Abstract
Background
Tumor necrosis factor (TNF)-α inhibitors increase the risk of tuberculosis (TB). The objective of the present study was to determine the rate of active TB infection in inflammatory bowel disease (IBD) patients receiving anti-TNF therapy and to determine the results of their latent TB infection (LTBI) screening tests during the follow up.
Methods
This is a retrospective observational study of IBD patients receiving anti-TNF therapy. Tuberculin skin test (TST), interferon-γ release assay (IGRA), and chest radiography were used to determine LTBI. Active TB infection rate during anti-TNF treatment was determined.
Results
Seventy-six IBD patients (25 with ulcerative colitis, 51 with Crohn’s disease; 53 male; mean age 42.0±12.4 years) were included. Forty-four (57.9%) patients received infliximab and 32 (42.1%) adalimumab. Their median duration of anti-TNF therapy was 15 months. Forty-five (59.2%) patients had LTBI and received isoniazid (INH) prophylaxis. During the follow-up period, active TB was identified in 3 (4.7%) patients who were not receiving INH prophylaxis. There was a moderate concordance between the TST and the IGRA (kappa coefficient 0.44, 95% CI 0.24-0.76). Patients with or without immunosuppressive therapy did not differ significantly with respect to TST (P=0.318) and IGRA (P=0.157).
Conclusion
IBD patients receiving anti-TNF therapy and prophylactic INH have a decreased risk of developing active TB infection. However, despite LTBI screening, the risk of developing active TB infection persists.
Keywords: Inflammatory bowel disease, anti-TNF therapy, tuberculosis, interferon-γ release assay, tuberculin skin test
Introduction
Anti-tumor necrosis factor agents are increasingly being used in conditions with a pathogenesis involving TNF-α, particularly rheumatic diseases and inflammatory bowel disease (IBD) [1]. Tuberculosis (TB) infection remains a major health problem in Asian and African countries, and its incidence has risen in parallel to the increasing incidence of HIV in the developed countries. Immunosuppressive treatments and anti-TNF agents have also contributed to the increased incidence of TB [2,3].
TNF-α is the key cytokine involved in host defense against TB playing a major role in the formation and durability of the granulomas that prevent the dissemination of TB bacilli. Anti-TNF treatment causes impairment to the structural integrity of granulomas which control the TB bacilli, and leads to the dissemination of the TB bacilli and TB reactivation [4]. Because of this, different algorithms and prophylactic treatment approaches are used to prevent TB reactivation caused by anti-TNF treatment [5-7].
The methods used to investigate latent TB infection (LTBI) prior to anti-TNF treatment vary geographically due to the prevalence of TB infection, the popularity of the Bacillus Calmette-Guérin (BCG) vaccination, and financial factors [8,9]. Tuberculin skin test (TST), chest x-ray, and interferon-γ release assay (IGRA) are the most commonly used LTBI screening tests [10].
According to the 2013 World Health Organization (WHO) report, in Turkey TB prevalence and incidence were 23/10000 and 22/10000 respectively. The WHO considers Turkey to belong among the Eastern European countries with respect to TB epidemiology. Within the context of the “plan to stop TB” project, Turkey is among the “18 high-priority countries in the WHO European Region” [11,12]. In our country, BCG vaccination is included in the Ministry of Health routine vaccination schedule.
This study aimed to determine TB rate in IBD patients receiving anti-TNF therapy and to determine the follow-up results of LTBI screening tests.
Patients and methods
Patient selection
This study included patients with Crohn’s disease (CD) and ulcerative colitis (UC) who received anti-TNF (infliximab, adalimumab) therapy between January 2007 and January 2014. Disease duration, IBD location, CD behavior pattern, anti-TNF agent, treatment duration, concomitant immunosuppressive treatment, and prophylactic isoniazid (INH) treatment (if any) were recorded.
LTBI screening tests
Purified protein derivative TST, IGRA, chest radiography, and/or chest tomography were used as screening methods for LTBI. TST was performed on the volar surface of the forearm using the intradermal Mantoux method, and the result was recorded after 72 h. An induration transverse diameter of ≥5 mm was considered as positive. TST anergic patients had the test repeated a week later. IGRA was studied using the QuantiFERON-TB Gold In-Tube. The blood sample used for the IGRA was collected before performing the TST. Evidence of LTBI included fibrotic changes in chest radiography, calcification areas larger than 5 mm, pleural thickening, and linear opacities.
Study design
Patients were considered as having LTBI if TST was ≥5 mm, IGRA was positive, or fibrotic changes were found in chest radiography. These patients were given prophylactic INH 300 mg/day for 9 months, one month of which was prior to onset of anti-TNF therapy. Over the course of the anti-TNF therapy, the occurrence of pulmonary and extra-pulmonary active TB infection was monitored clinically, radiologically, and microbiologically at 2-month intervals.
Patients who received azathioprine (for at least 3 months) or systemic steroids (a daily dose equivalent to ≥20 mg of prednisolone for ≥2 weeks) during LTBI screening were considered to be within the group of patients who received immunosuppressive therapy.
Patients who received INH for less than 9 months or those who did not use it regularly were considered to be patients without previous prophylactic treatment. Patients with previous TB history and those who received anti-TB treatment at sufficient duration and doses were not given INH prophylaxis.
The Ethics Committee of the Katip Çelebi University Faculty of Medicine, Izmir, Turkey approved this study.
Statistical analysis
Descriptive statistics were presented as numbers and percentages for categorical variables, and as mean±standard deviation or as medians and interquartile ranges for continuous variables. Fisher’s exact test was used to compare categorical data. Kappa statistics between IGRA and TST were used for the concordance analysis. Relationship analyses between LTBI screening tests and immunosuppressive treatment were estimated by chi-square test. All statistical analyses were performed using the SPSS 16.0 statistical package program and P values <0.05 were considered statistically significant.
Results
Patient characteristics
A total of 76 IBD patients (51 with CD, 25 with UC) were included. Mean age was 42.0±12.4 years, while 53 (69.7%) were male and 23 (30.3%) were female. Mean duration of IBD was 5 years. The demographic data of the patients evaluated in this study are presented in Table 1.
Table 1.
Patient characteristics

IBD treatment and INH prophylaxis data
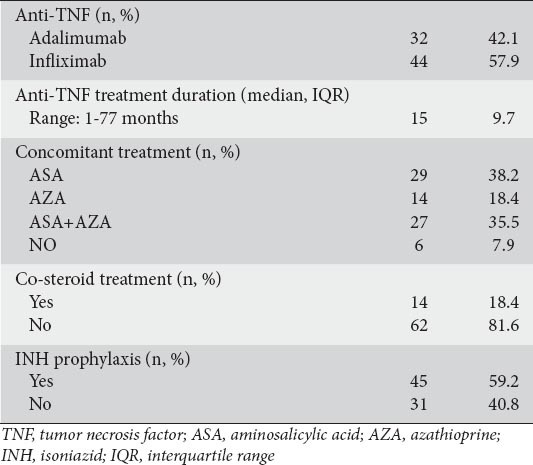
Forty-four (57.9%) of the patients were on infliximab and 32 (42.1%) on adalimumab. The median anti-TNF-α therapy duration was 15 months (range: 1-77). Forty-five (59.2%) patients, considered to have LTBI, were given INH prophylaxis. Only one of the patients who received INH prophylaxis had mildly elevated hepatic enzymes, but the levels returned to normal without requiring a reduction in INH dose. Data on agents co-administered with anti-TNF therapy are shown in Table 2.
Table 2.
Treatment characteristics
Results of LTBI screening tests
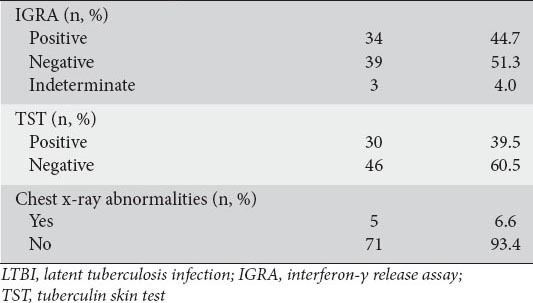
IGRA was positive in 34 (44.7%) patients, negative in 39 (51.3%), and indefinite in 3 (4%). TST was positive in 30 (39.5%) patients and negative in 46 (60.5%). Five (6.6%) patients had abnormal chest radiography. The results of the LTBI screenings are presented in Table 3.
Table 3.
LTBI screening tests results
IGRA and TST concordance analysis results
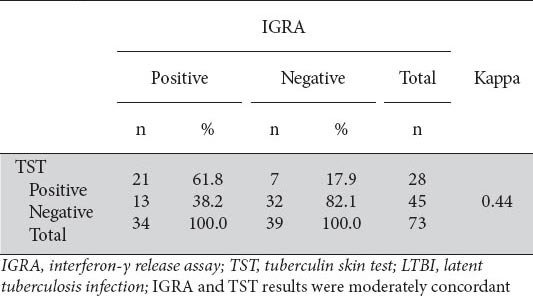
Twenty-one patients (27.6%) had TST (+)/IGRA (+), 13 (17.1%) had TST (-)/IGRA (+), 7 (9.2%) had TST (+)/IGRA (-), and 32 (42.1%) had TST (-)/IGRA (-) was. The IGRA and TST concordance analysis yielded a kappa coefficient of 0.44 (95%CI: 0.24-0.76), which indicated a moderate concordance between the two. Data used to evaluate the concordance between IGRA and TST are presented in Table 4.
Table 4.
Agreement between IGRA and TST
IGRA and TST results in patients with abnormal chest radiography
Five patients had abnormal chest radiography. Two of these patients were positive for both IGRA and TST, 2 were positive for TST only, and one was negative for both TST and IGRA.
Effect of immunosuppressive therapy on LTBI screening tests
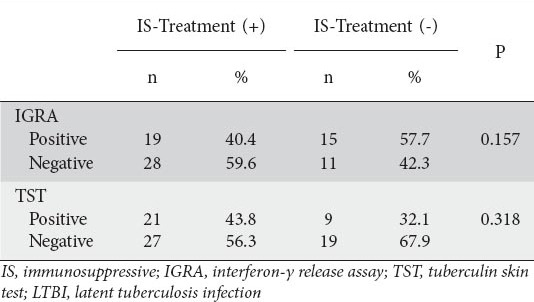
Whether patients did or did not receive immunosuppressive therapy at the time of the LTBI screening tests did not significantly affect the TST and IGRA results (P=0.318 for TST, and P=0.157 for IGRA). Data used to determine the effect of concomitant immunosuppressive therapy on LTBI screening tests are presented in Table 5.
Table 5.
Impact of immunosuppressive therapy on LTBI screening tests
Characteristics of patients developing active TB infection during anti-TNF-α therapy
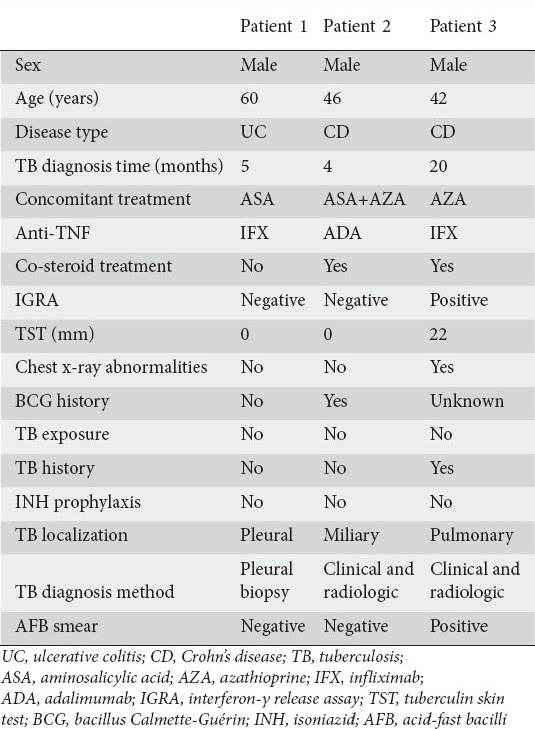
Active TB infection was detected in 3 (4.7%) patients receiving anti-TNF therapy (patients 1, 2, and 3). Patients 1 and 2 had negative IGRA and TST results, and no abnormalities were detected by pulmonary screening methods. These patients were not given prophylactic treatment with INH. Patient 1 developed pleural TB and patient 2 developed miliary TB. Active TB infection was detected at months 4 and 5 of the anti-TNF therapy in these patients, respectively. Patient 3 had a history of pulmonary TB (approximately 10 years before anti-TNF-α therapy), and received anti-TB treatment for 9 months. IGRA and TST tests for patient 3 were positive prior to treatment. However, because this patient had received treatment for TB with sufficient dose and duration, prophylactic INH was not administered. Active pulmonary TB was detected in month 20 of anti-TNF therapy in patient 3.
None of the patients receiving prophylactic INH developed active TB infection. Data for the patients who developed evidence of active TB during anti-TNF therapy are presented in Table 6.
Table 6.
Characteristics of patients who developed TB while receiving anti-TNF treatment
Discussion
The changes caused by anti-TNF therapy during the course of TB infection and the increasing prevalence of active TB infections are significant concerns for clinicians treating the conditions for which anti-TNF therapy is used (e.g., immune-mediated inflammatory diseases).
Currently, all guidelines indicate that at-risk patients should undergo screening for LTBI and should undergo prophylactic treatment approaches, if necessary, prior to commencing treatment with TNF-α inhibitors (5,6). However, the diverse epidemiological characteristics of TB infection in different geographical regions preclude a consensus regarding these guidelines. In addition, the occurrence of anti-TNF-associated TB infections cannot be completely prevented despite the LTBI screenings and prophylactic TB treatments recommended by national organizations [13,14].
Since there is no “gold standard” for LTBI screening, the capabilities of the individual LTBI screening tests cannot be precisely evaluated [15]. TST is the oldest and most common test used in LTBI screening. However, TST has a low specificity and often gives false-positive results in BCG recipients. In addition, it yields positivity in patients with non-TB mycobacteria [16]. Furthermore, false-negative results are also quite common among individuals receiving immunosuppressive therapy [17].
The recently developed IGRA method measures the amount of interferon-γ released from T cells stimulated by TB-specific antigens in vitro. IGRA is unaffected by the BCG vaccine and non-TB mycobacteria, making it a superior method for LTBI screening [18,19]. However, major disadvantages of this method include its high cost and requirement of laboratory substructure and equipment. In addition, IGRA may be negatively affected by immunosuppressive therapy, although not to the same extent as TST [20].
In the current study, the LTBI rate was approximately 60%. This high rate may be due to the high prevalence of TB in our country and/or false-positive TST results caused by routine BCG vaccination. Although the prevalence of LTBI in our country is not precisely known, it was reported to be 67.2% by Hanta et al and 83% by Çağatay et al [21,22].
Some published studies indicate that immunosuppressive therapy does not result in statistically significant differences in tests used in LTBI screenings [21,22]. However, other studies have shown that the results of both TST and IGRA tests are negatively affected by immunosuppressive therapy [23,24]. In our current study, TST and IGRA results were not significantly different between patients who did or did not receive immunosuppressive therapy. Data suggesting that the LTBI screening tests were affected by immunosuppressive status were obtained from studies performed with patients with HIV and TB co-infection [25]. However, as opposed to patients with HIV co-infection, another study reported that IBD patients receiving immunosuppressive therapy had CD4 cell counts above normal ranges [26].
There are contradictory data regarding the concordance between the TST and IGRA tests. Most of the studies suggest a poor concordance between these two tests [27,28]. İnanç et al (kappa=0.29) and Çobanoğlu et al (kappa=0.18) reported that the concordance between the IGRA and TST tests is not good in our country [29,30]. However, in their meta-analysis including a total of 9 studies and 1309 IBD patients, Shahidi et al reported a moderate to strong concordance between IGRA and TST [20]. In our current study, there was a moderate concordance between IGRA and TST (kappa=0.44). The variations in results from studies evaluating the concordance between IGRA and TST may be due to the different immunosuppressive therapy and BCG vaccination profiles of the patient groups included in the studies.
IGRA and TST results can change during the anti-TNF treatment, making this an important issue in TB screening. Papay and Bermejo declared that in patients using anti-TNF TST might undergo the process of conversion or revesion whereas IGRA might only reverse under INH prophylaxis [31,32]. However we have not performed TB testing during the anti-TNF treatment in our study.
Although LTBI treatment reduces the risk of active TB infection during anti-TNF therapy, active TB infections may develop, despite INH prophylaxis [14]. In our current study, none of the patients receiving prophylactic INH therapy developed active TB infection during anti-TNF therapy (within a median period of 15 months).
Three (4.7%) of the patients who did not receive prophylactic INH therapy developed active TB infection during anti-TNF therapy. This rate is higher than those previously reported from studies conducted in our country and in European countries, which have relatively lower TB prevalence [33,34]. The high rate of active TB infection in our current series could be due to false-negative LTBI results, associated with the immunosuppressive treatments (azathioprine and steroid) used by two patients who developed active TB infection at the time of the LTBI screening test.
We presume that the patient with a previous history of TB had reactivation. It does not seem possible to differentiate between reactivation or newly acquired TB in the other two patients in our study. It is assumed that most cases of TB in patients on anti-TNF-α are due to reactivation of LTBI. However, patients living in TB-endemic regions or with other high-risk exposure (e.g., active TB in the household) could also be at increased risk of newly acquired infection. In addition, in a study, it was suggested that some of the increased risk of TB in individuals in non-TB-endemic regions may be due to a new infection [35]. This hypothesis needs confirmation by studies using DNA typing. DNA fingerprinting to differentiate between tuberculosis relapse and reinfection is a newly used technique which might be available in clinical practice in the near future [36].
Anti-TNF therapy was discontinued as soon as active TB infection was detected in all 3 of our patients. The UC patient in whom pleural TB was detected was at month 4 of anti-TB therapy, and was using only oral and topical mesalazine for ulcerative colitis. The CD patient who developed miliary TB used only oral mesalazine up to month 7 of anti-TB therapy, and was in remission. However, systemic steroid and azathioprine were added to this patient’s treatment due to the worsening of CD at month 7 of anti-TB therapy. The patient’s anti-TB therapy was completed due to a previous history of TB. In addition, this patient used only 5-aminosalicylic acid during the first 3 months of anti-TB therapy, and the azathioprine was added at month 7.
Interestingly, the patients in our study who developed active TB infection and started receiving conventional anti-TB therapy had mild UC and CD disease activity, even though immunosuppressive therapy for IBD was discontinued during early anti-TB therapy.
Patients who did or did not receive the BCG vaccine could not be evaluated separately in our study because BCG vaccination data was not known. However, BCG vaccination is recommended as a part of the routine vaccination schedule of the Turkish Ministry of Health. In addition, since the mean age of the patients in our study was 42 years, the positive effect of BCG on TST may have been diminished. Due to our study protocol, LTBI screening tests were performed for patients with a previous history of TB who developed active TB infection.
In conclusion, LTBI screenings and prophylactic INH prior to anti-TNF therapy reduces the risk of active TB infection. However, immunosuppressive therapy during LTBI screening decreases the sensitivity of the tests, including TST and IGRA. Therefore, in a best-case scenario, LTBI screening should be performed before the onset of immunosuppressive therapy.
Summary Box.
What is already known:
Anti-tumor necrosis factor (TNF) therapy increases the risk of tuberculosis (TB) infection
Screening for latent TB infection (LTBI) is recommended prior to anti-TNF therapy
Isoniazid (INH) prophylaxis prevents TB infection during anti-TNF therapy
What the new findings are:
Immunosuppressive therapy does not only interfere with tuberculin skin test, but may also affect interferon-γ release assay results
TB reactivation can be detected in patients with negative LTBI screening tests
LTBI screening tests can be recommended to be performed prior to the initiation of immunosuppressive therapy, conceivably in the first visit of the patient
Biography
Katip Çelebi University, Atatürk Training and Research Hospital, İzmir, Turkey
Footnotes
Conflict of Interest: None
References
- 1.Jo KW, Hong Y, Jung YJ, et al. Incidence of tuberculosis among anti-tumor necrosis factor users in patients with a previous history of tuberculosis. Respir Med. 2013;107:1797–802. doi: 10.1016/j.rmed.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD, Meintjes G, McIlleron H, Harries AD, Wood R. Management of HIV-associated tuberculosis in resource-limited settings: a state-of-the-art review. BMC Med. 2013;11:253. doi: 10.1186/1741-7015-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre-Cisneros J, Doblas A, Aguado JM, et al. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis. 2009;48:1657–1665. doi: 10.1086/599035. [DOI] [PubMed] [Google Scholar]
- 4.Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(Suppl 3):S189–S193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 5.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 6.British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005;60:800–805. doi: 10.1136/thx.2005.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Tuberculosis associated with blocking agents against tumor necrosis factoralpha: California, 2002-2003. MMWR Morb Mortal Wkly Rep. 2004;53:683–686. [PubMed] [Google Scholar]
- 8.Lawrance IC, Radford-Smith GL, Bampton PA, et al. Serious infections in patients with inflammatory bowel disease receiving anti-tumor-necrosis-factor-alpha therapy: an Australian and New Zealand experience. J Gastroenterol Hepatol. 2010;25:1732–1738. doi: 10.1111/j.1440-1746.2010.06407.x. [DOI] [PubMed] [Google Scholar]
- 9.Navarra SV, Tang B, Lu L, et al. Risk of tuberculosis with anti-tumor necrosis factor-α therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis. 2014;17:291–298. doi: 10.1111/1756-185X.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solovic I, Sester M, Gomez-Reino JJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010;36:1185–1206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO). Global tuberculosis report 2013. http://apps.who.int/iris/bitstream/10665 /91355/1/9789241564656_eng.pdf .
- 12.Plan to Stop TB in 18 High-priority Countries in the WHO European Region 2007–2015. http://www.euro.who.int/__data/assets/pdf_file/0005/68180/E91049.pdf .
- 13.Jauregui-Amezaga A, Turon F, Ordás I, et al. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013;7:208–212. doi: 10.1016/j.crohns.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int J Tuberc Lung Dis. 2006;10:1127–1132. [PubMed] [Google Scholar]
- 15.Cattamanchi A, Smith R, Steingart KR, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56:230–238. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tissot F, Zanetti G, Francioli P, Zellweger JP, Zysset F. Influence of bacille Calmette-Guérin vaccination on size of tuberculin skin test reaction: to whatsize? Clin Infect Dis. 2005;40:211–217. doi: 10.1086/426434. [DOI] [PubMed] [Google Scholar]
- 17.Mow WS, Abreu-Martin MT, Papadakis KA, Pitchon HE, Targan SR, Vasiliauskas EA. High incidence of anergy in inflammatory bowel disease patients limits the usefulness of PPD screening before infliximab therapy. Clin Gastroenterol Hepatol. 2004;2:309–313. doi: 10.1016/s1542-3565(04)00060-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Choi HJ, Park IN, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006;28:24–30. doi: 10.1183/09031936.06.00016906. [DOI] [PubMed] [Google Scholar]
- 19.Richeldi L. An update on the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2006;174:736–742. doi: 10.1164/rccm.200509-1516PP. [DOI] [PubMed] [Google Scholar]
- 20.Shahidi N, Fu YT, Qian H, Bressler B. Performance of interferon-gamma release assays in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18:2034–2042. doi: 10.1002/ibd.22901. [DOI] [PubMed] [Google Scholar]
- 21.Hanta I, Ozbek S, Kuleci S, Kocabas A. The evaluation of latent tuberculosis in rheumatologic diseases for anti-TNF therapy: experience with 192 patients. Clin Rheumatol. 2008;27:1083–1086. doi: 10.1007/s10067-008-0867-3. [DOI] [PubMed] [Google Scholar]
- 22.Cagatay T, Aydin M, Sunmez S, et al. Follow-up results of 702 patients receiving tumor necrosis factor-αantagonists and evaluation of risk of tuberculosis. Rheumatol Int. 2010;30:1459–1463. doi: 10.1007/s00296-009-1170-6. [DOI] [PubMed] [Google Scholar]
- 23.Papay P, Eser A, Winkler S, et al. Factors impacting the results of interferon-γ release assay and tuberculin skin test in routine screening for latent tuberculosis in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:84–90. doi: 10.1002/ibd.21427. [DOI] [PubMed] [Google Scholar]
- 24.Schoepfer AM, Flogerzi B, Fallegger S, et al. Comparison of interferon-gamma release assay versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease. Am J Gastroenterol. 2008;103:2799–27806. doi: 10.1111/j.1572-0241.2008.02050.x. [DOI] [PubMed] [Google Scholar]
- 25.Oni T, Gideon HP, Bangani N, et al. Risk factors associated with indeterminate gamma interferon responses in the assessment of latent tuberculosis infection in a high-incidence environment. Clin Vaccine Immunol. 2012;19:1243–1247. doi: 10.1128/CVI.00166-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debeuckelaere C, De Munter P, Van Bleyenbergh P, et al. Tuberculosis infection following anti-TNF therapy in inflammatory bowel disease, despite negative screening. J Crohns Colitis. 2014;8:550–557. doi: 10.1016/j.crohns.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Matulis G, Jüni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 28.Behar SM, Shin DS, Maier A, Coblyn J, Helfgott S, Weinblatt ME. Use of the T-SPOT. TB assay to detect latent tuberculosis infection among rheumatic disease patients on immunosuppressive therapy. J Rheumatol. 2009;36:546–551. doi: 10.3899/jrheum.080854. [DOI] [PubMed] [Google Scholar]
- 29.Inanc N, Aydin SZ, Karakurt S, Atagunduz P, Yavuz S, Direskeneli H. Agreement between Quantiferon-TB gold test and tuberculin skin test in the identification of latent tuberculosis infection in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2009;36:2675–2681. doi: 10.3899/jrheum.090268. [DOI] [PubMed] [Google Scholar]
- 30.Cobanoglu N, Ozcelik U, Kalyoncu U, et al. Interferon-gamma assays for the diagnosis of tuberculosis infection before using tumour necrosis factor-alpha blockers. Int J Tuberc Lung Dis. 2007;11:1177–1182. [PubMed] [Google Scholar]
- 31.Papay P, Primas C, Eser A, et al. Retesting for latent tuberculosis in patients with inflammatory bowel disease treated with TNF-α inhibitors. Aliment Pharmacol Ther. 2012;36:858–865. doi: 10.1111/apt.12037. [DOI] [PubMed] [Google Scholar]
- 32.Bermejo F, Algaba A, Chaparro M, et al. How frequently do tuberculosis screening tests convert in inflammatory bowel disease patients on anti-tumour necrosis factor-alpha? A pilot study. Dig Liver Dis. 2013;45:733–737. doi: 10.1016/j.dld.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Kurt OK, Kurt B, Talay F, et al. Intermediate to long-term follow-up results of INH chemoprophylaxis prior to anti-TNF-alpha therapy in a high-risk area for tuberculosis. Wien Klin Wochenschr. 2013;125:616–620. doi: 10.1007/s00508-013-0417-0. [DOI] [PubMed] [Google Scholar]
- 34.Elbek O, Uyar M, Aydin N, et al. Increased risk of tuberculosis in patients treated with antitumor necrosis factor alpha. Clin Rheumatol. 2009;28:421–426. doi: 10.1007/s10067-008-1067-x. [DOI] [PubMed] [Google Scholar]
- 35.Wallis RS. Mathematical modeling of the cause of tuberculosis during tumor necrosis factor blockade. Arthritis Rheum. 2008;58:947–952. doi: 10.1002/art.23285. [DOI] [PubMed] [Google Scholar]
- 36.Marx FM, Dunbar R, Enarson DA, et al. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin Infect Dis. 2014;58:1676–1683. doi: 10.1093/cid/ciu186. [DOI] [PubMed] [Google Scholar]


