Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39:1033–1042. doi: 10.1111/apt.12728. [DOI] [PubMed] [Google Scholar]
- 15.Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Sero- tonin and Altered Microbiome. J Neurogastroenterol Motil. 2012;18:258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quigley EM. Small intestinal bacterial overgrowth: what it 77 is and what it is not. Curr Opin Gastroenterol. 2014;30:141–146. doi: 10.1097/MOG.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 17.Pimentel M, Lembo A, Chey WD, et al. TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 18.Crouzet L, Gaultier E, Del’Homme C, et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25:e272–e282. doi: 10.1111/nmo.12103. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20:14105–14125. doi: 10.3748/wjg.v20.i39.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667–672. doi: 10.1016/j.coph.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Barbara G, Stanghellini V, Brandi G, et al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 22.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain and behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 23.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 24.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and Behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrams GD, Bishop JE. Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med. 1967;126:301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- 26.Iwai H, Ishihara Y, Yamanaka J, Ito T. Effects of bacterial flora on cecal size and transit rate of intestinal contents in mice. Jpn J Exp Med. 1973;43:297–305. [PubMed] [Google Scholar]
- 27.Caenepeel P, Janssens J, Vantrappen G, Eyssen H, Coremans G. Interdigestive myoelectric complex in germ-free rats. Dig Dis Sci. 1989;34:1180–1184. doi: 10.1007/BF01537265. [DOI] [PubMed] [Google Scholar]
- 28.Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 29.Wostmann E, Bruckner-Kardoss E. Development of cecal distention in germ-free baby rats. Am J Physiol. 1959;197:1345–1346. doi: 10.1152/ajplegacy.1959.197.6.1345. [DOI] [PubMed] [Google Scholar]
- 30.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 31.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 32.Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011;4:492–494. doi: 10.4161/cib.4.4.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishino, Mikami K, Takahashi H, et al. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil. 2013;25:521–528. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- 34.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 36.Al-Qudah M, Anderson CD, Mahavadi S, et al. Brain-derived neurotrophic factor enhances cholinergic contraction of longitudinal muscle of rabbit intestine via activation of phospholipase C. Am J Physiol Gastrointest Liver Physiol. 2014;306:G328–G337. doi: 10.1152/ajpgi.00203.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saulnier DM, Ringel Y, Heyman MB, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4:17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 40.Distrutti E, O’Reilly JA, McDonald C, et al. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One. 2014;9:e106503. doi: 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One. 2013;8:e63893. doi: 10.1371/journal.pone.0063893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain Communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ait-Belgnaoui A, Colom A, Braniste V, et al. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil. 2014;26:510–520. doi: 10.1111/nmo.12295. [DOI] [PubMed] [Google Scholar]
- 44.Kunze WA, Mao YK, Wang B, et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13:2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004;20:292–299. doi: 10.1016/j.tig.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Asano Y, Hiramoto T, Nishino R, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 47.Sobko T, Huang L, Midtvedt T, et al. Generation of NO by probiotic bacteria in the gastrointestinal Tract. Free Radic Biol Med. 2006;41:985–991. doi: 10.1016/j.freeradbiomed.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 48.Schicho R, Krueger D, Zeller F, et al. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 49.Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci U S A. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292:G429–G437. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- 51.Vecsey CG, Hawk JD, Lattal KM, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Dowd SE, Scurlock B, Acosta-Martinez V, Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol Behav. 2009;96:557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Uribe A, Alam M, Johansson O, Midtvedt T, Theodorsson E. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology. 1994;107:1259–1269. doi: 10.1016/0016-5085(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 55.Tortorella C, Neri G, Nussdorfer GG. Galanin in the regulation of the hypothalamic-pituitary-adrenal axis (Review) Int J Mol Med. 2007;19:639–647. [PubMed] [Google Scholar]
- 56.Giordano R, Pellegrino M, Picu A, et al. Neuroregulation of the hypothalamus-pituitary-adrenal (HPA) axis in humans: effects of GABA-, mineralocorticoid-, and GH-Secretagogue-receptor modulation. Sci World J. 2006;17:1–11. doi: 10.1100/tsw.2006.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verdú EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito T, Bunnett NW. Protease-activated receptors: regulation of neuronal function. Neuromolecular Med. 2005;7:79–99. doi: 10.1385/NMM:7:1-2:079. [DOI] [PubMed] [Google Scholar]
- 59.Biancheri P, Di Sabatino A, Corazza GR, MacDonald TT. Proteases and the gut barrier. Cell Tissue Res. 2013;351:269–280. doi: 10.1007/s00441-012-1390-z. [DOI] [PubMed] [Google Scholar]
- 60.Gecse K, Róka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 61.Carroll IM, Ringel-Kulka T, Ferrier L, et al. Fecal protease activity is associated with compositional alterations in the intestinal microbiota. PLoS One. 2013;8:e78017. doi: 10.1371/journal.pone.0078017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 63.Theodorou V, Ait Belgnaoui A, Agostini S, Eutamene H. Effect of commensals and probiotics on visceral sensitivity and pain in irritable bowel syndrome. Gut Microbes. 2014;5:430–436. doi: 10.4161/gmic.29796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Budzyński J, Kłopocka M. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World J Gastroenterol. 2014;20:5212–5225. doi: 10.3748/wjg.v20.i18.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcerdyspepsia. Cochrane Database Syst Rev. 2006 Apr 19;2:CD002096. doi: 10.1002/14651858.CD002096.pub4. Review. Update in: Cochrane Database Syst Rev 2011;(2): CD002096. [DOI] [PubMed] [Google Scholar]
- 66.Galley JD, Nelson MC, Yu Z, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6:111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guthrie GD, Nicholson-Guthrie CS. Gamma-Aminobutyric acid uptake by a bacterial system with neurotransmitter binding characteristics. Proc Natl Acad Sci U S A. 1989;86:7378–7381. doi: 10.1073/pnas.86.19.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A. 2006;103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macfarlane S, Dillon JF. Microbial biofilms in the human gastrointestinal Tract. J Appl Microbiol. 2007;102:1187–1196. doi: 10.1111/j.1365-2672.2007.03287.x. [DOI] [PubMed] [Google Scholar]
- 71.Rubio CA, Huang CB. Quantification of the sulphomucin-producing cell population of the colonic mucosa during protracted stress in rats. In Vivo. 1992;6:81–84. [PubMed] [Google Scholar]
- 72.Gué M, Peeters T, Depoortere I, Vantrappen G, Buéno L. Stress-induced changes in gastric emptying, postprandial motility, and plasma gut hormone levels in dogs. Gastroenterology. 1989;97:1101–1107. doi: 10.1016/0016-5085(89)91678-8. [DOI] [PubMed] [Google Scholar]
- 73.Gué M, Junien JL, Bueno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991;100:964–970. doi: 10.1016/0016-5085(91)90270-u. [DOI] [PubMed] [Google Scholar]
- 74.Demaude J, Salvador-Cartier C, Fioramonti J, Ferrier L, Bueno L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55:655–661. doi: 10.1136/gut.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santos J, Saperas E, Nogueiras C, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- 76.Söderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- 77.Varghese AK, Verdú EF, Bercik P, et al. Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology. 2006;130:1743–1753. doi: 10.1053/j.gastro.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 78.Park AJ, Collins J, Blennerhassett PA, et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733–e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alonso C, Guilarte M, Vicario M, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163–172.e1. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 80.Alverdy J, Holbrook C, Rocha F, et al. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 2000;232:480–489. doi: 10.1097/00000658-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cogan TA, Thomas AO, Rees LE, et al. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut. 2007;56:1060–1065. doi: 10.1136/gut.2006.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock. 2002;18:465–470. doi: 10.1097/00024382-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 83.Freestone PP, Haigh RD, Williams PH, Lyte M. Involvement of enterobactin in norepinephrine-mediated iron supply from transferrin to enterohaemorrhagic Escherichia coli. FEMS Microbiol Lett. 2003;222:39–43. doi: 10.1016/S0378-1097(03)00243-X. [DOI] [PubMed] [Google Scholar]


