Abstract
Background
Celiac disease is an immune-mediated small bowel disorder that develops in genetically susceptible individuals upon exposure to dietary gluten. Celiac disease could have extra-intestinal manifestations that affect women’s reproductive health. The aim of this study was to investigate fertility and outcomes of pregnancy among women with celiac disease.
Methods
In a retrospective cohort study, we analyzed information collected from patients at a tertiary care celiac center and from members of 2 national celiac disease awareness organizations. Women without celiac disease were used as controls. Women completed an anonymous online survey, answering 43 questions about menstrual history, fertility, and outcomes of pregnancy (329 with small bowel biopsy-confirmed celiac disease and 641 controls).
Results
Of the 970 women included in the study, 733 (75.6%) reported that they had been pregnant at some point; there was no significant difference between women with celiac disease (n=245/329, 74.5%) and controls (488/641, 76.1%; P=0.57). However, fewer women with celiac disease than controls (79.6% vs. 84.8%) gave birth following 1 or more pregnancies (P=0.03). Women with celiac disease had higher percentages of spontaneous abortion than controls (50.6% vs. 40.6%; P=0.01), and of premature delivery (23.6% vs. 15.9% among controls; P=0.02). The mean age at menarche was higher in the celiac disease group (12.7 years) than controls (12.4 years; P=0.01).
Conclusions
In a retrospective cohort analysis examining reproductive features of women with celiac disease, we associated celiac disease with significant increases in spontaneous abortion, premature delivery, and later age of menarche.
Keywords: Celiac disease, women’s health, fertility, nutrition, gluten
Introduction
Celiac disease (CD) is an immune-mediated small bowel disorder that occurs in genetically susceptible people upon exposure to dietary gluten [1]. The only treatment for CD is strict and life-long adherence to a gluten-free diet (GFD), which can lead to mucosal recovery [2]. The accuracy of prevalence estimations of CD has been greatly improved with the development of reliable serological testing. Prevalence rates vary widely across different regions, which reflect varying population risks for disease. Serological based testing in the U.S. estimates prevalence of 1:105 (0.95%) in “not-at-risk” adults, 1:322 (0.31%) in children, and 1:133 overall (0.75%) [3]. The male-to-female ratio of disease is roughly 1:2.8 [4].
The pathophysiology of CD involves the environmental trigger gluten in genetically susceptible individuals. The HLA-DQ2 and DQ8 haplotypes are expressed on the surface of antigen-presenting cells in the gut lamina propria and bind activated gliadin peptides, eliciting an inflammatory reaction. This inflammatory state leads to changes in the small bowel mucosa architecture including increased infiltration of lymphocytes into the epithelial cells, villous atrophy and crypt distortion [5]. These intestinal changes can lead to malabsorption of macro- and micro- nutrients, resulting in symptoms of malabsorption such as weight loss and diarrhea. Additionally, CD is associated with a number of extraintestinal manifestations, and resultant morbidity and mortality [6]. An association between CD and reproductive abnormalities was first made in 1970 when Morris et al described three patients with untreated CD and infertility, all of whom became pregnant after initiating a GFD [7]. However, since this case report, the literature addressing complications of CD in women, specifically rates of infertility, length of fertile life span, perinatal complications and adverse pregnancy outcomes, has been inconsistent [8-16,23-27].
The aim of this study was to help clarify celiac patients’ experiences with fertility and pregnancy outcomes. This study constitutes the largest women’s health survey to date of U.S. patients with CD.
Patients and methods
In this retrospective cohort study, female subjects were recruited from the Jefferson Celiac Center and from members of two national CD awareness organizations. The National Foundation for Celiac Awareness posted an on-line hyperlink to the study in their “Research News Feed” and on their social media websites. The Gluten Intolerance Group also promoted the study via social media. Patients at the Jefferson Celiac center were recruited with in-office fliers and received a copy of the hyperlink in the Jefferson Celiac Center newsletter. The patients were asked to complete an anonymous, online survey. Females who did not carry a diagnosis of CD were recruited as a comparison group via social media. These women were asked to complete the same survey, described as a women’s health survey.
The 43-question study queried whether patients were diagnosed with CD. Patients who reported CD were then asked to identify the method of diagnosis specifically whether they were diagnosed with small bowel biopsy, serology and/or trial of GFD. Patients defined their fertile life span by entering their age at menarche and menopause. Patients were asked to describe their fertility history, including whether they had at some time sought to conceive a pregnancy and whether they had been successful in delivery of one or more child. Additionally, patients answered questions about pregnancy complications, including miscarriages, preterm births (delivery before 37 weeks gestational age), birth weights, and methods of delivery.
Only patients who were told they had CD by a physician after a small bowel biopsy were included in the analysis and were compared with the patients recruited without a history of CD. Differences between means for the two groups were evaluated using independent t-tests. Differences for proportions were evaluated using z-tests. Calculations were performed using Stata 11.0, College Station, Texas. The Institutional Review Board of Thomas Jefferson University approved the study protocol.
Results
A total of 1757 women responded to the survey and 1156 reported that they had at some time sought to conceive a pregnancy. Of these women, 329 patients reported that their CD had been diagnosed by a physician and confirmed with small bowel biopsy. A total of 186 women who reported a history of CD diagnosed by serologies alone or by trial of GFD but without a biopsy were excluded from the study. For the purpose of this study, only the 329 patients with biopsy-proven CD who intended to conceive a pregnancy were considered. The remaining 641 patients with no history of CD were used as a comparison group for the 329 patients with biopsy-proven CD (CD group) for a total of 970 women.
Fertility
Overall, 733 of the 970 women included in the study (75.6%) reported that they had been pregnant at some point, and there was no difference (P=0.57) between the CD (245 of 329 women, 74.5%) cohort and non-CD (488 of 641 women, 76.1%) cohort. Of the 733 women who became pregnant, 609 had a successful delivery (195 CD, 414 non-CD). Fewer (195 of 245, 79.6%) of the women with CD who sought to conceive a pregnancy eventually gave birth, as compared to 414 of 488 women (84.8%) in the comparison group (P=0.03). Fig. 1 describes the women who were included in the study and their pregnancy outcomes.
Figure 1.

Flow diagram of women included in the study. 970 women (329 women with CD, 641 controls) had attempted pregnancy. Of these 733 (75.6%) reported that they had been pregnant at some point, and there was no difference (P<0.57) between the CD (245 of 329 women, 74.55%) cohort and non-CD (488 of 641 women, 76.1%) cohort. Of these patients 609 had a successful delivery (195 CD, 414 non-CD). More women in the CD group (46 of 195 women, 23.6%) reported at least one premature delivery than women without CD (66 of 414 women, 15.9%)
Pregnancy outcomes
The rate of spontaneous abortions was 50.6% (124 of 245 women) in the CD group, which was significantly (P=0.01) higher than the rate of 40.6% (198 of 488 women) in the control group. Of the 124 women in the CD group who reported spontaneous abortions, 105 (84.7%) reported that it was prior to their diagnosis with CD. Of the 609 women who had delivered a child, significantly (P=0.05) more women in the CD group (46 of 195 women, 23.6%) reported at least one premature delivery (delivery before 37 weeks gestational age) than women without CD (66 of 414 women, 15.9%) (Fig. 2). Women with CD had more cesarean sections (70 of 195, 35.9%) compared to women without CD (129 of 414, 31.2%), but this was not statistically significant (P=0.24).
Figure 2.
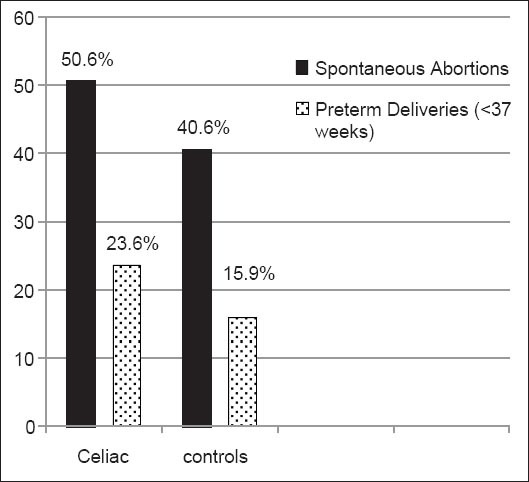
Significantly more (P<0.01) women with celiac disease (CD) reported spontaneous abortions (124 of 245 women, 50.6%) than in the comparison group 40.6% (198 of 488 women). Of the women who had had a successful delivery, significantly more (P<0.05) women in the CD group (46 of 195 women, 23.6%) reported at least one premature delivery than women without CD (66 of 414 women, 15.9%)
Fertile life span
The mean age at menarche for the CD group was 12.7 years, which was significantly (P<0.01) later than the mean of 12.4 years for the control group. Seventy-seven of the patients with CD had reached menopause at a mean age of 48.4 years, which was not significantly earlier than then 148 control patients who had reached menopause at a mean age of 48.8 years (P=0.57).
Discussion
This retrospective cohort study examines the fertility experience of a large number of women in the U.S. with biopsy-proven CD. CD has been reported to have a number of reproductive complications. It has been suggested that maternal infertility and perinatal morbidity in untreated maternal CD may be related to malabsorption of iron and/or folate, leading to vitamin deficiency in the mother [8]. The mean age of diagnosis of CD is advancing, and the diagnosis is being made more commonly in women who present without classic malabsorptive symptoms [9]. This silent presentation of CD combined with delayed diagnosis may result in prolonged dietary gluten exposure and an extended effect of the disease on women’s fertile life span. A previous study from Sher and colleagues was performed to determine the incidence of infertility, abortions and perinatal mortality in CD patients. The investigators compared 68 CD patients with paired controls and found patients with CD had later menarche (13.6 vs. 12.7 years) [10]. This study is similar to our study suggesting that patients with CD may have a shorter fertile life span.
Recent studies investigating the association of maternal CD with infertility and poor fetal outcomes have been inconsistent, probably secondary to low statistical power. A study screened 121 California women with unexplained infertility for serologic markers of CD, and found a prevalence rate (0.8%) similar to that of the general U.S. population (approx. 1%) [11]. A Swedish population-based cohort found that women with CD had normal fertility, but their fertility had decreased in the 2 years preceding the diagnosis of CD [12]. These findings may reflect an improvement in fertility once starting a GFD. Further support of fertility improving on a GFD was demonstrated in an Italian study that found women with CD who had had trouble conceiving got pregnant as early as 2 months after starting a GFD [13]. In contrast, a study from Meloni et al screened 99 couples being evaluated for infertility for serologic and histologic evidence of CD [14]. The prevalence of subclinical CD among the infertile women was significantly higher than that of the general population of Sardinia (3.03% vs. 1.06%) and was higher in a 25-patient subgroup of the test population with unexplained infertility (8%). A case-control study by Collin screened 98 women with unexplained infertility or multiple miscarriages for CD and found a prevalence of 4.1%, compared with 0% in the control group. Two of these women had iron deficiency anemia [15]. A recent cross-sectional study of infertile women in Brazil identified celiac seropositivity in 10.3% of patients with unexplained infertility [16]. In our national survey, significantly fewer women with biopsy-proven CD who sought to become pregnant reported successful delivery of one or more pregnancies, suggesting that U.S. women with CD may have a significantly lower rate of fertility.
Our study also found a significantly higher prevalence of spontaneous abortions in the CD population compared to controls. Similarly, a cross-sectional study from India investigated the prevalence of undiagnosed CD by checking serologic markers in women who had experienced idiopathic recurrent abortion, unexplained stillbirths, unexplained infertility or idiopathic intrauterine growth restriction. The investigators found a statistically significant increased seroprevalence of IgA anti-tissue transglutaminase (tTG) antibody in the group with recurrent abortion, in the group with stillbirth, in the group with infertility, and in the group with intrauterine growth restriction, as compared to the control group [17]. The pathogenesis of spontaneous abortions in CD patients has been linked to vitamin and mineral deficiencies of zinc, selenium, iron and folate [18-21]. It has also been suggested that placental tTG may be bound by maternal antibody to tTG, which may adversely affect placental function [22,23].
Our results also show that most of the spontaneous abortions (85%) in women with CD occurred prior to initiation of a GFD. A case-control study by Ciacci found similar results when comparing women with undiagnosed CD to women with treated CD and found that undiagnosed women have an 8.9-relative risk of abortion and a 5.84-relative risk of low birth weight baby compared with treated patients. In a small population before-after study, the same investigators demonstrated that the GFD resulted in a 9.18-fold reduction in the abortion rate and a decrease in the prevalence of low birth weight babies from 29.4% to zero [24]. Martinelli et al previously aimed to determine the prevalence of undiagnosed CD in pregnant women and to determine the association with unfavorable pregnancy outcomes. After screening 845 pregnant women, 12 were found to have serologic and histologic evidence of CD. Seven of the 12 women experienced an unfavorable pregnancy outcome (breech presentation, pre-eclampsia, premature delivery, newborn morbidity, small for gestational age baby). They found that of the multiparous undiagnosed women, 4 of the 5 had experienced a prior miscarriage [25]. These same investigators later questioned their original findings in another cohort study from 2004 and demonstrated that while undiagnosed CD is frequent among pregnant women (roughly the same prevalence as the general population), there is no association with unfavorable outcomes of pregnancy [26]. Our large U.S. cohort study confirms the original Italian study and suggests that women with untreated CD are at an increased risk of pregnancy complications.
Our study has demonstrated that women with CD have a higher prevalence of preterm deliveries. Similar results were found in a large, multi-year, national cohort study by Ludvigsson in 2005, who examined the risk of adverse fetal outcomes in women with CD diagnosed prior to pregnancy and in women not diagnosed with CD until after delivery. They found that undiagnosed CD prior to pregnancy was associated with a statistically significant increased risk of intrauterine growth retardation, low birth weight (<2500 g), very low birth weight (<1500 g), and preterm birth [27]. Our results did not demonstrate an increased prevalence of cesarean section in women with CD. By comparison, a cohort study by Tata published in 2005 found the risks of cesarean section (odds ratio, 1.33; 95% confidence interval, 1.03-1.70) and miscarriage (rate ratio, 1.31; 95% confidence interval, 1.06-1.61) were moderately higher in women with CD, but risks of assisted birth, breech birth, pre-eclampsia, postpartum hemorrhage, ectopic pregnancy, stillbirth, and termination were similar. The authors of the study concluded the increased risk of cesarean section and miscarriage were more likely related to socioeconomic advantage of women with CD [28]. Since our study did not assess the socioeconomic status of the polled patients, a similar conclusion cannot be made.
Limitations of this study include the online nature of the questionnaire. Questionnaires in general are limiting as there may be recall bias, particularly when addressing specific aspects of CD. Due to a concern of possible recall bias and in order to keep the language of the survey at a patient level understanding, we did not ask patients for specific details of their celiac diagnosis such as Marsh score or which celiac serologies were tested. We included only patients who had a small bowel biopsy in order to increase the likelihood of including only patients with a correct diagnosis of CD. However, the comparison group may have included both women without CD as well as women with undiagnosed CD. Another limitation to the study is that we did not query patients regarding their race, the age of spontaneous abortions or other factors and comorbidities that could impact pregnancy outcomes. Furthermore, the fact the questionnaire was accessed on-line and via social media may have led to sample bias.
In conclusion, this is the largest reported study, performed in the U.S., which examined the reproductive health of women with CD. Compared with women in the general population, women with CD have increased spontaneous abortions and preterm delivery and fewer successful pregnancies. With the increase in the prevalence of CD [29] over the last several decades and the advent of celiac centers in the U.S. and across the world, the fertility experience of these patients is an important aspect of women’s health that needs increased awareness among patients and physicians. Though there are conflicting data regarding the relative risk of infertility and other reproductive complications, undiagnosed CD should be considered as an etiology in patients with recurrent complications of pregnancy, and these women should be screened for serologic markers. Finally, given that CD affects 1% of the American population and a large population of undiagnosed women with CD likely exists, women’s health specialist need to be aware of the pregnancy complication faced by women with untreated CD and have a low threshold for testing high risk patients.
Summary Box.
What is already known:
The prevalence of celiac disease is increasing
Celiac disease is associated with a number of extraintestinal manifestations
What the new findings are:
Women with untreated celiac disease may have increases in: a) spontaneous abortion; b) premature delivery; and c) earlier age of menarche
Biography
Thomas Jefferson University Hospital, Philadelphia; Cleveland Clinic, Cleveland; Jefferson Medical College, Philadelphia, USA
Footnotes
Conflict of Interest: None
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.AGA Institute. AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006;131:1977. doi: 10.1053/j.gastro.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 4.Green PH, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126–131. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 5.Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr. 2014;168:272–8.5. doi: 10.1001/jamapediatrics.2013.3858. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;20:2419–2426. doi: 10.1056/NEJMcp1113994. [DOI] [PubMed] [Google Scholar]
- 7.Morris JS, Adjukiewicz AB, Read AE. Coeliac infertility: an indication for dietary gluten restriction. Lancet. 1970;1:213. doi: 10.1016/s0140-6736(70)90572-6. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson R, Holmes K, Cooke WT. Coeliac disease, fertility, and pregnancy. Scand J Gastroenterol. 1982;17:65–68. doi: 10.3109/00365528209181045. [DOI] [PubMed] [Google Scholar]
- 9.Choi JM, Lebwohl B, Wang J, et al. Increased prevalence of celiac disease in patients with unexplained infertility in the United States. J Reprod Med. 2011;56:199–203. [PMC free article] [PubMed] [Google Scholar]
- 10.Sher KS, Mayberry JF. Female fertility, obstetric and gynaecological history in coeliac disease: a case control study. Digestion. 1994;55:243–246. doi: 10.1159/000201155. [DOI] [PubMed] [Google Scholar]
- 11.Jackson JE, Rosen M, McLean T, et al. Prevalence of celiac disease in a cohort of women with unexplained infertility. Fertil Steril. 2008;89:1002–1004. doi: 10.1016/j.fertnstert.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 12.Zugna D, Richiardi L, Akre O, et al. A nationwide population-based study to determine whether coeliac disease is associated with infertility. Gut. 2010;59:1471–1475. doi: 10.1136/gut.2010.219030. [DOI] [PubMed] [Google Scholar]
- 13.Nenna R, Mennini M, Petrarca L, et al. Immediate effect on fertility of a gluten-free diet in women with untreated coeliac disease. Gut. 2011;60:1023–1024. doi: 10.1136/gut.2010.232892. [DOI] [PubMed] [Google Scholar]
- 14.Meloni GF, Dessole S, Vargiu N, et al. The prevalence of coeliac disease in infertility. Hum Reprod. 1999;14:2759–2761. doi: 10.1093/humrep/14.11.2759. [DOI] [PubMed] [Google Scholar]
- 15.Collin P, Vilska S, Heinonen PK, et al. Infertility and coeliac disease. Gut. 1996;39:382–384. doi: 10.1136/gut.39.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machado AP, Silva LR, Zausner B, et al. Undiagnosed celiac disease in women with infertility. J Reprod Med. 2013;58:61–66. [PubMed] [Google Scholar]
- 17.Kumar A, Meena M, Begum N, et al. Latent celiac disease in reproductive performance of women. Fertil Steril. 2011;95:922–927. doi: 10.1016/j.fertnstert.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Bedwal RS, Bahuguna A. Zinc, copper and selenium in reproduction. Experientia. 1994;50:626–640. doi: 10.1007/BF01952862. [DOI] [PubMed] [Google Scholar]
- 19.Bougle D, Proust A. Iron and zinc supplementation during pregnancy: interactions and requirements. Contracept Fertil Steril. 1999;27:537–543. [PubMed] [Google Scholar]
- 20.Singhal N, Alam S, Sherwani R, et al. Serum zinc levels in celiac disease. Indian Pediatr. 2008;45:319–321. [PubMed] [Google Scholar]
- 21.Hirson C. Coeliac infertility-folic-acid therapy. Lancet. 1970;1:412. doi: 10.1016/s0140-6736(70)91537-0. [DOI] [PubMed] [Google Scholar]
- 22.Anjum N, Baker PN, Robinson NJ, et al. Maternal celiac disease autoantibodies bind directly to syncytiotrophoblast and inhibit placental tissue transglutaminase activity. Reprod Biol Endocrinol. 2009;7:16. doi: 10.1186/1477-7827-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Simone N, Silano M, Castellani R, et al. Anti-tissue transglutaminase antibodies from celiac patients are responsible for trophoblast damage via apoptosis in vitro. Am J Gastroenterol. 2010;105:2254–2261. doi: 10.1038/ajg.2010.233. [DOI] [PubMed] [Google Scholar]
- 24.Ciacci C, Cirillo M, Auriemma G, et al. Celiac disease and pregnancy outcome. Am J Gastroenterol. 1996;91:718–722. [PubMed] [Google Scholar]
- 25.Martinelli P, Troncone R, Paparo F. Coeliac disease and unfavourable outcome of pregnancy. Gut. 2000;46:332–335. doi: 10.1136/gut.46.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greco L, Veneziano A, Di Donato L. Undiagnosed coeliac disease does not appear to be associated with unfavourable outcome of pregnancy. Gut. 2004;53:149–151. doi: 10.1136/gut.53.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterol. 2005;129:454–463. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 28.Tata LJ, Card TR, Logan RF. Fertility and pregnancy-related events in women with celiac disease: a population-based cohort study. Gastroenterology. 2005;128:849–855. doi: 10.1053/j.gastro.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248–1255. doi: 10.1038/ajg.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]


