Abstract
Background
Crohn’s disease (CD) activity index (CDAI) is still widely used for monitoring clinical activity in CD patients, but is of little value as indicator of persistent inflammation in symptomless patients. Fecal calprotectin levels ≥150 µg/g are strongly indicative of endoscopically and/or histologically active disease. Our aim was to study, in a large cohort of CD patients, the relationship between CDAI and fecal calprotectin levels.
Methods
CDAI and fecal calprotectin levels were evaluated in consecutive patients from a CD outpatient clinic.
Results
We enrolled 193 CD patients, of whom 38% with CDAI <150 had a calprotectin value ≥150 µg/g, suggestive of active disease. A logistic regression model showed that for CDAI levels between 100 and 150, the estimated logistic probability of calprotectin ≥150 µg/g increased progressively to 76%, reaching 94% where disease activity was localized in the colon. With a CDAI cut-off >120, we found a high diagnostic accuracy of 72%, with 88% specificity and 50% sensitivity (positive predictive value: 76%, negative predictive value: 71%) to identify a calprotectin value ≥150 µg/g.
Conclusion
CDAI scores between 100 and 150 display an acceptable ability to quantify the risk of persistent inflammation as expressed by the high calprotectin level.
Keywords: Crohn’s disease, Crohn’s disease activity index, fecal calprotectin, inflammatory bowel diseases
Introduction
Crohn’s disease (CD) activity index (CDAI), first described by Best et al in 1976 [1], has been the gold standard for monitoring clinical activity in CD patients and for defining clinical relapses in trials testing new drugs [2]. Attempts have been made to explore the potential utility of the CDAI in reflecting the endoscopic appearance of disease activity [3,4], but the index is reported to be of little use as an indicator of persistent inflammation in symptomless patients [5,6].
Nowadays, the new endpoint for medical therapy in CD patients is not only the improvement in symptoms and/or the induction of clinical remission but also the achievement of mucosal healing or “deep” remission to prevent clinical evolution of the disease and to change the CD “history” of cumulative damage, thereby preventing disability [7].
Fecal calprotectin, a calcium-binding protein derived from neutrophils, monocytes and reactive macrophages [8], has been shown to be a good marker of subclinical, persistent mucosal inflammation in CD, demonstrating a positive linear correlation with both endoscopic appearance and histological activity in CD patients [9-11]. Although the debate on the most appropriate cut-off value is still ongoing, fecal calprotectin level ≥150 µg/g seems appropriate to identify endoscopically evident, active disease and to define a subgroup of patients in clinical remission, but with active disease and prone to clinical relapses [12,13].
Previous small series of CD patients [4,9,14] showed that, when CDAI ≥150 (i.e. clinically active disease), fecal calprotectin values are high, but in more than 50% of patients in clinical remission according to their CDAI score (i.e. CDAI <150), fecal calprotectin remains elevated (>150 µg/g), reflecting the presence of an ongoing, endoscopically and histologically evident inflammation.
We evaluated a large cohort of CD patients attending our CD outpatient clinic, determining the CDAI and fecal calprotectin levels at the same visit, aiming to show a possible relationship between these two variables.
Patients and methods
Study population
In this study, carried out between January and December 2013, we enrolled consecutive CD patients attending an outpatient clinic in accordance with the following inclusion criteria: age from 16 to 89 years, and CD diagnosed at least 6 months previously. Exclusion criteria were patients with overlapping infectious enterocolitis from Salmonella, Shigella, Campylobacter, Clostridium difficile, and Cytomegalovirus, patients with a pouch, patients with a colostomy, and patients with known neoplasia; non-steroid anti-inflammatory drug use was not allowed. The study was carried out in accordance with the Helsinki Declaration.
Sample collection
During the same visit blood and feces samples were collected. The fecal sample was stored in a refrigerator at 2-8° C for a maximum of 48 h before sending it to the central laboratory of Sant’Orsola Hospital, Bologna, where calprotectin assay was performed using a quantitative enzyme-linked immunosorbent assay (ELISA). The blood sample was also analyzed at Sant’Orsola Hospital’s central laboratory to determine hematocrit.
Clinical scores
During the same visit, clinical parameters were recorded using validated questionnaires, which, together with the hematocrit value, were used to determine CDAI [1].
Statistical analysis
The Shapiro-Wilk and Pearson correlation tests were used to study the distribution and correlation of the two variables, fecal calprotectin and CDAI. Medians were analyzed using the Wilcoxon-Mann-Whitney U test. Logistic regression models were used for studying the dichotomous outcome variable (calprotectin) with CDAI as the continuous variable. The goodness of fit for the estimated logistic regression model was assessed with the Osius-Rojek test. Statistical significance was set at P<0.05 for all tests. A receiver operating characteristic (ROC) analysis was performed to evaluate the best CDAI cut-off value.
In the present paper, all analyses were performed using the R Core Team environment for statistical computing (R Foundation for Statistical Computing, 2013, Vienna, Austria).
Results
Patients’ characteristics
We enrolled 193 CD patients whose clinical characteristics are reported in Table 1. The median age of patients was 36.5 years (18-75 years) and the mean duration of disease was 7.1±10 years. Forty-five percent of patients were male and 30% were current smokers.
Table 1.
Clinical characteristics of Crohn’s disease patients
The median CDAI was 84.8 (range 32-401.6) and the median fecal calprotectin value was 112 (range 4-1231) µg/g.
Twenty-three patients had CDAI ≥150; the remaining 170 patients were in clinical remission (i.e. CDAI <150) with a mean remission time of 6±4.2 months (data not shown).
Frequency histograms for CDAI and fecal calprotectin (Fig. 1) give some indication of a departure from normal distribution that is confirmed by the Shapiro-Wilk normality test: W=0.9238, P <0.001 for CDAI data; W=0.761, P<0.001 for fecal calprotectin data.
Figure 1.

Distribution histograms of Crohn’s disease activity index (CDAI) (A) and fecal calprotectin (B) in the total population
CDAI and fecal calprotectin correlation and analysis
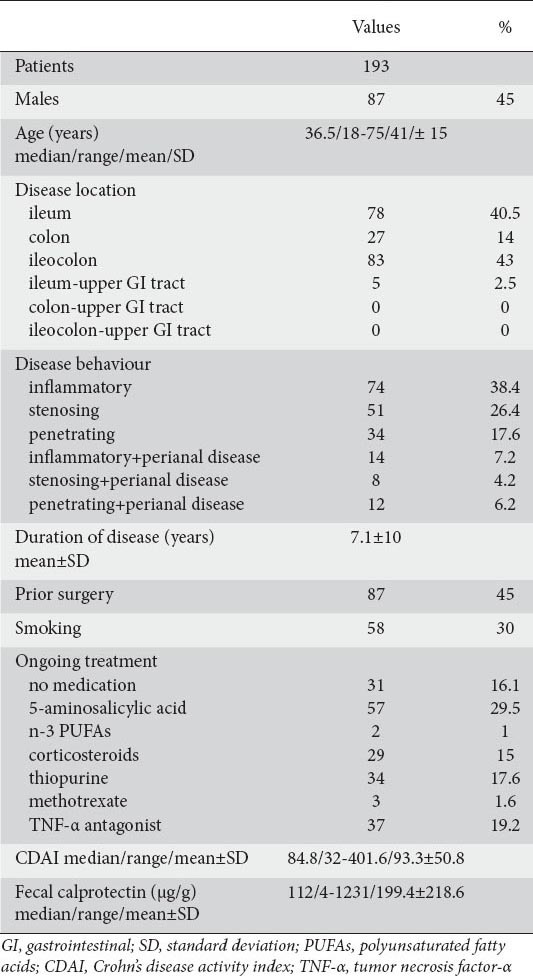
Comparison of the observed values of CDAI and calprotectin yielded a significant Pearson correlation coefficient r=0.5365 (P<0.001). However, the scatter diagram (Fig. 2) does not suggest the existence of a linear relationship between the two variables.
Figure 2.
Scatter plot of Crohn’s disease activity index (CDAI) and fecal calprotectin
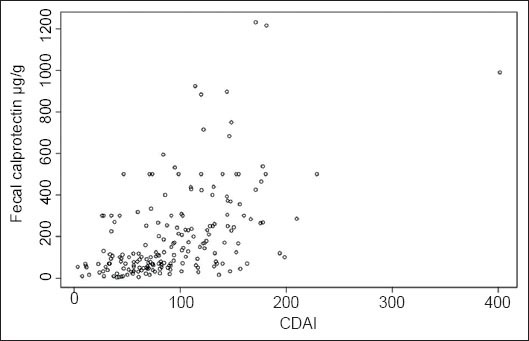
To better understand the relationship between CDAI and fecal calprotectin, we divided our sample into two groups according to the CDAI threshold value usually adopted in clinical practice to identify clinically active disease: Group 1 (n1=170) subjects with CDAI <150; Group 2 (n2=23) subjects with CDAI ≥150. The box plots for fecal calprotectin in the two groups are shown in Fig. 3.
Figure 3.
Box plots of fecal calprotectin for Crohn’s disease activity index (CDAI): cut-off 150
As expected, there was a highly significant difference between the median calprotectin levels for the two groups. The median for Group 1 was 100 µg/g and that for Group 2 was 300 µg/g, (Wilcoxon-Mann-Whitney test W=836.5 with a P<0.001).
In agreement with previous studies (4,9,14), in the group with CDAI <150, we found that 37.65% of patients had a fecal calprotectin ≥150 µg/g. In these patients, we can reasonably assume the presence of persistent symptomless inflammatory activity, confirming that a subgroup of patients with CDAI <150 has some degree of mucosal inflammation.
In view of the non-linear relationship between CDAI and fecal calprotectin, the next step of our analysis was to consider a logistic regression model and to focus on the CDAI values for the subgroup of 37.65% CD patients with calprotectin ≥150 µg/g. Adopting a fecal calprotectin threshold of 150 µg/g, we defined the variable “dichotomized calprotectin” that assumed the following values: 0 if subject had a value of fecal calprotectin <150 µg/g; 1 if the subject had fecal calprotectin ≥150 µg/g.
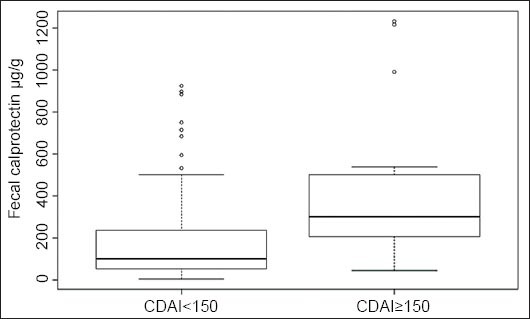
Fig. 4A depicts the values of dichotomized calprotectin (open circles) versus the values of CDAI. It also shows the sample proportions (solid circles) obtained by grouping the data into CDAI categories ([<25], [25-50], [50-75],….[375-400], [400-425]) and by computing for each category the proportions of subjects having fecal calprotectin ≥150 µg/g.
Figure 4.
(A) Scatter plot of dichotomized calprotectin versus Crohn’s disease activity index (CDAI) (open circles) and sample proportions for CDAI categories (solid circles). (B) Predicted event probability with 95% confidence limits
Fig. 4A suggests a positive relationship between CDAI and fecal calprotectin since it appears that calprotectin ≥150 µg/g tends to occur relatively more often at higher CDAI values. Therefore, we proceeded to fit a logistic regression model for the dichotomized calprotectin, with CDAI as a continuous variable. The results of parameters estimation are reported (Table 2).
Table 2.
Fitted logistic regression model for n=193 subjects classified by Crohn’s disease activity index (CDAI) and dichotomized calprotectin
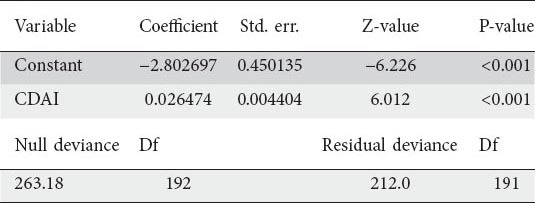
Fig. 4B depicts the estimated logistic curve (continuous black line), with the relative 95% confidence limits (dashed lines). Examining Fig. 4B, it can be seen that the estimated curve tends to follow the pattern of the sample proportions reasonably well (Osius-Rojek test, P=0.07).
Our model shows that when CDAI = 150 the estimated logistic probability that calprotectin ≥150 µg/g is 0.76285 (95% CI 0.64346-0.85149), meaning that, among patients with CDAI = 150, we estimate that 76.3% of them have calprotectin >150 µg/g. When CDAI = 120, the estimated logistic probability is 0.59246 (95% CI 0.49520-0.68298). When CDAI = 106 the estimated logistic probability is 0.5 (95% CI 0.41541-0.58459). When CDAI = 100 the estimated logistic probability is 0.46124 (95% CI 0.38072-0.54384).
According to estimates of the odds ratio (OR), for every 10 points increase in CDAI score, the odds of having calprotectin ≥150 µg/g increases by 30 % (OR 1.30309, 95% CI 1.19534-1.42055).
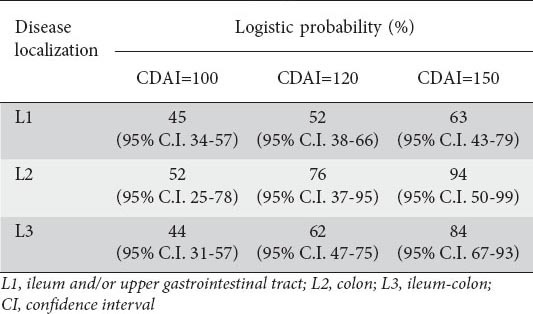
Consistent results were obtained by applying the same analysis model when patients are subdivided according to disease localization (ileum and/or upper gastrointestinal tract-L1, colon-L2, and ileum-colon-L3) (Table 3).
Table 3.
Estimated logistic probability of having calprotectin ≥150 µg/g according to Crohn’s disease activity index (CDAI) in different disease localization subgroups
The ROC curve for the fitted model is shown in Fig. 5 and the area under the ROC curve is c=0.793.
Figure 5.

Receiver operating characteristic curve for prediction of calprotectin ≥150 µg/g for the fitted logistic regression model containing Crohn’s disease activity index. Area under curve: 0.793
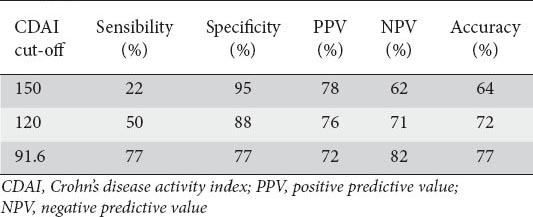
Table 4 shows for different CDAI cut-off values the corresponding sensitivity, specificity and accuracy estimates in predicting calprotectin levels consistent with the presence of mucosal inflammation. With a CDAI cut-off >120 we have a high diagnostic accuracy of 72%; 88% for specificity and 50% for sensitivity (positive predictive value: 76%, negative predictive value: 71%).
Table 4.
CDAI cut-off analysis based on fecal calprotectin value ≥150 μg/g
Discussion
To our knowledge, this is the first study that investigated the relationship between CDAI and fecal calprotectin in a large cohort of CD patients. A cut-off value of fecal calprotectin ≥150 µg/g was chosen as a marker for detecting “deep” active disease.
In previous studies [3,4,9,10,14], attempts were made to correlate CDAI and endoscopic activity but the authors’ conclusion was that relying on indices of clinical activity, such as the CDAI, means underestimating a significant proportion of CD patients with considerable inflammatory activity.
Furthermore, a subanalysis from the SONIC trial [15] suggests that half the patients under azathioprine and infliximab in clinical remission have endoscopic evidence of residual, active CD and that CDAI is not a reliable measure of this underlying inflammation.
CDAI has important limitations and confounding elements since approximately 40% of the index is derived from 3 subjective criteria such as diarrhea, abdominal pain and sense of well-being which account for 80% of the responsiveness to change [1]. These symptoms are also part of irritable bowel syndrome which often overlaps with the clinical picture of CD patients and affects the final CDAI score [16]. For these reasons, the interpretation of CDAI values <150 have never been investigated, though, intuitively, it seems that a CDAI of 40 reflects a very mild disease activity, whereas a value of 140 mirrors a more active disease.
Fecal calprotectin is an easily performed test that reflects the degree of mucosal inflammation [9-11]. Fecal calprotectin cut-off level of 150 µg/g seems to be the most widely adopted value corresponding to endoscopic and/or histological activity [12,13,17].
Sipponen [9] first showed, in a small series, that more of 50% of CD patients with CDAI <150 had high calprotectin levels, but further investigation on a possible relationship between these two variables was not made.
Data on the relationship between fecal calprotectin and CDAI are variable, with a coefficient of correlation ‘r’ ranging from 0.23 to 0.39 [3,9]. In our study, a better correlation between CDAI and fecal calprotectin was found (r = 0.54).
We herein demonstrated that a CDAI value from 100 to 150 corresponds to an increased probability of an associated high level of fecal calprotectin (i.e. ≥150 µg/g) and we developed a mathematical model analysis showing that the data support a new way for interpreting CDAI values <150 which, conventionally, define a CD patient as being in clinical remission.
In particular, the “grey area” of CDAI values over the range 100 to 150, conventionally an indicator of “clinically non-active” disease, is actually associated with an increasing probability of having symptomless mucosal inflammation, reaching 94% in patients with colonic localization of the disease when the CDAI is equal to 150.
To translate numbers into clinical relevance, a symptomless CD patient with a CDAI = 120 has a 60% chance of having endoscopically/histologically-evident “active disease”, accordingly to the corresponding calprotectin level. Considering the same value of CDAI as the cut-off (>120), we found a high diagnostic accuracy (72%), with 88% specificity and 50% sensitivity (positive predictive value: 76%, negative predictive value: 71%) of a fecal calprotectin value >150 µg/g.
Only two studies have attempted to identify CDAI cut-off values that would identify CD patients with endoscopically active disease: 1) Langhorst et al [14] found, in a cohort of 43 CD patients, a diagnostic accuracy of CDAI of 41.9% (sensitivity 24.2%, specificity 100%) with a cut-off value >150. This improved to 67.4% (sensitivity 66.7%, specificity 70%) with an optimized cut-off value >80; 2) af Björkesten et al [4], identified in 64 patients a CDAI cut-off value >55 with a sensitivity of 71%, specificity of 64% and area under the curve of 0.73. Both these papers [4,14] have limitations due to small sample sizes and, in the paper by af Björkesten et al [4], the clinical score was not determined at the same time with the endoscopy.
A strong limitation in our study is the lack of simultaneous endoscopic and fecal calprotectin assessment, but an increasing body of evidence [9-13,17] indicates that fecal calprotectin represents a reliable marker of profound disease activity.
From our results it is worth noting that CDAI values <150 display an interesting ability to quantify the risk of persistent inflammation, since the proposed model allows us to estimate, for a given value of CDAI, the probability of having high calprotectin levels.
CDAI, a clinical index, may give additional information whereby >150 indicates a clinical relapse, but values in the “grey area” between 100 and 150 may also quantify the inflammatory activity of the disease and help in the correct management of CD patients.
Summary Box.
What is already known:
Crohn’s disease (CD) activity index (CDAI) monitors clinical activity in CD patients
Fecal calprotectin levels ≥150 µg/g are strongly indicative of endoscopically and/or histologically evident disease activity
When CDAI is ≥150 (i.e. clinically active disease), fecal calprotectin values are high, but in more than 50% of patients who have CDAI <150 (i.e. clinical remission), fecal calprotectin remains elevated (>150 µg/g), reflecting the presence of an ongoing, endoscopically and histologically evident inflammation
What the new findings are:
A positive relationship between CDAI and fecal calprotectin has been found since it appears that calprotectin ≥150 µg/g tends to occur more often at higher CDAI values
CDAI scores between 100-150 display an interesting ability to quantify the risk of persistent inflammation as expressed by a high calprotectin level
A CDAI cut-off of 120 has a high diagnostic accuracy (72%) with 88% specificity and 50% sensitivity (positive predictive value: 76%, negative predictive value: 71%) of intercepting active disease defined by a fecal calprotectin value >150 µg/g
Biography
Sant’Orsola-Malpighi Hospital; University of Bologna, Italy
Footnotes
Conflict of Interest: None
References
- 1.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 2.Yoshida EM. The Crohn's disease activity index, its derivatives and the Inflammatory Bowel Disease Questionnaire: a review of instruments to assess Crohn's disease. Can J Gastroenterol. 1999;13:65–73. doi: 10.1155/1999/506915. [DOI] [PubMed] [Google Scholar]
- 3.Jones J, Loftus EV, Jr, Panaccione R, et al. Relationships between disease activity and serum and faecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 4.af Björkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn's disease. Scand J Gastroenterol. 2012;47:528–537. doi: 10.3109/00365521.2012.660542. [DOI] [PubMed] [Google Scholar]
- 5.Brignola C, Lanfranchi GA, Campieri M, et al. Importance of laboratory parameters in the evaluation of Crohn's disease activity. J Clin Gastroenterol. 1986;8:245–248. doi: 10.1097/00004836-198606000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–235. doi: 10.1136/gut.35.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50–54. doi: 10.1080/00365529950172835. [DOI] [PubMed] [Google Scholar]
- 9.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn's disease activity assessed by faecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 10.Schoepfer AM, Beglinger C, Straumann A, et al. Faecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 11.D’Haens G, Ferrante M, Vermeire S, et al. Faecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 12.Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut. 2005;54:364–368. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gisbert JP, Bermejo F, Pérez-Calle JL, et al. Faecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–1198. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 14.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Non invasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of faecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 15.Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut. 2014;63:88–95. doi: 10.1136/gutjnl-2013-304984. [DOI] [PubMed] [Google Scholar]
- 16.Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–1482. doi: 10.1038/ajg.2012.260. [DOI] [PubMed] [Google Scholar]
- 17.Mooiweer E, Fidder HH, Siersema PD, Laheij RJ, Oldenburg B. Faecal calprotectin testing can identify ineffective colorectal cancer surveillance procedures in patients with longstanding colitis. Inflamm Bowel Dis. 2014;20:1079–1084. doi: 10.1097/MIB.0000000000000054. [DOI] [PubMed] [Google Scholar]


