Abstract
Background
Botulinum toxin (BT) injection reduces lower esophageal sphincter pressure and alleviates symptoms in idiopathic achalasia (IA). Ethanolamine oleate (EO) has also been introduced for the treatment of IA. We compared the long-term efficacy of BT and EO injections in the treatment of IA.
Methods
A total of 189 IA patients were evaluated prospectively, of whom 21 were unwilling to undergo or were poor candidates for pneumatic balloon dilation and Heller myotomy and were enrolled in the study. Eleven patients were treated by BT, and 10 by EO injections. Patients were followed up by achalasia symptom score (ASS), timed barium esophagogram (TBE), and high-resolution manometry at baseline and post-treatment. A good initial response was defined as a decrease in ASS to 4 or less, and a reduction in barium column height and volume in TBE by >50%.
Results
All 10 EO group patients and 10 of 11 BT group patients showed a good initial response. Four EO group relapsers and 6 BT group relapsers were managed effectively by re-injections. Mean duration of follow up was 27.38 months. On completion of the study, a sustained good response was seen in 9 and 6 patients in EO and BT groups, respectively (P=0.149).
Conclusion
This study revealed that BT and EO have comparable efficacy in the treatment of IA. However, the cost of EO is about 2 times lower than BT.
Keywords: Achalasia, botulinum toxin, ethanolamine oleate, esophagus
Introduction
Idiopathic achalasia (IA) is a chronic disease of the esophagus characterized by absence of peristalsis and incomplete relaxation of the lower esophageal sphincter (LES). Esophageal emptying is impaired in IA due to the inflammatory degeneration of inhibitory ganglion cells in the myenteric esophageal plexus. This causes an imbalance between the excitatory and inhibitory neurons, leading to aperistalsis, increased basal LES pressure, and incomplete relaxation of LES [1,2].
The treatment of the disease is only palliative, aiming to reduce LES pressure thereby facilitating esophageal emptying by gravity. Current therapeutic modalities include pharmacologic treatment, pneumatic balloon dilation (PBD) of LES, Heller myotomy, and intrasphincteric injection of botulinum toxin (BT) [1,2] or ethanolamine oleate (EO) [4,5].
Intrasphincteric injection of BT as an alternative to PBD or Heller myotomy was introduced by Pasricha [6]. BT is a potent inhibitor of acetylcholine release from nerve endings. Injection of BT into the LES under direct endoscopic vision is a safe and effective treatment and decreases LES pressure by inhibiting acetylcholine release from excitatory neurons and relaxing the muscle fibers [6-8].
EO, a sclerosant agent, has been introduced as an effective alternative treatment of achalasia [9]. EO is a salt, a synthetic mixture of ethanolamine and oleic acid, with an empirical formula of C20H41NO3. EO injection has been used in the treatment of bleeding esophageal varices, varicose veins in the legs and reactive vascular lesions [10-12]. EO is a sclerosant agent inducing inflammatory response and fibrosis in the tissues; thus, the injection of EO into the LES causes excitatory neuron damage and decreases the LES pressure [4,5].
To the best of our knowledge, there is no study in the literature comparing sclerotherapy with BT injection for the treatment of IA. Therefore, we decided to compare the long-term efficacy of these treatment options in IA patients.
Patients and methods
Study population and design
In this prospective study, we evaluated 189 patients with IA, referred to our center over a period of 4 years, i.e. from 2009 to 2013.
The diagnosis of IA was based on clinical symptoms as well as radiologic and endoscopic findings, and confirmed by manometric criteria. Patients who were poor candidates for PBD (due to presence of sigmoid esophagus, epiphrenic diverticula, or severe comorbidities) or Heller myotomy (due to high perioperative risk), and those unwilling to undergo the latter procedures were enrolled in this study. Exclusion criteria comprised: being a good candidate for PBD or Heller myotomy; secondary achalasia; poor cooperation; pregnancy; breast-feeding; age <18 years; use of illicit drugs or alcohol abuse; and history of allergic reactions to sclerosant agents.
Twenty one eligible patients with newly diagnosed IA were enrolled in the study, of whom 3 unwilling to undergo Heller myotomy or PBD, and 18 poor candidates for these procedures. Patients were randomly assigned into two groups. Eleven patients were treated with BT and 10 with EO injection.
The symptom scores of all patients were assessed by one single physician (N.F.) timed barium esophagograms (TBE) were performed by one single radiologist (Sh. Sh.); and BT and EO were injected by the same gastroenterologist (J.M.).
Symptomatic response
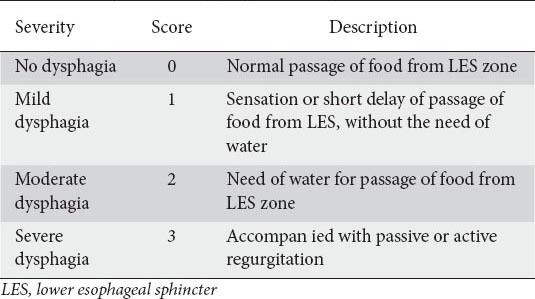
Clinical data was collected using a standard questionnaire measuring achalasia symptom score (ASS) similar to our previous studies [5,16]. The severity of each symptom was recorded on a scale of 0-3, depending on each symptom’s frequency. The total symptom score was the sum of five cardinal symptom scores and the severity score of dysphagia (Tables 1, 2). Therefore, the highest possible score was 18. Regurgitation was defined as the returning of food material from the esophagus; active in standing or sitting, and passive in supine or lying positions. The ASS was calculated for each patient at the following intervals: pretreatment, 1.5, 3, 6, 9, and 12 months after the last injection, then every 6 months, and finally at the end of the follow up. The patients were asked to come back for a reevaluation if they felt severe dysphagia or regurgitation between follow-up periods. A good clinical response was defined as a decrease in ASS to a score of 4 or less, while a clinical relapse was defined as an increase in the severity score of dysphagia of about 2 or more points after the initial good response was achieved. A sustained good response was defined as clinical remission (ASS ≤4) at the follow-up intervals without requiring any reinjection over the last 6 months.
Table 1.
Cardinal symptom score
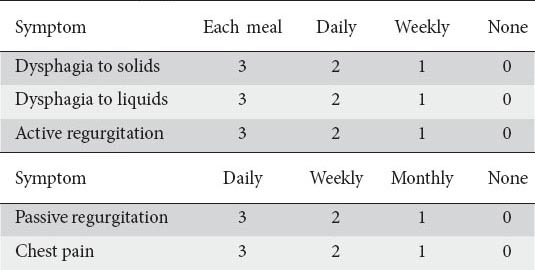
Table 2.
Severity score of dysphagia for every swallow
TBE
TBE was used as an objective tool to assess esophageal emptying. All patients underwent TBE at baseline and 1.5 months post-treatment. Patients swallowed 200 mL of barium sulfate suspension (81% weight/volume) [13] in the upright position, and then radiographies were taken at 1, 3, and 5 min after swallowing from the left posterior oblique view. The barium column height, i.e. the distance from the most distal part of the esophagus to the most proximal barium level, was measured in centimeters. The volume of retained barium in milliliter (mL) was calculated as follows: (mean radius)² × 3.14 × height of the column. These calculations have been used in similar studies [5,13].
The initial TBE in our patients showed barium retention at 1, 3, and 5 min, a dilated esophagus and beak-like narrowing at gastroesophageal (GE) junction. Severe dilation and tortuosity of the esophagus were seen in 9 patients. The difference in the retained barium height and volume at 5 min was calculated between the pretreatment and post-treatment TBEs. A good response was defined as a reduction >50% from the baseline in the barium column height and volume at 1.5 months after the last injection, whereas if the mentioned outcomes were not achieved patients were considered as poor responders.
Endoscopy
Endoscopic evaluation was performed in all patients to confirm IA and exclude malignancies. In our study group, the endoscopic findings included dilated esophagus, retained foamy secretion and/or food particles in the esophagus, hypertonic LES that did not open spontaneously but could be traversed by gentle pressure of the endoscope, and some erythema and irritation of esophageal mucosa in cases with sigmoid esophagus.
High-resolution manometry (HRM)
The HRM (Solar Gastrointestinal [GI] HRM, Medical Measurement Systems MMS, The Netherlands) was done with 22 water-perfused catheter. Abnormal LES relaxation was defined as a mean integrated relaxation pressure (mean IRP) >15 mmHg. Patients were classified in three IA types based on HRM. In the BT group, 1 patient had type I, 8 patients had type II, and 2 patients had type III IA. In the EO group, 2 patients had type I, 7 patients had type II, and 1 patient had type III IA. All patients underwent HRM at baseline and 1.5 months post-treatment.
Endoscopic injections
Patients were randomly assigned into two groups. Eleven patients were treated in two sessions of BT injection (Dysport, Ipsen, UK) with an interval of 4 weeks. After a1-day liquid diet and an overnight fasting, patients were sedated using intravenous diazepam (5-10 mg) and meperidine (25-50 mg) and the esophagogastric junction was identified through upper GI endoscopy. Dysport powder (one vial of 500 U) was dissolved in 5 mL of normal saline, and an aliquot of 0.5 mL was used in each injection. Ten injections were performed into the LES around the GE junction up to 1 cm above it, using a 5-mm sclerotherapy needle. BT injection was repeated 4 weeks later. The number of injection sessions was determined using data from a previous multicenter study [7].
In the second group, one vial of EO (5%, 5 mL) (Martindale Pharma Ltd., Harold Hill, UK) was diluted by 5 mL of normal saline and an aliquot of 1 mL was used in each injection. Ten injections of diluted EO (2.5%) were performed into the LES around the GE junction up to 1 cm above it, using a 5 mm sclerotherapy needle. Patients received identical doses of EO 2 and 4 weeks later. The number of injection sessions was determined based on previous studies [4,5-9]. Patients were observed for 3 h after the procedure and were then discharged. They were allowed to start eating soft food on the same day.
Ethical considerations
The study was approved by the Digestive Disease Research Center Ethics Committee and was conducted according to the ethical guidelines of the declaration of Helsinki. This study was not blinded. Possible benefits and risks of EO or BT injections were explained to the patients. Informed consent was obtained from all patients and their participation in the study was voluntary. This study was registered in Iranian Registry Center of Clinical Trials. Registration number was IRCT201108157335N1.
Study endpoints
The primary study endpoint was the reduction in ASS and TBE values 6 weeks after the last injection compared with baseline values. The secondary endpoint was defined as a sustained good response during follow-up periods. Furthermore, voluntary withdrawal for any reason, development of severe complications or side-effects leading to the discontinuation of the injection, poor cooperation, poor or no response to treatment protocols, recurrences of more than 3 times, and death were considered as a failure of treatment.
Statistical analysis
This study is a parallel randomized controlled, open-label, not blinded trial. A central randomization center used even numbers for EO and odd numbers for BT treatment. The results were analyzed using SPSS version 19.00 for Windows. Continuous variables were expressed as mean ± standard deviation. Comparisons in each group were performed using the paired t-test for symptom scores and height of barium and Wilcoxon sign rank test for the volume of barium. Categorical data was summarized as relative and absolute frequency, and the differences between groups were tested by the independent t-test for symptom scores and height of barium column, and the Mann–Whitney test for barium volume. We used chi-square based on Fisher’s exact test for evaluation of the end of follow up. A P<0.05 was chosen for the rejection of the null hypothesis.
Results
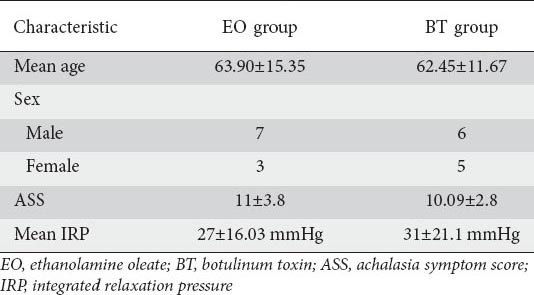
We prospectively evaluated 189 IA patients. One hundred and sixty-eight patients were excluded from the study since they fell under the exclusion criteria, defined previously. The remaining –21 patients were enrolled in this study, of whom 18 were poor candidates for PBD (due to sigmoid esophagus, epiphrenic diverticula, and severe comorbidities) and/or Heller myotomy (due to high perioperative risk) and 3 were unwilling to undergo Heller myotomy and PBD. Patients were randomly assigned to EO (n=10) and BT (n=11) groups. Mean age was 63.14±13.2 (range 26-81) years, and 13 patients were male (Table 3). Patients were assessed by changes in symptom scores and TBE. Moreover, side-effects of the injections, relapses, and the cumulative remission rates were recorded.
Table 3.
Baseline characteristic of the patients
In both groups, the treatment protocols (BT and EO injection) were completed. The mean duration of follow up was 27.38±16.49 months (range: 12-64).
All 10 patients in the EO group and 10 of 11 patients in the BT group showed a good initial response. One patient, who initially refused to undergo PBD or Heller myotomy, later changed his decision and opted for the surgery owing to the poor initial response in BT treatment (>Fig. 1).
Figure 1.
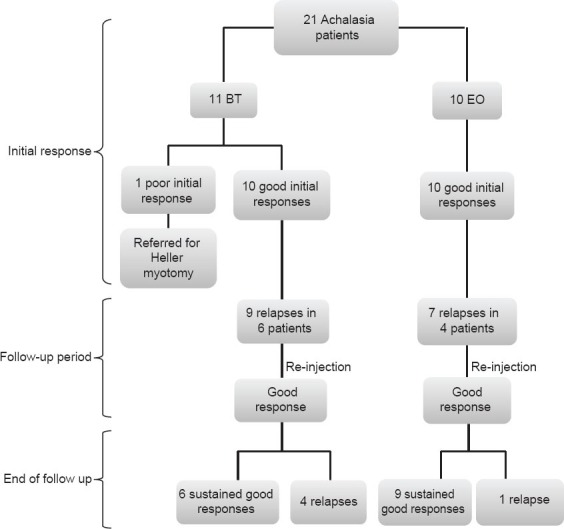
Flow diagram of the study
Age and sex were not correlated with the responses of the treatment (P=0.120, P=0.180, respectively).
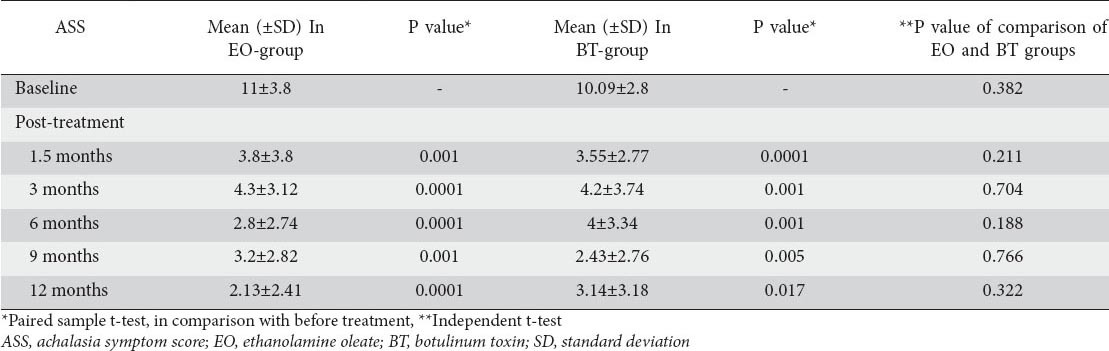
In BT-treated patients, the mean symptom scores at baseline and at 1.5 months post-treatment (second injection) was 10.09±2.80 vs. 3.55±2.77, respectively (P=0.0001). In the EO group, the mean symptom score decreased from 11±3.8 at baseline to 3.8±3.8 at 1.5 months post-treatment (third injection) (P=0.001). The mean symptom scores in both groups were also calculated at 3, 6, 9, and 12 months after the last injections (Table 4).
Table 4.
The comparison of ASS at baseline and post-treatment
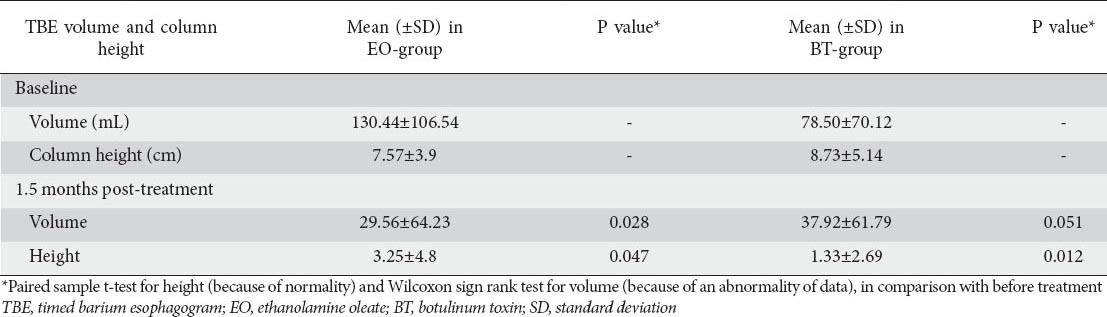
In TBE of the BT group, the mean retained barium volume at 5th min, at baseline and at 1.5 months post-treatment were 78.50±70.12 mL vs. 37.92±61.79 mL, respectively (P=0.051), while, in the EO group, these values were 130.44±106.54 mL vs. 29.56±64.23 mL, respectively (P=0.028) (Table 5).
Table 5.
The comparison of retained barium volume (mL) and column height (cm), in 5th min of TBE, at baseline and post-treatment
The mean IRP decreased from 31 to 25 mmHg in BT group, and from 27 to 15 mmHg in EO group (P=0.764 vs. 0.008).
A relapse was defined as an increase in the severity score of dysphagia by 2 or more points after an initial good response.
In the follow-up period, symptoms relapsed in 4 patients in the EO group (1 patient 3 times, 1 patient 2 times, and 2 patients 1 time) and 6 patients in the BT group (1 patient 3 times, 1 patient 2 times, and 4 patients 1 time) (P=0.24). All of them underwent reinjections (7 times in the EO group and 9 times in the BT group). The median duration to the first relapse was 15 and 10 months in the EO and BT groups, respectively (P=0.432).
Transient chest pain after the injection was reported by 5 patients in the BT and 3 patients in the EO group (P=0.020). Some erosions (<5 mm) were seen in the distal esophagus during the second and third injections in 2 and 4 patients in the BT and EO groups, respectively (P=0.155). The erosions seen after injections may have been secondary to GE reflux or local injury of the previous injections. We did not perform complementary studies (e.g. 24 h esophageal pH-metry) to differentiate them. All of these minor complications were managed conservatively. No severe complications (perforation, bleeding, ulcer, and fibrotic stenosis) were noticed.
In the short term, neither BT nor EO had any significant therapeutic advantages over each other (Tables 4 and 5). However, at the end of the follow up, in the BT group, 6 patients had good sustained response, and 5 patients had poor responses (<4 scores in ASS), while in the EO group, these figures were 9 and 1, respectively (P=0.149, based on Fisher’s exact test).
Discussion
In this study, after the completion of the treatment schedule, we compared for the first time the efficacy of BT and EO injections in two groups of IA patients in long-term follow up. In 1995, Pasricha et al compared BT injection with placebo for the treatment of IA patients. Symptom scores decreased by 5.4 points in the BT group versus 0.5 point in the placebo group. Nineteen of 21 patients in the BT group showed initial good responses and 14 patients remained in remission 6 months post-treatment. They concluded that BT injection is safe and effective for treating IA patients [6].
Annese et al reported a good response to BT injection in the short-term follow up in the majority (97 of 118) of their IA patients [7].
D’Onofrio et al treated 19 IA patients with BT injection and reported a good response in 14 patients. They concluded that one or two intrasphincteric injections of BT are effective in 74% of IA patients in long-term follow up [14]. In another study, significant improvement in clinical symptoms was seen in 12 elderly IA patients after two intraspincteric BT injections over a 1-year follow up. They suggested that BT is a treatment option for patients unsuitable for surgery or PBD [15].
In 1996, Moretó et al introduced EO as a treatment for IA. They treated 33 IA patients with EO injections and reported good or excellent response in 31 of them in the short-term follow up. In another study with long-term follow up (mean: 72 months), EO treatment failed in only 6 of 65 patients [9].
In our previous study, we evaluated the efficacy of EO injection in 13 IA patients, and all of them showed good response according to ASS and TBE in the short-term follow up (mean duration: 17.83±1.12 months) [5]. In a recent study, we investigated the long-term efficacy of EO injection in 31 IA patients. At the end of the follow up, 26 patients (83%) had sustained good response or good responses after reinjections (mean duration of follow up: 30.16±11.3 months) [16].
Thus, it seems that EO injection is an effective agent for the treatment of IA.
In a recent study, the cumulative expectancy of being free of recurrence was 90% at 50 months with EO injections. Patients who did not respond to EO were treated with 30 mm balloon dilation [17].
Our study results showed that EO and BT are equally effective in the short term based on ASS and TBE criteria, although the mean IRP was significantly reduced in EO compared to BT group (P=0.008 vs. P=0.764).
ASS decreased significantly in both EO and BT groups at 1.5 months after the injections, although in the long term, some recurrences occurred in both groups that required retreatment.
Both groups had comparable relapse rates (approximately 40% at 27 months), but median duration to the first relapse was longer in the EO group compared to the BT group (Table 6).
Table 6.
Cardinal symptom score
At the end of follow-up, 9 patients in the EO group versus 6 patients in the BT group were in sustained good response according to ASS, but this difference was not statistically significant (P=0.149).
Since both BT and EO injections may cause fibrosis in the esophageal wall, we enrolled the patients who were poor candidates for PBD and Heller myotomy with low probability to undergo these invasive procedures in the future. Only 3 patients who did not accept to undergo Heller myotomy or PBD were enrolled in this study.
Fibrotic stricture was not developed in any of our patients, and it could be the advantage of deep injections of diluted EO (2.5%) but the duration of the follow up in this study may not have been long enough to rule out this possibility.
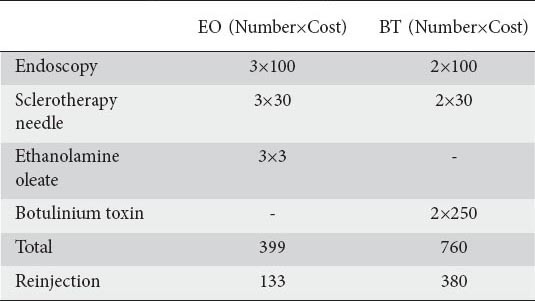
On the other hand, BT is more expensive than EO (Table 6). BT costs approximately 20 times more than EO (for each injection session), and this is an important advantage of EO over BT. Except for the initial injection protocol including 3 sessions for EO and 2 sessions for BT, additional endoscopies and injections for relapses were comparable in both groups.
Since IA is a chronic and progressive disease, it would be reasonable to choose the most practical method for its lifelong treatment.
Given that the comparison between these two groups has been performed for the first time, it can be a clue for further studies to find better ways to treat IA and improve the quality-of-life in IA patients.
As a result of the rarity of IA and the limited inclusion criteria, only a small number of patients were enrolled in this study. The studies with large sample sizes may reveal more details in comparison between these treatments.
Summary Box.
What is already known:
Botulinum toxin (BT) and ethanolamine oleate (EO) have comparable efficacy in the treatment of idiopathic achalasia (IA)
The cost of EO is approximately 20 times lower than BT
Both EO and BT groups had comparable relapse rates, but median duration to the first relapse was longer in the EO group compared to the BT group
What the new findings are:
EO and BT have comparable efficacy in the treatment of IA
Biography
Digestive Disease Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Footnotes
Conflict of Interest: None
References
- 1.Mikaeli J, Islami F, Malekzadeh R. Achalasia: A review of Western and Iranian experiences. World J Gastroenterol. 2009;15:5000–5009. doi: 10.3748/wjg.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrokhi F, Vaezi MF. Idiopathic (primary) achalasia. Orphanet J Rare Dis. 2007;2:38. doi: 10.1186/1750-1172-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spechler SJ. Clinical manifestations and diagnosis of achalasia. UpToDate, Wolters Kluwer Health. 2013. Available at: http://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-achalasia .
- 4.Moretó M, Ojembarrena E, Rodríguez ML. Endoscopic injection of ethanolamine as a treatment for achalasia: A first report. Endoscopy. 1996;28:539–545. doi: 10.1055/s-2007-1005551. [DOI] [PubMed] [Google Scholar]
- 5.Niknam R, Mikaeli J, Mehrabi N, et al. Ethanolamine oleate in resistant idiopathic achalasia: A novel therapy. Eur J Gastroenterol Hepatol. 2011;23:1111–1115. doi: 10.1097/MEG.0b013e328349647e. [DOI] [PubMed] [Google Scholar]
- 6.Pasricha PJ, Ravich WJ, Hendrix TR, et al. Treatment of achalasia with intrasphincteric injection of botulinum toxin. A pilot trial. Ann Intern Med. 1994;121:590–591. doi: 10.7326/0003-4819-121-8-199410150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Annese V, Bassotti G, Coccia G, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study Group. Gut. 2000;46:597–600. doi: 10.1136/gut.46.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaninotto G, Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239:364–370. doi: 10.1097/01.sla.0000114217.52941.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moretó M, Barturen A, Arechavala A, et al. Treatment of achalasia by two sclerosing agents: A long-term experience and analysis. Gastrointest Endosc. 2009;69:AB207. [Google Scholar]
- 10.Kiripolsky MG. More on ethanolamine oleate as a vascular sclerosant. Dermatol Surg. 2010;36:1153–1154. doi: 10.1111/j.1524-4725.2010.01588.x. [DOI] [PubMed] [Google Scholar]
- 11.Hong SK, Lee HJ, Seo JK, et al. Reactive vascular lesions treated using ethanolamine oleate sclerotherapy. Dermatol Surg. 2010;36:1148–1152. doi: 10.1111/j.1524-4725.2010.01599.x. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira LR, Vargas FS, Carmo AO, et al. Effectiveness of ethanolamine oleate as a pleural sclerosing agent in rabbits. Respiration. 1998;65:304–308. doi: 10.1159/000029281. [DOI] [PubMed] [Google Scholar]
- 13.Montazeri G, Nouri N, Estakhri A, et al. Lower oesophageal sphincter pressure and timed barium oesophagogram: Two objective parameters in the non-invasive assessment of primary achalasia. Aliment Pharmacol Ther. 2005;22:261–265. doi: 10.1111/j.1365-2036.2005.02557.x. [DOI] [PubMed] [Google Scholar]
- 14.D’Onofrio V, Miletto P, Leandro G, et al. Long-term follow-up of achalasia patients treated with botulinum toxin. Dig Liver Dis. 2002;34:105–110. doi: 10.1016/s1590-8658(02)80238-9. [DOI] [PubMed] [Google Scholar]
- 15.Dughera L, Battaglia E, Maggio D, et al. Botulinum toxin treatment of oesophageal achalasia in the old old and oldest old: A 1-year follow-up study. Drugs Aging. 2005;22:779–783. doi: 10.2165/00002512-200522090-00006. [DOI] [PubMed] [Google Scholar]
- 16.Niknam R, Mikaeli J, Fazlollahi N, et al. Ethanolamine oleate as a novel therapy is effective in resistant idiopathic achalasia. Dis Esophagus. 2014;27:611–616. doi: 10.1111/dote.12122. [DOI] [PubMed] [Google Scholar]
- 17.Moretó M, Ojembarrena E, Barturen A, et al. Treatment of achalasia by injection of sclerosant substances: A long-term report. Dig Dis Sci. 2013;58:788–796. doi: 10.1007/s10620-012-2476-x. [DOI] [PubMed] [Google Scholar]


