Abstract
Aims: The in vivo pharmacology of the sigma 1 receptor (σ1R) is certainly complex; however, σ1R antagonists are of therapeutic interest, because they enhance mu-opioid receptor (MOR)-mediated antinociception and reduce neuropathic pain. Thus, we investigated whether the σ1R is involved in the negative control that glutamate N-methyl-d-aspartate acid receptors (NMDARs) exert on opioid antinociception. Results: The MOR C terminus carries the histidine triad nucleotide-binding protein 1 (HINT1) coupled to the regulator of G-protein signaling RGSZ2-neural nitric oxide synthase assembly. Activated MORs stimulate the production of nitric oxide (NO), and the redox zinc switch RGSZ2 converts this signal into free zinc ions that are required to recruit the redox sensor PKCγ to HINT1 proteins. Then, PKCγ impairs HINT1-RGSZ2 association and enables σ1R-NR1 interaction with MOR-HINT1 complexes to restrain opioid signaling. The inhibition of NOS or the absence of σ1Rs prevents HINT1-PKCγ interaction, and MOR-NMDAR cross-regulation fails. The σ1R antagonists transitorily remove the binding of σ1Rs to NR1 subunits, facilitate the entrance of negative regulators of NMDARs, likely Ca2+-CaM, and prevent NR1 interaction with HINT1, thereby impairing the negative feedback of glutamate on opioid analgesia. Innovation: A redox-regulated process situates MOR signaling under NMDAR control, and in this context, the σ1R binds to the cytosolic C terminal region of the NMDAR NR1 subunit. Conclusion: The σ1R antagonists enhance opioid analgesia in naïve mice by releasing MORs from the negative influence of NMDARs, and they also reset antinociception in morphine tolerant animals. Moreover, σ1R antagonists alleviate neuropathic pain, probably by driving the inhibition of up-regulated NMDARs. Antioxid. Redox Signal. 22, 799–818.
Introduction
The mu-opioid receptor (MOR) is a G-protein-coupled receptor (GPCR) that selectively controls the perception of nociceptive sensorial signals. Unfortunately, the frequent administration of opioids such as morphine and derivatives typically leads to the development of analgesic tolerance. These drugs promote little recycling/resensitization of their receptors (12), and then recruit other adaptive processes that result in MOR desensitization on the cell surface (14). In animals, tolerance to the antinociceptive effects of opioids can be observed even after a single and adequate dose. Thus, morphine can induce acute strong tolerance via the glutamate N-methyl-d-aspartate acid receptor (NMDAR)/neural nitric oxide synthase (nNOS)/zinc metabolism (45, 47). In this scenario, the physical association between MORs and NMDARs within a specialized protein assembly facilitates their functional cross-regulation (49).
Innovation.
In neural cells, the sigma 1 receptor (σ1R) binds to NMDAR NR1 subunits and it co-operates with the redox-regulated histidine triad nucleotide-binding protein 1 (HINT1) protein to bring mu-opioid receptor (MOR) signaling under the regulation of the NMDAR, thereby contributing to tolerance. In this protein assembly, opioids promote the binding of the redox sensor PKCγ to the HINT1 histidine residues, via nitric oxide (NO) and zinc metabolism, whereby the kinase recruits NMDAR activity proportional to MOR signaling. In naïve mice, the σ1R antagonists disrupt σ1R-NR1 interaction and uncouple the NMDAR from MOR activity, enhancing morphine analgesia and reducing the development of acute opioid tolerance. In mice rendered tolerant to morphine, σ1R antagonists promote the inhibition of NMDARs via Ca2+-CaM and they then increase the strength of the MOR signaling, rescuing morphine analgesia from tolerance. Thus, selective σ1R antagonists could be therapeutically exploited as adjuvants of opioid analgesia, reducing the risk of adverse effects.
The sigma 1 receptor (σ1R) has been proposed as a tonic anti-opioid system (39) that modulates the activity-induced sensitization in nociceptive pathways (8). The σ1Rs are widely expressed in nervous tissue, presenting high levels in areas that are associated with pain control (28). Whereas σ1R agonists facilitate nociception (27, 69), σ1R antagonists reduce the allodynia and hyperalgesia that accompany neuropathy in different animal models, improving the activity of opioids against nociceptive stimuli (8, 52, 53, 70). The σ1R was initially considered a type of opioid receptor (35); however, the σ1R lacks glycosylation, and its molecular structure suggests a different class of regulatory function, most likely that of chaperones (21). The σ1R constitutes a unique class of linear proteins that only has two transmembrane (TM) domains (3), with both N and C terminal sequences projecting to the same side, cytosol (59), or extracellular space (4), similar to the hairpin-like structure of caveolins, which are non-neural scaffold proteins (42).
The σ1R activity is modulated through a series of endogenous and exogenous substances. The pharmacology of the σ1R is complex, with exogenous ligands showing different profiles depending on the system under study (38). Notwithstanding this drawback, σ1R ligands are of therapeutic interest for the treatment of neurological diseases (31), substance abuse syndromes (46), and NMDAR-related neuropsychiatric disorders (22) or as adjuvants of opioid analgesia (25, 39, 64). According to the anti-opioid function of the σ1R (39), σ1R antagonists enhance the analgesic effect of systemic morphine, which is prevented by σ1R agonists, and also restore morphine analgesia in tolerant mice (64). As expected, σ1R−/− mice exhibit an increased response to morphine antinociception that cannot be regulated by σ1R ligands (57). Importantly, the opioid effects that are enhanced by σ1R antagonists are those regulated by the NMDAR/NOS/CaMKII pathway (70); thus, σ1R ligands do not modify morphine-induced hyperlocomotion or gastrointestinal transit inhibition. The positive features of the highly selective σ1R antagonist S1RA make this drug a good candidate for the treatment of neuropathic pain (53), and this treatment has satisfactorily completed phase I safety and pharmacokinetic evaluation in humans (1).
The σ1R ligands modulate NMDAR functions both in vivo and in vitro (36, 41, 55). Indeed, in cellular expression systems and in vitro assays, the σ1R displays calcium-dependent binding with NMDAR NR1 subunits (55). Because σ1Rs also associate with MORs (25), it is possible that these proteins regulate opioid function within the protein assembly that, via the histidine triad nucleotide-binding protein 1 (HINT1), redox signaling and zinc metabolism, support the MOR-NMDAR physical association and functional cross-regulation (48–50).
Within this background, this study analyzed the potential role of σ1Rs in the cross-regulation between MORs and NMDARs in the mesencephalic periaqueductal grey (PAG) matter, a supraspinal region that regulates spinal nociceptive signals. The σ1Rs associate with NMDAR NR1 subunits, and σ1R antagonists promote the binding of negative regulators of NMDAR activity. The negative feedback that NMDARs display on MOR signaling requires the nitric oxide (NO)- and zinc-dependent recruitment of PKCγ to the HINT1 proteins followed by σ1R-NR1 binding to the MOR-HINT1 complex. Consequently, σ1R antagonists uncouple NMDAR effects from MOR signaling, thereby enhancing morphine analgesia and reducing the development of opioid tolerance.
Results
Organization of the σ1R receptor in the cell membrane
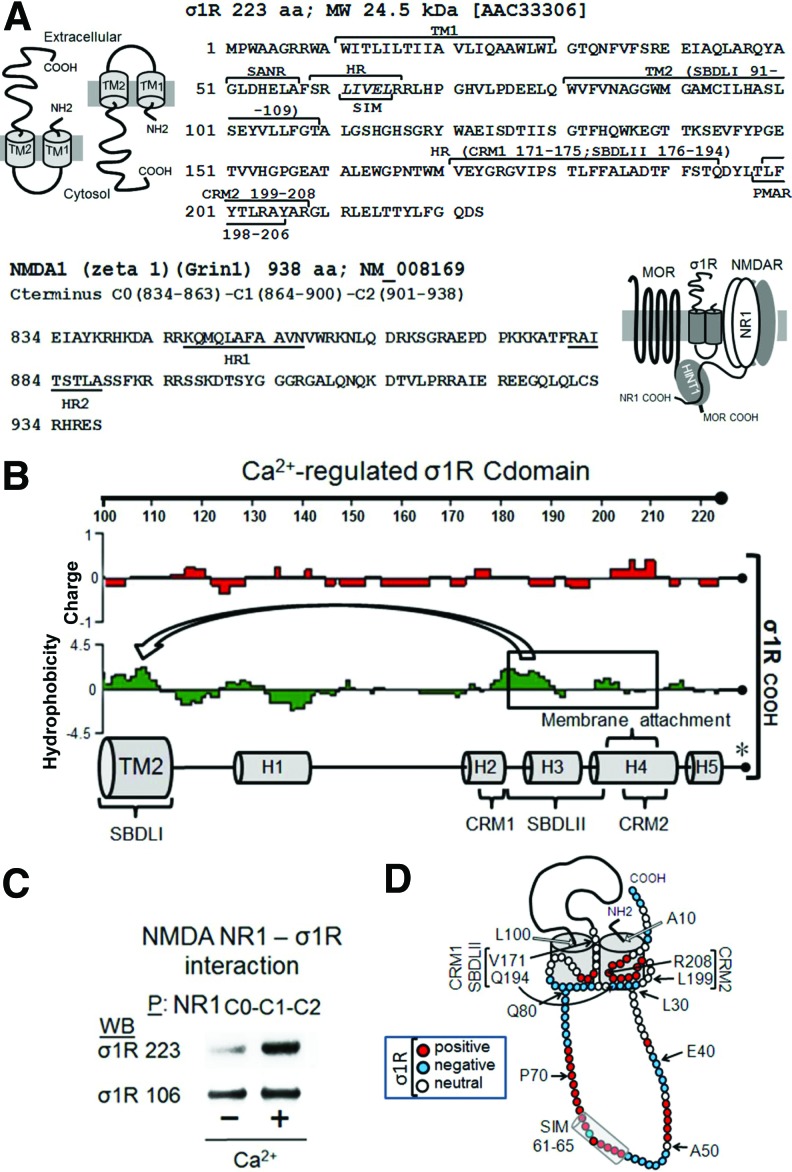
In the nervous tissue, the σ1R exists as long and short isoforms (21, 58). The long form of σ1R comprises 223 amino-acid residues, while the short form contains 106 amino acids. The σ1R has a hairpin structure with a short N terminal sequence before the first hydrophobic TM domain that leads to the loop region, followed by the second TM domain and the long C-terminal domain (cd). The short form of σ1R contains a clipped C-terminal sequence that differs from that of the long form in the four last residues. There is some controversy regarding the potential arrangement of σ1R in the plasma membrane, specifically with respect to whether both the N- and C-terminal sequences are directed to the cytosolic side leaving the loop in the extracellular milieu, or in the opposite orientation with the loop facing the cytosolic side (4, 44) (Fig. 1A).
FIG. 1.
The σ1R in the cell membrane: interaction with the glutamate NMDAR. (A) The protein domains of the murine σ1R and of the NMDAR NR1 cytosolic C terminal sequence. The long isoform of σ1R contains 223 residues, with two hydrophobic TMs, TM1 and TM2. The hairpin loop contains a SIM 61–65 within a HR and a SANR. The C-terminal domain comprises another HR, which includes cholesterol-binding motifs (CRM1 and CRM2), a PMAR, and SBDLII. The NMDAR NR1 subunit C terminus C0-C1-C2 contains 104 residues with two HRs (HR1 and HR2; Lasergene Protean Software DNASTAR, Inc., Madison, WI). (B) The σ1Rcd region, charge average, and hydrophobicity map (the images were created using Lasergene Protean Software). The binding of this region to Bip is regulated through calcium (* taken from Ref. 43), and the SBDLII associates with SBDLI in the TM2 domain, forming the neurosteroid-binding pocket. (C) The binding of recombinant σ1R long and short forms to GST-NR1 C0-C1-C2. The recombinant NR1 C-terminal sequence C0-C1-C2 and σ1 receptor variants were used at 100 nM. The assay was performed in the presence or absence of 2.5 mM calcium. The bait protein (GST-NR1 C0-C1-C2) was immobilized by covalent attachment to NHS-activated sepharose. The prey proteins alone did not bind to either NHS-sepharose (negative control) or recombinant GST (negative control). After incubation, the proteins were resolved by SDS-PAGE chromatography, followed by Western blotting analysis. P stands for the precipitation of immobilized NR1 C-terminal sequences. (D) The proposed arrangement of the long σ1R in the cell membrane is shown with the loop directed to the cytosol, the cd HR situated in the inner region of TM1 and TM2, and the N- and C-terminal regions arranged toward the extracellular space, key indicates amino acid charge. σ1R, sigma 1 receptor; cd, C-terminal domain; H, helical region; HR, hydrophobic region; NMDAR, glutamate N-methyl-D-aspartate acid receptor; PMAR, potential membrane attachment region; SANR, sumo-associated negative region; SBDLII, steroid-binding-like domain II; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; SIM, SUMO-interacting motif; SUMO, small ubiquitin-related modifier; TM, transmembrane. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The σ1R in the endoplasmic reticulum membrane binds Bip in a calcium-dependent manner, and this binding is retained through the cd region (residues 112–223; σ1R) (43). In cell expression systems, σ1R shows a calcium-dependent interaction with the NR1 subunit of NMDAR, and both proteins can be co-immunoprecipitated from brain synaptosomes (4, 55). In in vitro assays, σ1R binds to the NR1 cytosolic C-terminal region C0-C1-C2 (55). The σ1R loop has a SUMO-interacting motif (SIM) domain (LIVEL: 61–65) that is typical of intracellular interactions (Fig. 1A and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars), and the long σ1R binds to the NMDAR NR1 subunit via fluctuations in calcium levels, such as those that are observed in the cytosol (55). Thus, at least a part of the C-terminal region should be located on the cytosolic side. Indeed, the ligand-binding pocket of the σ1R comprises the two TM domains and the hydrophobic region of the σ1Rcd (44), containing a potential membrane attachment amino-acid sequence (43). SBDLI and SBDLII (steroid-binding-like domains) are located in the σ1R TM2 and cd regions, and although the average charge of SBDLI is mostly neutral, that of SBDLII is negative, offering the potential for calcium regulation (Fig. 1B). The short σ1R displayed calcium-independent binding to NR1 C0-C1-C2; however, this binding in the long isoform was calcium dependent (Fig. 1C), suggesting that the cd impairs the binding of the long σ1R form to the NR1 subunit and that calcium eliminates this barrier. A tentative model for σ1R arrangement is presented in Figure 1D.
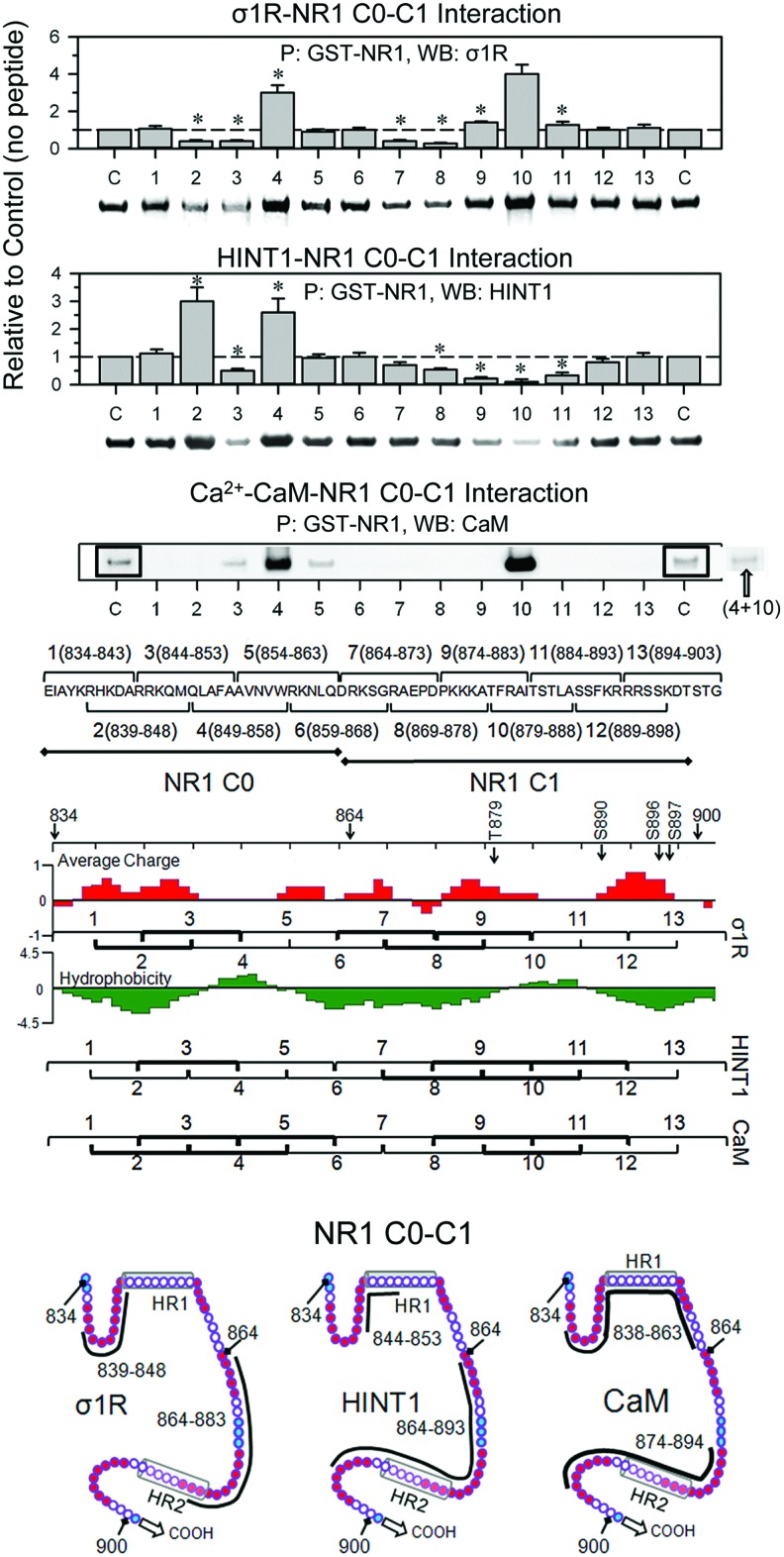
Regarding the arrangement of σ1R in the cell membrane, we examined the calcium-dependent binding of σ1Rs to NR1 subunits through peptide interference (Fig. 2 and Supplementary Fig. S2). This approach suggested that NR1 C0 (839–853) and NR1 C1 (864–878) bind to the σ1R. Peptide mapping of the hydrophobic regions in the NR1 C0 and C1 segments greatly enhanced the interaction between the σ1Rs and NR1 subunits, suggesting that both of the NR1 hydrophobic domains interact and that σ1R disrupts this interaction to achieve NR1 binding. It is therefore possible that peptide 9 interferes with σ1R binding in the NR1 region of the 883 residue; however, this interference could be masked by peptide 9 better exposing the NR1 C0-C1 domain for interaction with third-part proteins. Peptide interference indicated that two regions of the σ1R loop could be implicated in its interaction with NR1 subunits; the first region lies between residues 40 and 60, and the second lies between residues 71 and 80. Mapping the σ1Rcd (174–223) indicated that the HR1 region of NR1 C0 does not interact with σ1R 184–203 sequence, and then the NR1 C0 region that is most likely involved in σ1R binding is 839–848. Notably, peptides mapping the σ1Rcd hydrophobic helical regions H3 and H4 impaired the binding of this receptor to the NR1 subunit. The interaction of these hydrophobic regions with the TM1 and TM2 domains is essential to maintain the conformation of the σ1R as being able to bind to the NR1 C0-C1 domains (43, 44). Thus, an excess of these peptides would remove the σ1R H2 and H3 regions from the TM domains, disorganizing the structure of the σ1R and consequently reducing its interaction with the NR1 subunit (Supplementary Fig. S2).
FIG. 2.
Interference assay of the association between NR1 C0-C1 and σ1R, HINT1 and CaM. A series of overlapping peptides (30 μM) mapping the C0 and C1 region (834–903) of NR1 subunits were incubated with 100 nM σ1R, 200 nM HINT1, or 100 nM Ca2+-CaM before the addition of 100 nM NR1. The NR1 was precipitated, and the associated protein was evaluated through ECL-densitometry. These data were obtained from three independent assays. *Significantly different from the σ1R or HINT1 signals in the absence of interfering peptides, ANOVA-Student–Keuls test; p<0.05. The peptide mapping regions that were tentatively implicated in the interaction of NR1 C0-C1 with these proteins are indicated in bold. Ca2+-CaM basal binding was low and greatly increased in the presence of peptides mapping to the HR1 and HR2 of NR1 subunits. This binding was abrogated when NR1 was sequentially incubated with peptides 4 and 10 before adding Ca2+-CaM. To increase the readability, the frames indicate higher exposition of the blots. The potential regions of Ca2+-CaM binding to the NR1 C0-C1 are indicated (Calmodulin Target Database; http://calcium.uhnres.utoronto.ca/ctdb/ctdb/sequence.html). Amino acid charge, see key in Fig. 1D. HINT1, histidine triad nucleotide-binding protein 1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
A similar approach revealed that the HINT1-binding region on NR1 C0-C1 overlaps with the σ1R-binding region. Thus, peptides mapping to the NR1 C0 hydrophobic region enhanced the HINT1-NR1 interaction. However, peptides mapping to the corresponding region in the NR1 C1 segment diminished this interaction. These observations indicate that HINT1 unfolds the NR1 but primarily binds to the NR1 C1 hydrophobic region, ignoring the hydrophobic region on the NR1 C0 segment. In the absence of peptides 4 and 10, the σ1R and HINT1 proteins exhibited noticeable binding to NR1 C0-C1, whereas the binding of Ca2+-CaM was certainly weak. It is possible that the disruption of the NR1 intrahydrophobic interaction through Ca2+-CaM is weaker than that achieved by σ1R or HINT1; thus, the Ca2+-CaM binding to NR1 subunits likely requires the assistance of third-party proteins. This possibility has been previously suggested through the binding of the cytoskeletal protein α-actinin2 with the C0 region of the NR1 subunits (40). The NR1 subunit contains two putative regulatory sites for Ca2+-CaM, with one site in the C0 segment and the other in the C1 region (11), and the disruption of the NR1 C0-C1 hydrophobic interaction through either peptide 4 or 10 exposed one of the NR1 sites for Ca2+-CaM binding. Accordingly, the presence of both peptides eliminated Ca2+-CaM binding to the NR1 subunits (Fig. 2).
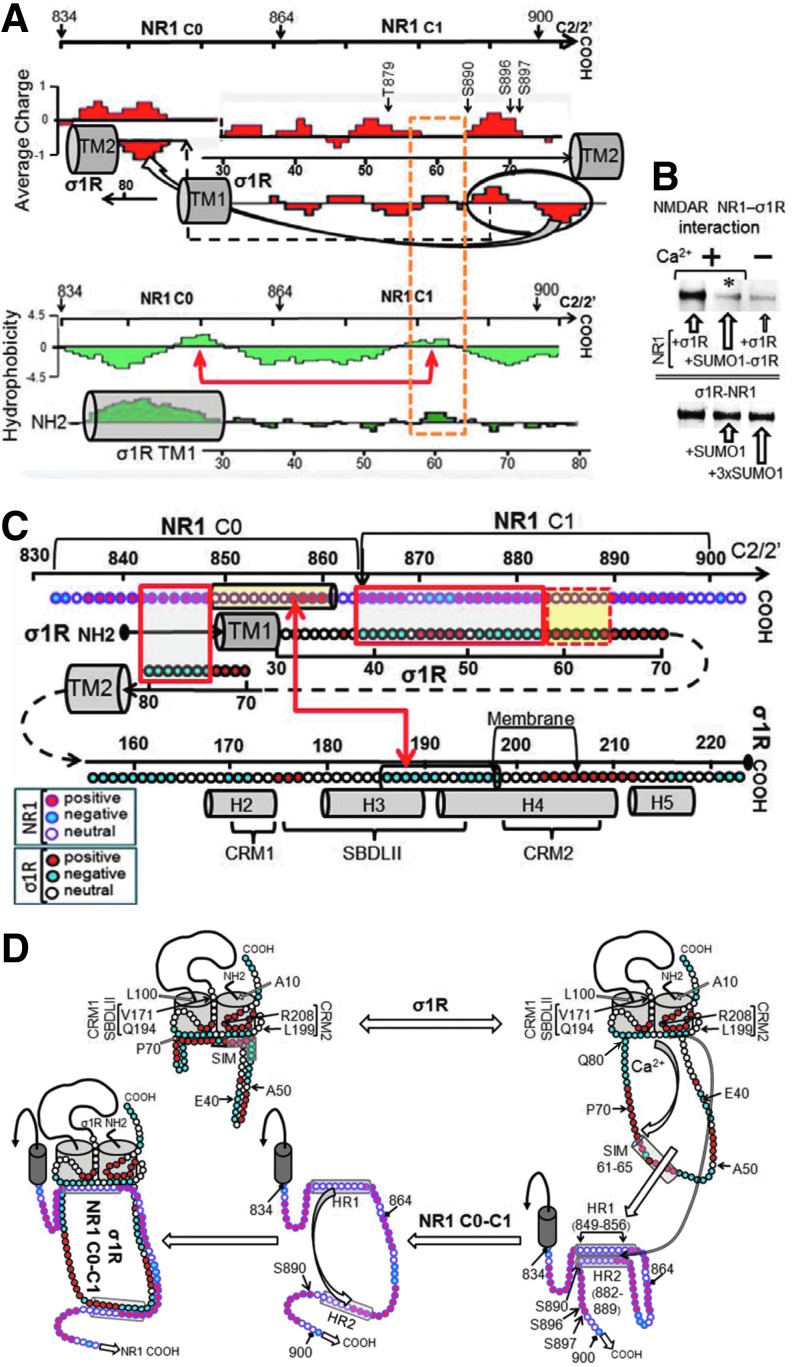
The juxtaposition of the σ1R loop and NR1 C0-C1 regions revealed complementary charges between the loop emerging from TM1 and the upper half of the NR1 C1 segment, followed by putative hydrophobic interactions between the SIM-containing sequence 58–66 and the NR1 hydrophobic region S890 (Fig. 3A). In addition, the preincubation of small ubiquitin-related modifier 1 (SUMO1) with σ1R impaired subsequent binding to the NR1 subunits, and SUMO1 weakly disrupted the existing σ1R-NR1 association (Fig. 3B). Thus, the SIM-containing loop is involved in σ1R binding to NR1, and this region is hardly available during their association, reflecting the interaction of the SIM-associated negative region with complementary charges in the NR1 C1 segment, subsequently weakening the possible SUMO-SIM interaction (24).
FIG. 3.
Juxtaposition of the σ1R and NR1 C0-C1 regions based on their charge complementarities and hydrophobicity. (A) Proposed interaction of the σ1R loop with the NMDAR NR1 C0-C1 region. The discontinuous yellow frame indicates possible hydrophobic interactions. TM stands for transmembrane region. The arrows on the NR1 sequence suggest a possible intramolecular interaction between the HR1 and HR2 hydrophobic regions (Lasergene Protean V8 DNASTAR). (B) Influence of SUMO1 on the σ1R association with NR1 subunits. The recombinant σ1R protein (100 nM) was preincubated with agarose-SUMO1. After the removal of the free σ1Rs, the NR1 subunits (100 nM) were added to the incubation milieu. In a set of assays, preformed agarose-NR1-σ1R complexes (100 nM) were incubated with free SUMO1. The agarose pellets containing the bound proteins were analyzed by Western blotting. (C) Model describing the potential interaction regions of the complete σ1R with NR1 C0-C1 segments. Closed boxes indicate charge complementarities, and open boxes indicate potential hydrophobic interaction. The arrows connect the HR on NR1 C0 with another region in σ1Rcd corresponding to SBDLII, a negative region that could bind calcium. TM stands for transmembrane domain, and the helix organization (H2–H5) is taken from Ortega-Roldan et al. (43). Key indicates amino acid charge for NR1 and s1R. (D) Diagram of calcium-dependent σ1R binding to the NR1 C terminal C0-C1 region. The σ1R loop in the cell membrane is oriented toward the cytosolic side, and both the N and C terminal sequences face the extracellular space. The hydrophobic cd region is oriented toward the inner side of the membrane and between the TM domains, forming the ligand-binding pocket. This hydrophobic and negative cd region interacts with a positive region of the σ1R loop. The position of certain residues is indicated in the figure. On calcium binding, this negative cd region becomes neutral and subsequently releases the positive loop region to bind other proteins, such as the NR1 subunit. Peptide interference mapping suggests that the NR1 HR1 and HR2 hydrophobic regions establish an intramolecular interaction. The calcium-bound σ1Rcd region would disrupt this internal interaction to bind to the NR1 subunit.
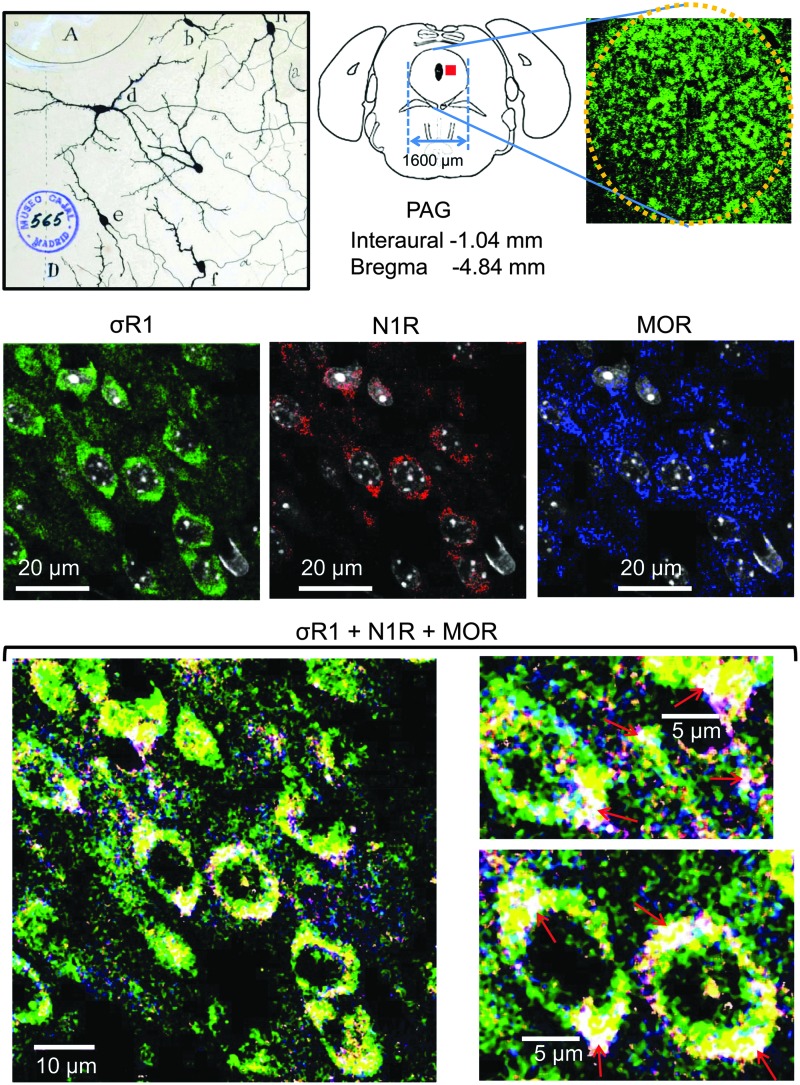
Considering our (Fig. 3C) and other (4, 55) data, we constructed the model of the σ1R-NR1 interaction that is shown in Figure 3D, in which σ1R is arranged in the plasma membrane with the SIM-containing loop facing the cytosol and the N-terminal sequence facing the extracellular space. The orientation of the short C-terminal sequence that is outside of the TM2 is tentatively orientated to the extracellular space; however, this sequence could also be facing the cytosolic side. The region of the σ1Rcd that is potentially regulated by calcium (SBDLII) is located on the cytosolic surface of the cell membrane. Thus, our arrangement of the σ1R in its interaction with the NMDAR NR1 subunit maintains the essential described features for σ1Rs in the cell membrane (4, 44) but with the cd and loop hydrophobic regions, which show complementary charges, situated on the cytosolic side of the membrane. In the absence of calcium, the interaction between the σ1R and the NR1 subunit is occluded. The positive calcium ions bind to the hydrophobic region of the σ1Rcd and neutralize the negative charge that is required to bind the SIM-containing positive region of the loop. Thus, the calcium-bound σ1Rcd region can now disrupt the internal hydrophobic interaction NR1 C0 (HR1)-NR1 C1 (HR2), exposing the required binding surface to the σ1R loop. We could detect these signaling proteins in mouse PAG neural cells using antibodies against the MOR, σ1R, and NMDAR NR1 subunits. Immunohistochemical analysis revealed a high degree of co-localization in the lateral PAG between the NR1 subunit and its regulator, the σ1R. In this region, the MOR showed a discrete co-localization with these proteins. The triple co-localization of the MOR, σ1R, and NR1 subunits could be observed as a series of white spots located at the cell periphery and also in fibers. This distribution/co-localization is compatible with σ1Rs regulating MOR and NMDAR function (Fig. 4 and Supplementary Fig. S3).
FIG. 4.
Co-localization of the NMDAR NR1 subunit with σ1R and MOR in the mouse PAG. Upper panel, Original Cajal drawing showing cells of the PAG, Golgi method. A, cerebral aqueduct; cell shape is varied, with predominance of the fusiform type, and the orientation is oblique or transversal. Mouse PAG (yellow dotted circle) and Coronal drawing of mouse brain showing the PAG region analyzed in this triple co-localization of σ1R, NMDAR NR1 subunit and MOR (red square in the diagram). Middle panel, Confocal laser-scanning microphotographs taken from coronal histological sections (10 μm) through the midbrain PAG showing individual labeling for σ1R (green), NR1 (red), and MOR (blue) antigens. Immunoreactivity was visualized with Alexa Fluor 488, 555 and 647, respectively. Lower panel, Triple co-localization of the MOR, σ1R, and NR1 subunits could be observed at the cell periphery and fibers (nuclei were removed). There was a high degree of coincidence between NR1 and σ1R (yellow color). The MOR co-localizes with σ1R (light green color) and NR1 (purple color) in a discrete pattern. Notice that high-power magnification panels show triple-co-localization as white structures (red arrows; for details, see Materials and Methods section and Supplementary Fig. S3). MOR, mu-opioid receptor; PAG, periaqueductal grey.
These observations situate σ1R in the protein assembly that controls MOR activity via NMDARs. Thus, we addressed whether the antinociception and molecular changes that are produced by morphine in the presence of σ1R antagonists fit this model.
Antagonists of σ1Rs enhance morphine-evoked supraspinal antinociception
The MOR in the mesencephalic PAG plays the most relevant role in the antinociception produced by opioids when injected by the intracerebroventricular (icv) route. The icv administration of all the substances studied circumvents the possibility that the drugs reach receptors beyond the brain. Subsequently, the capacity of morphine to produce supraspinal antinociception, and that of the studied drugs to modulate this effect, was assessed through the warm water tail flick test (see Materials and Methods section). The distribution of the thermal stimulus over two-thirds of the tail and the cut-off of 10 s protect the mice from tissue damage, as well as prevent the particular thermal sensitivity of a discrete point of the tail from compromising the data. Moreover, the mice practically do not anticipate their responses in using this analgesic test when they are studied several times during a time-course study.
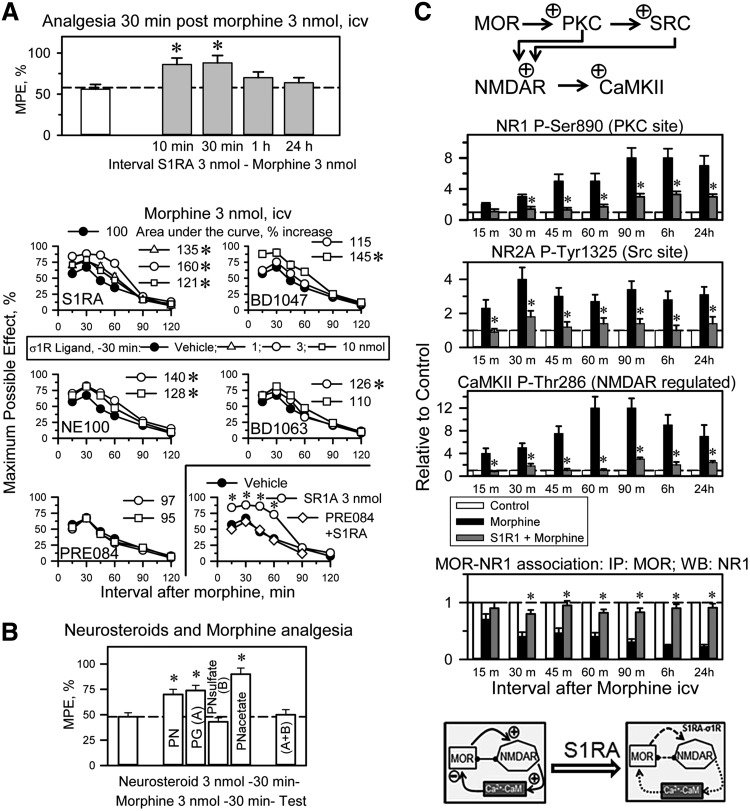
The icv administration of the highly selective σ1R antagonist S1RA [E-52862; (53)] at 10 or 30 min before morphine treatment (3 nmol, icv) increased the analgesic activity when evaluated at 30 min postopioid injection. S1RA given 1 h or longer before morphine produced no such effect. Therefore, 30 min was selected to study the effect of σ1R ligands on the capacity of morphine to promote supraspinal analgesia in the thermal tail-flick test. We have reported a model for the detection of σ1R antagonist activity, and S1RA is likely the most potent and pure σ1R antagonist available (55). S1RA at 3 nmol increased the analgesic activity of morphine; other antagonists, such as BD1047 and NE100, also performed well in this paradigm, whereas BD1063 weakly increased morphine analgesia. The agonist PRE084 did not affect morphine analgesia but prevented S1RA from enhancing opioid antinociception (Fig. 5A).
FIG. 5.
Effect of icv σ1R ligands on the morphine supraspinal analgesia and NMDAR activity. (A) Influence of the interval between the selective antagonist of σ1R, S1RA, and morphine in the production of analgesia. Mice received icv 3 nmol S1RA at the indicated time intervals before 3 nmol morphine, and analgesia was evaluated in the thermal (water 52°C) “tail-flick” test 30 min later. The bars represent the mean±SEM of the data from six mice. *Significantly different from the group that received only morphine, p<0.05. A series of σ1R antagonists and the agonist PRE084 were icv-injected at 30 min before 3 nmol morphine, and antinociception was evaluated at the indicated postopioid intervals. Each point represents the mean±SEM of data from 10 mice. The area under the curve in the postmorphine interval at 15–90 min was calculated using the trapezoidal rule for each σ1R ligand and dose (Sigmaplot/Sigmastat v12.5, Erkrath, Germany). *The analgesia produced by the σ1R antagonist-morphine combination was significantly different from that of morphine alone, ANOVA-Student–Newman–Keuls, p<0.05. The σ1R agonist PRE084 prevented S1RA from enhancing morphine analgesia. Mice received a combined injection of 3 nmol PRE084 and 3 nmol S1RA 30 min before morphine treatment. *Significantly different from the group that received vehicle and morphine or PRE084+S1RA and morphine, p<0.05. (B) The neurosteroids PG, PN, PN acetate, and PN sulfate (sulf) were used at 3 nmol, icv. The enhancing effects of progesterone on morphine analgesia were abolished by pregnenolone sulfate. *Significantly different from the group that received morphine and vehicle instead of the neurosteroid, p<0.05. (C) The selective σ1R antagonist S1RA impairs the MOR-mediated activation of NMDARs. Groups of 42 mice each received an icv dose of 10 nmol morphine alone or 3 nmol S1RA 30 min before the opioid. Control mice were treated with saline instead of morphine. Subsequently, for each group, six mice were sacrificed at the indicated intervals. PAG synaptosomes were obtained to determine ex vivo the presence of CaMKII P-Thr286, NR1 C1 P-Ser890, and NR2A P-Tyr 1325. The MOR proteins were immunoprecipitated, and the associated NR1 subunits were determined by Western blotting. Immunosignals (average optical density of the pixels within the object area/mm2, Quantity One Software; Bio-Rad, Madrid, Spain) were expressed as the change relative to the control group (attributed an arbitrary value of 1, dashed lines). Each bar represents the mean±SEM of the data from three determinations that were performed using different gels and blots. *For every postopioid interval, indicates that S1RA produced a significant difference from the group receiving only morphine, p<0.05. We observed no differences in the levels of the nonphosphorylated proteins. Representative blots are shown in Supplementary Figure S4. The diagram indicates that S1RA impairs the MOR to NMDAR pathway, which is required to build up the negative feedback via kinases such as CaMKII on opioid signaling. icv, intracerebroventricular; PG, progesterone; PN, pregnenolone.
Neurosteroids, putative endogenous regulators of the σ1R, were also studied. Pregnenolone is a σ1R agonist, and its metabolite progesterone is a σ1R antagonist. Pregnenolone and its acetate derivative enhance morphine antinociception; however, the sulfate form of pregnenolone does not show this effect. Progesterone enhances morphine antinociception, an effect that is reduced by pregnenolone sulfate (Fig. 5B), suggesting that pregnenolone, but not its sulfate form, is rapidly converted into progesterone.
Morphine increases NMDAR activity: effect of σ1R antagonism
At the molecular level, the icv administration of 10 nmol morphine increased the function of the NMDAR-CaMKII pathway. This opioid increased the phosphorylation of NR1 subunits (PKC on S890) and NR2 subunits (Src on Y1325). Moreover, morphine increased the T286 autophosphorylation of the Ca2+- and CaM-dependent serine and threonine kinase CaMKII, an NMDAR effector. As a result of the MOR-induced activation of NMDARs, the MOR-NR1 association was weakened, and these changes persisted beyond the morphine analgesic time course (49). The presence of 3 nmol S1RA, administered 30 min before morphine treatment, nearly abolished CaMKII activation, reduced NR1/NR2 phosphorylation, and preserved the MOR-NR1 inhibitory association during the intervals that corresponded to the morphine analgesic time-course and beyond (Fig. 5C and Supplementary Fig. S4).
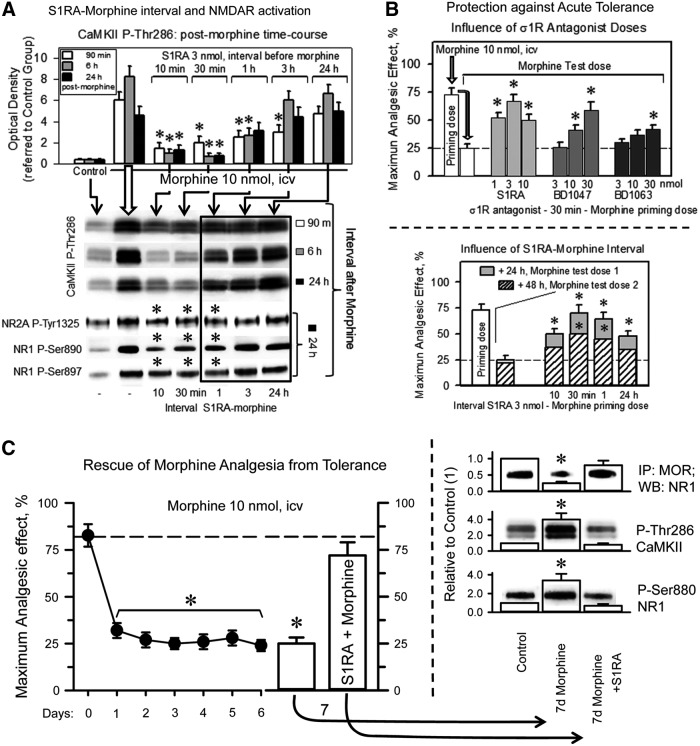
The capacity of S1RA to enhance morphine antinociception or alter MOR-induced NMDAR changes decreased as the S1RA-morphine interval increased (Fig. 6A). The σ1R antagonists S1RA, BD1047, and, to a minor extent, BD1063, when given before the first dose of morphine (priming dose), protected the analgesic effects of a subsequent dose of morphine (test dose) against acute tolerance (Fig. 6B). In this paradigm, the S1RA-morphine intervals of 30 min and 1 h were more successful than that of 10 min. S1RA icv-injected between 1 and 24 h before morphine neither enhanced morphine analgesia nor prevented the activation of MOR-coupled NMDARs (Figs. 5A and 6A); however, the σ1R antagonist still moderately decreased the antinociceptive acute tolerance (Fig. 6B). In a previous study, systemic S1RA was found to restore morphine analgesia in mice rendered tolerant by repeated subcutaneous administration of this opioid (64). Thus, we addressed whether S1RA was capable of such positive effects on mice that had received a daily icv dose of 10 nmol morphine for 7 days (Fig. 6C). Antinociception was evaluated at 30 min after the delivery of each morphine dose. The analgesic response diminished during the chronic morphine protocol; however, on day 7, the icv administration of 3 nmol S1RA 20 min before morphine enabled the opioid to produce an analgesic effect comparable to that of the first 10 nmol dose delivered on day 0 of the assay. At the molecular level, chronic morphine weakened the association of MORs with NMDAR NR1 subunits, whereas it enhanced the PKC-mediated phosphorylation of NR1 serine 890 and the activating autophosphorylation of CaMKII. These changes revealed an increase in NMDAR activity that returned to control levels when the chronic morphine-tolerant mice received a single icv dose of S1RA (Fig. 6C). Thus, the rescue of morphine analgesia from tolerance correlated with the deactivation of NMDARs.
FIG. 6.
Influence of the interval S1RA-morphine on the MOR-induced activation of NMDARs. (A) Influence of the interval S1RA-morphine. Groups of 36 mice each received icv a desensitizing dose of 10 nmol morphine alone or 3 nmol S1RA at 10, 30 min, 1, 3, or 24 h before the administration of the opioid. Subsequently, for each group receiving morphine, six mice were sacrificed at 90 min, 6, or 24 h after opioid treatment. The control mice received saline instead of morphine. PAG synaptosomes were obtained to determine the presence of CaMKII P-Thr286, NR1 C1 P-Ser890, P-Ser897, and NR2A P-Tyr 1325. The assay was repeated at least twice. The data from the S1RA-morphine intervals that failed to enhance analgesia but conferred protection from tolerance are framed. *For every postopioid interval, the symbol indicates that S1RA produced a significant difference from the group receiving only morphine, ANOVA-Student–Newman–Keuls test, p<0.05. The details are shown in Figure 5. (B) Protection against morphine acute antinociceptive tolerance. A priming icv dose of 10 nmol morphine reduced the analgesic response to a second and identical test dose of the opioid when administered 24 h later. Increasing doses of S1RA, BD1047, and BD1063 were icv-injected at 30 min before the morphine priming dose, and the analgesic effect of the test dose was evaluated 24 h later. Analgesia was measured in the thermal tail-flick test at the peak effect for morphine analgesia, 30 min postopioid treatment. Effect of the interval between S1RA and the morphine priming dose on acute tolerance. The σ1R antagonist S1RA was administered icv at 10, 30 min, 1, or 24 h before the morphine priming dose (10 nmol), and the analgesic effects of identical doses were evaluated 24 h (test dose 1) and 48 h (test dose 2) later. Each bar indicates the mean±SEM of the analgesia; n=6 mice. *Significantly different from the group that received saline instead of the σ1R ligand before morphine priming dose, p<0.05. (C) Mice were administered morphine daily for 6 days (10 nmol, icv), and antinociception was evaluated by the tail flick test 30 min after injection. On day 7, the administration of S1RA (3 nmol) to morphine tolerant mice restored the antinociceptive effect of the opioid. Each point/bar represents the mean±SEM of the data from 10 mice. *Significantly different from the group that received the first dose of morphine (day 0), p<0.05. Immunodetection of NMDAR-related signals in the PAG of mice not exposed to morphine (control), mice that received morphine for 7 days and mice that after 6 days of morphine treatment received 3 nmol S1RA plus 10 nmol morphine on day 7. *Significantly different from the control group that received saline instead of morphine, p<0.05. Details as in Figure 5C.
The σ1Rs controls the interaction of NR1 subunits with NMDAR inhibitors and the redox-regulated HINT1 protein
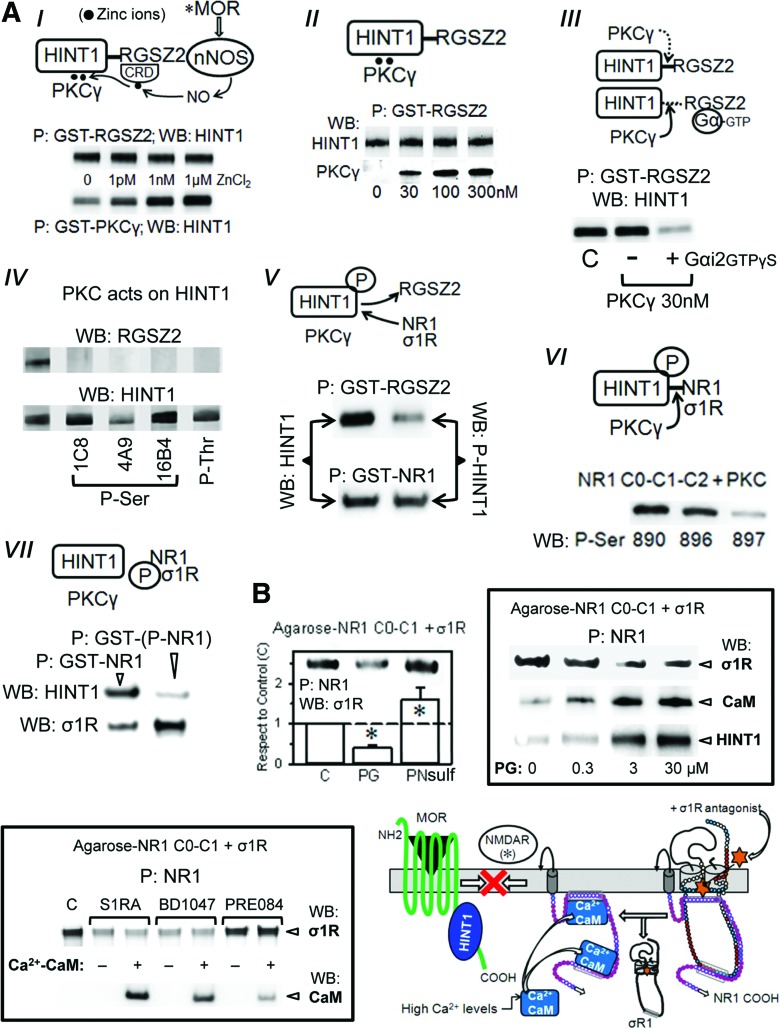
The MOR C terminus interacts with HINT1, which carries the regulator of the G-protein signaling RGSZ2-nNOS complex (2, 15, 20). The RGSZ2 binds to the HINT1 protein in a zinc-independent manner and prevents the entrance of NMDAR NR1 subunits. Thus, activated MORs release the RGSZ2-negative control on nNOS and stimulate the production of NO to release zinc ions from RGSZ2 zinc domain (54) (Fig. 7A). This activity promotes the zinc-dependent binding of the redox sensor PKCγ to HINT1 proteins through zinc ions bridging HINT1 histidines with the cysteines of the PKCγ regulatory domain (15, 51), and both RGSZ2 and PKCγ bind simultaneously to the redox-regulated scaffold HINT1 protein (Fig. 7A). The MORs provide activated GαGTP subunits that bind to the RGS domain of RGSZ2, exposing the HINT1 protein to the effect of PKCγ (48) (Fig. 7A). The phosphorylation of HINT1 is the switch that releases RGSZ2, bringing NMDARs under MOR control. Within the MOR-HINT1-σ1R-NR1 protein assembly, the NMDAR displays low activity (63). PKCγ increases the σ1R-NR1 interaction and weakens that of HINT1 with NR1 subunits (Fig. 7A). This concatenated process situates NMDARs close to MORs to build together the negative feedback on opioid signaling.
FIG. 7.
The redox-regulated coupling of NMDARs with MORs: effect of σ1R ligands. (A) MOR-mediated activation of NMDARs: (I) Zinc-mediated association of PKCγ with the HINT1 protein. Zinc was removed from the recombinant proteins (TPEN-EDTA buffer) before incubation of 200 nM HINT1 with 100 nM GST-PKCγ in presence of ZnCl2. GST alone did not bind to the HINT1 protein. CRD stands for cysteine-rich domain and P for precipitation of the GST protein with GS. A similar study was conducted between 100 nM GST-RGSZ2 and HINT1. (II) The RGSZ2, PKCγ, and HINT1 form a ternary complex: 200 nM HINT1 were incubated with 100 nM GST-RGSZ2 in the presence of 100 nM ZnCl2 and increasing concentrations of PKCγ. Details as in (I). (III) PKCγ disrupts HINT1-RGSZ2 association in presence of GαGTPγS subunits. GST-RGSZ2 (100 nM) and HINT1 (200 nM) were incubated in the absence or presence of 100 nM Gαi2GTPγS and of PKCγ. (IV) Effect of PKCγ on RGSZ2 and HINT1. RGSZ2 (100 nM) or HINT1 (100 nM) were incubated for 20 min at RT with 30 nM PKCγ. The PKCγ-induced phosphorylation of RGSZ2 and HINT1 was evaluated using specific anti-phospho antibodies. (V) Effect of HINT1 phosphorylation on its association with RGSZ2 and the C-terminal cytosolic region of NR1 subunit (C0-C1-C2). Native and PKC-phosphorylated HINT1 proteins (200 nM) were incubated with either 100 nM GST-RGSZ2 or GST-NR1. (VI) Phosphorylation of NR1 C0-C1-C2 by PKC, and (VII) its association with HINT1 and σ1R. Further details in Materials and Methods section. (B) Left: Effect of PG and PN sulfate on the association σ1R-NR1 C0-C1-C2. Agarose-NR1 was preincubated in the presence of 2.5 mM CaCl2 (30 min, RT) with the σ1R (100 nM) before the addition of 30 μM PG or PN (30 min, RT). P indicates that agarose was recovered and washed before the analysis of the NR1-bound σ1R through SDS-PAGE and WB. Each bar represents the mean±SEM of three determinations using different gels and blots. *Significant differences with respect the control without neurosteroid, ANOVA-Student–Newman–Keuls test; p<0.05. Right: Influence of increasing concentrations of PG on the association of NR1 with σ1Rs. Agarose-NR1 was incubated with the σ1R before the addition of PG. Agarose-NR1 carrying the associated σ1Rs was recovered and washed before the addition of 200 nM HINT1 or 100 nM CaM (in the presence of 2.5 mM CaCl2 and of 30 μM peptide 4). Low: This assay was also conducted with σ1R antagonists (S1RA and BD1047) and the agonist PRE084 in the absence or presence of CaM. Diagram: S1RA removes the σ1R from the NR1 subunit, favoring the binding of Ca2+-CaM, which impairs the interaction of NMDARs with MOR-HINT1 complexes. As a result, morphine only recruits a fraction of the NMDAR activity that is required to control its effects. Amino acid charge, see key in Fig. 3C. GS, glutathione-sepharose; WB, Western blotting. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The binding of the σ1R to the NR1 subunit covers a region that is critical for the high-affinity binding of negative regulators of NMDAR function, such as HINT1 and Ca2+-CaM (11, 63). The in vitro assays indicated that pregnenolone sulfate enhanced and progesterone diminished the association of NR1 with the long isoform of the σ1R. On the reduction of σ1Rs binding to NR1 subunits, the NMDAR subunit was made available for CaM (in the presence of peptide 4 and 2.5 mM CaCl2) or HINT1 binding. This pattern was also observed for the exogenous ligands of the σ1R receptor, and while the antagonists S1RA and BD1047 stimulated CaM binding, the agonist PRE084 did not (Fig. 7B). In addition, the peptide interference assay showed that σ1Rcd peptides 7 and 10 could couple to the steroid-binding domain, reducing the σ1R-NR1 interaction. It is possible that these peptides and σ1R antagonists share a common mechanism that alters the structure of the σ1R and then diminishes its affinity for NR1 binding (Supplementary Fig. S2). Thus, by affecting the interaction of σ1Rs with NR1 subunits, σ1R antagonists regulate the activity of NMDARs.
The σ1R is essential for MOR-NMDAR cross-regulation
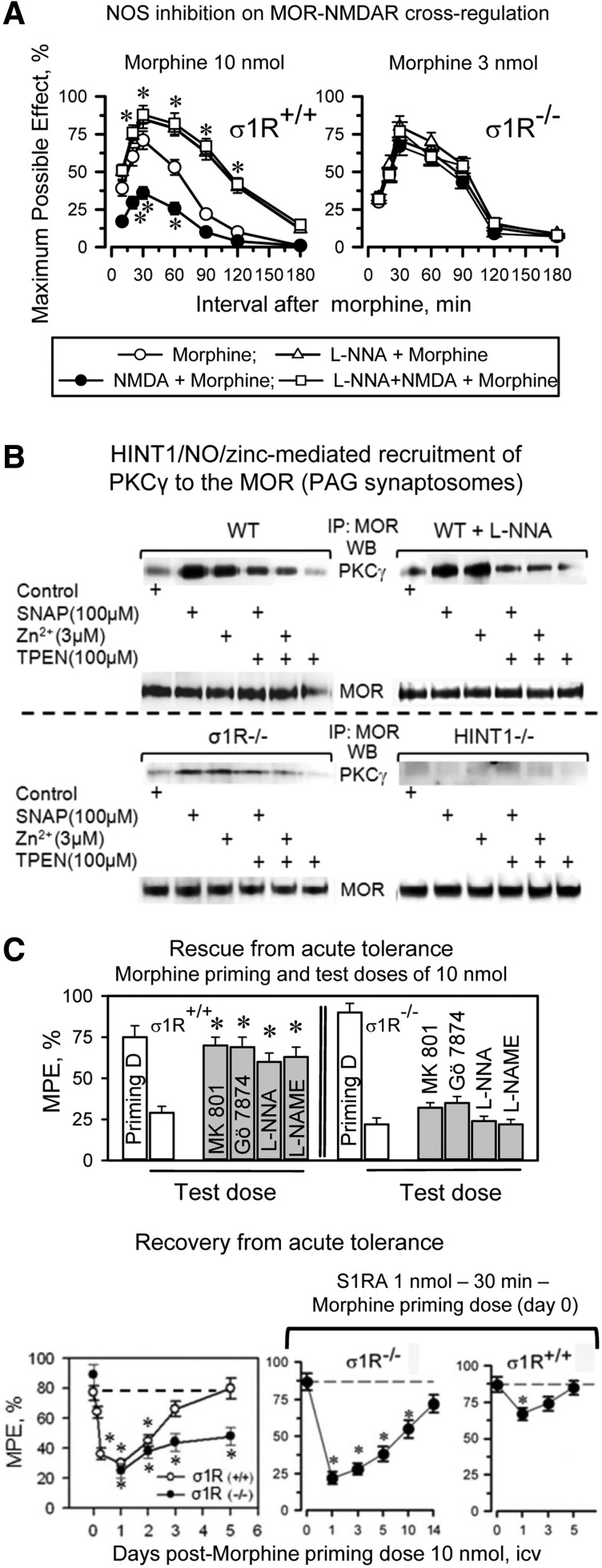
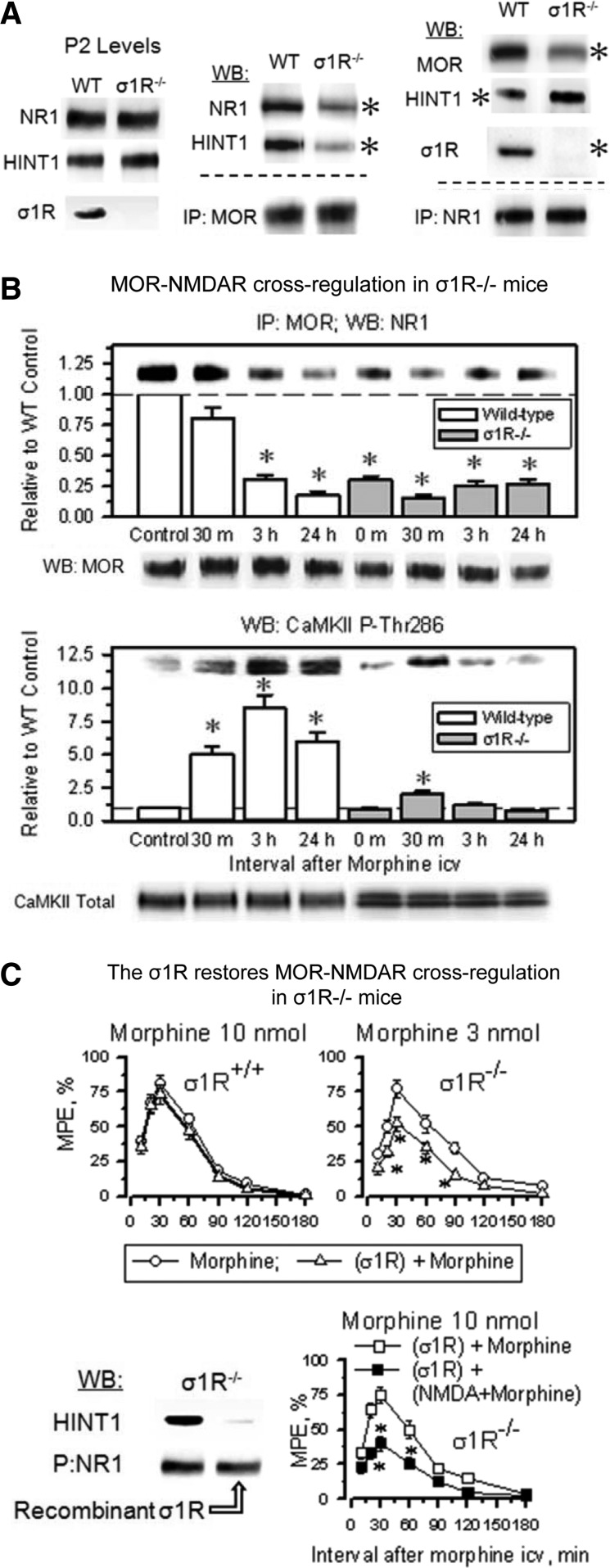
In σ1R−/− mice, the opioids showed an enhanced capacity to produce antinociception, and in these mice, the antinociceptive peak effect of an icv dose of 3 nmol morphine was comparable to that produced by 10 nmol morphine in wild-type mice (Fig. 8A). The administration of an agonist of NMDARs, NMDA, to wild-type mice significantly reduced the capacity of morphine to produce antinociception; however, NMDA failed to do so in σ1R−/− mice. Because MOR-NMDAR cross-regulation is a redox-regulated process that depends on NO and zinc metabolism (51, 54), in wild-type mice, the inhibition of NOS enhanced morphine antinociception and prevented NMDA from reducing the antinociceptive capacity of morphine; however, this approach was not effective in the σ1R−/− mice (Fig. 8A). At the molecular level, the inhibition of NOS prevented morphine from recruiting PKCγ in the MOR environment, and then opioid- and PKCγ-triggered activation of the NMDAR-CaMKII pathway, as well as the weakening of MOR-NR1 interaction were not observed (Supplementary Fig. S5). Synaptosomal membranes from mice that were treated in vivo with NOS inhibitors responded to SNAP, NO donor, or ZnCl2, recruiting PKCγ to the MOR environment. This effect was prevented by the co-incubation of the PAG membranes with TPEN, a zinc chelator. These observations indicate that the in vivo administration of NOS inhibitors specifically prevented morphine from stimulating NO production but left operative the NO-mediated removal of zinc ions from zinc fingers. The MOR-coupled HINT1 protein binds PKCγ through zinc ions (54). Accordingly, in PAG synaptosomes from HINT1−/− mice, SNAP or zinc ions did not stimulate PKCγ arrival at the MOR. The absence of σ1Rs greatly impaired the arrival of PKCγ to MORs, suggesting an altered MOR-HINT1 relationship, but some response to SNAP and zinc ions could still be observed (Fig. 8B).
FIG. 8.
The MOR-NMDAR cross-regulation requires NO and zinc metabolism: the role of σ1Rs. (A) Because σ1R−/− mice display enhanced antinociception to opioids, the effect of the direct activation of NMDARs by icv-injection of NMDA and of NOS inhibition, 3 nmol morphine was studied instead of 10 nmol used in wild-type mice. Saline or 50 pmol NMDA, 7 nmol L-NNA were icv-injected at 20 min before morphine treatment into wild-type and σ1R−/− mice, and analgesia was determined using in the warm water tail-flick test. *Significantly different from the group that received saline and morphine, ANOVA-Student–Newman–Keuls test, p<0.05. (B) NOS provides the zinc ions that are required for the recruitment of PKCγ to the HINT1 protein in the MOR environment. PAG synaptosomes obtained from wild-type mice with and without in vivo inhibition of NOS, σ1R−/− mice, and HINT1−/− mice were incubated for 4 h at 4°C with ZnCl2, the NO generator SNAP, and the metal ion chelator TPEN. Subsequently, free zinc ions were removed by centrifugation and extensive washing. The synaptosomal membranes were then solubilized and incubated with affinity-purified IgGs raised against extracellular sequences in MOR. The MOR-associated proteins were then separated by SDS-PAGE and analyzed by Western blotting. Doses of reactives and the incubation time were taken from Refs. (51, 54). IP signifies immunoprecipitation and WB analysis. (C) Rescue of morphine acute analgesic tolerance via inhibition of NMDAR, NOS, or PKC. A morphine priming dose of 10 nmol was icv-injected, and analgesia was evaluated 30 min later in the “tail-flick” test. The noncompetitive NMDAR antagonist MK801 (1 nmol), the NOS inhibitors L-NNA (7 nmol) and L-NAME (20 nmol), or the PKC inhibitor Gö7874 (1 nmol) were icv-injected at 30 min before administering 24 h later an identical morphine test dose of 10 nmol, and analgesia was again measured 30 min later. Each bar indicates the mean±SEM of the analgesia; n=8 mice. *Significantly different from the group that received saline before the morphine test dose, p<0.05. Recovery from morphine acute antinociceptive tolerance produced by a single dose of 10 nmol morphine in wild-type and σ1R−/− mice: effect of the antagonist of σ1Rs, S1RA. After icv-injecting all of the mice with the morphine priming dose of 10 nmol, the analgesia was evaluated in the thermal tail-flick 30 min later. At the time intervals that are indicated in the figure, a different group of four mice received a test dose of 10 nmol morphine, and analgesia was subsequently evaluated 30 min later. *Significantly different from the control group that received the priming dose of morphine, ANOVA, Student–Newman–Keuls test, p<0.05. In a parallel assay, the mice received 1 nmol S1RA at 30 min before the morphine priming dose. The evaluation of antinociceptive tolerance was as described earlier. *Significantly different from the control group that received S1RA and the priming dose of morphine, p<0.05. NO, nitric oxide.
The analgesic acute tolerance that an icv-dose of morphine produces in wild-type mice can be reverted by NMDAR antagonists, PKC inhibitors, and NOS inhibitors. However, this effect could not be produced in σ1R−/− mice (Fig. 8C). In σ1R−/− mice, the NMDAR-mediated regulation of MOR signaling is impaired, and this control is apparently taken beyond the receptor level, most likely at the effectors. Thus, in σ1R−/− mice, this protective desensitization affects not only MORs but also other GPCRs, such as α2-adrenoceptors and cannabinoids (Supplementary Fig. S6A). Recovery from this state of heterologous tolerance requires longer periods than those that are produced by MOR-coupled NMDAR activity, and in σ1R−/− mice, the restoration of analgesic effects could not be accelerated σ1R antagonists (Fig. 8C).
The earlier observations indicate that the σ1R plays an essential role in bringing MOR signaling under the negative control of NMDAR activity. Thus, we analyzed whether the deletion of σ1R influences the MOR-HINT1-NR1 association. Indeed, in σ1R−/− mice, the MOR-NR1 association was greatly diminished, most likely because HINT1 primarily associates with NR1 subunits (Fig. 9A). Thus, in the absence of σ1Rs, the MOR-NMDAR cross-regulation is impaired, and morphine produced almost no recruitment of NMDAR activity, that is, CaMKII autophosphorylation or weakening of the residual MOR-NR1 association, molecular events that were not altered by the administration of S1RA (Fig. 9B and Supplementary Fig. S6B). Interestingly, the in vivo icv-injection of the recombinant σ1R restored the negative influence of NMDAR activation and diminished MOR-mediated morphine analgesia in σ1R−/− mice, and this effect was associated with a decrease in the binding of HINT1 proteins to NR1 subunits (Fig. 9C).
FIG. 9.
The σ1R connects MOR with the negative regulation of NMDAR. (A) Presence of NR1 subunits, HINT1 and σ1R in the synaptosomes of PAG obtained from wild-type and σ1R−/− mice. IP: MOR or NR1 was immunoprecipitated, and the co-precipitated proteins were detected by WB. *Significantly different from the paired group (n=3), ANOVA, Student-Newman–Keuls test, p<0.05. WT (wild-type mice), KO (σ1R−/− mice), and P2 (synaptosomal fraction). (B) Morphine promotes little activation of the NMDAR-CaMKII pathway in σ1R−/− mice. Mice were icv-injected with 10 nmol morphine, and ex vivo determinations were performed at the indicated postopioid time intervals. In σ1R−/− mice, MOR binding to NR1 subunits was impaired, and then morphine hardly altered the association that remained. Moreover, in the absence of σ1Rs, morphine recruited little CaMKII activity and only for a short interval. Details in Figure 5C and Supplementary Figure S4. (C) The icv-injection of the recombinant σ1R in σ1R−/− mice reduced the antinociceptive potency of morphine. The mice were icv-injected with 0.5 nmol recombinant σ1R, and the effect of morphine was evaluated 24 h later. *Significantly different from the group that received saline instead of the σ1R, p<0.05. The icv-injection of the σ1R restored the NMDAR negative regulation on MORs. The σ1R−/− mice that had received the σ1R were icv-injected with 50 pmol NMDA and 10 nmol morphine 24 h later. *Significantly different from the group that received saline instead of NMDA, p<0.05. Inset: Solubilized brain membranes from σ1R−/− mice were incubated with biotinylated IgGs directed against the NR1. After recovery with streptavidin-sepharose (P), the NR1-containing complexes were exposed to σ1R recombinant protein (200 nM) before detecting HINT1-associated proteins by SDS-PAGE chromatography and WB. This study was repeated at least twice using different preparations. Equal loading was determined from the NR1 signal.
The σ1R prevents the swapping of HINT1 proteins between MOR and NR1 subunits
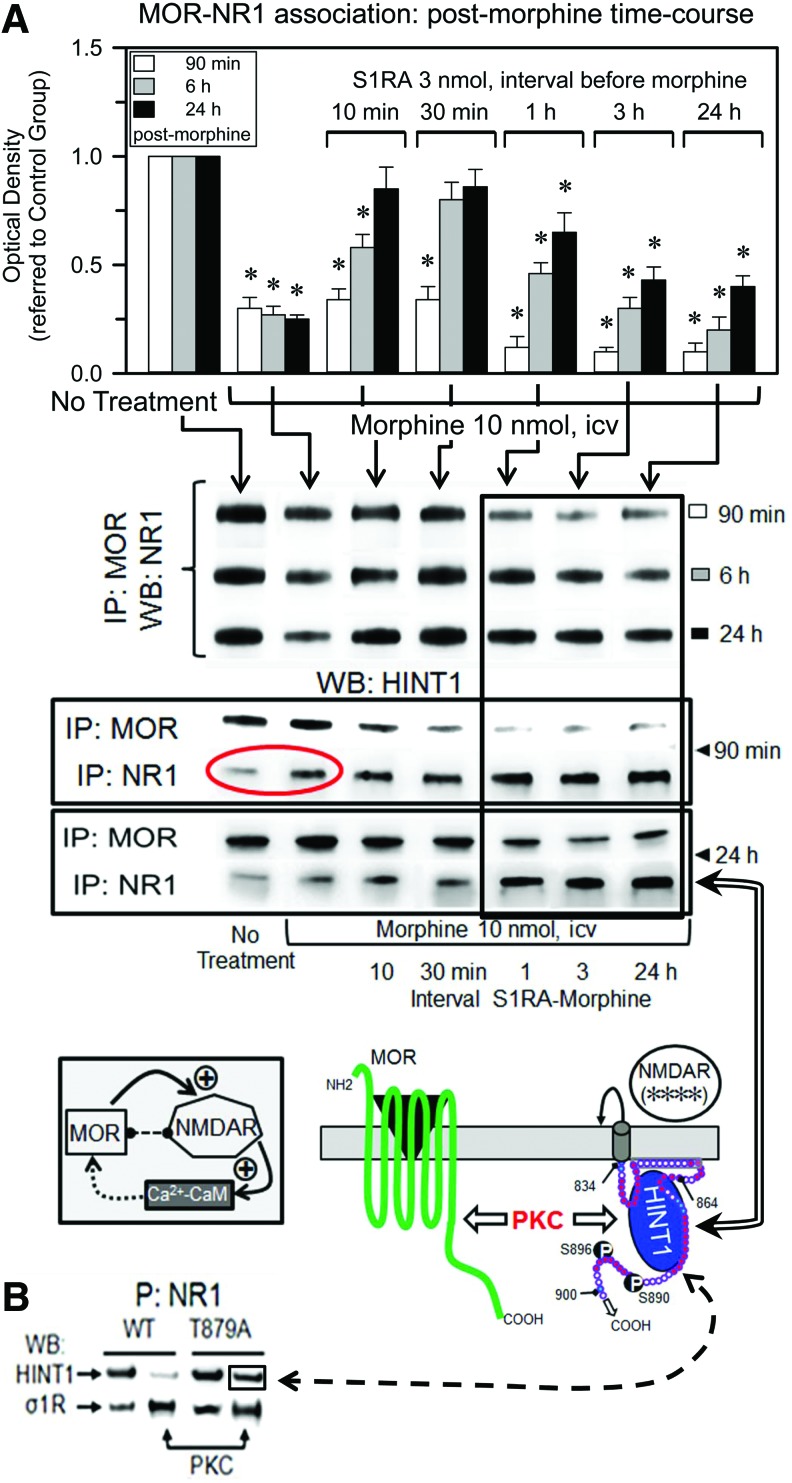
The administration of S1RA at 10 min or 30 min before morphine administration reduced the opioid-induced phosphorylation of the NR1/NR2 subunits and promoted an early re-association of NR1 subunits under MOR-HINT1-negative control, thereby limiting the activity of the NMDAR-CaMKII pathway (Figs. 5C, 6A and 10A). However, longer S1RA-morphine intervals, that is, 1, 3, and 24 h, brought about a delayed re-association between the morphine-activated MORs and NR1 subunits (Fig. 10A). Interestingly, in wild-type mice, morphine alone promoted certain transfer of HINT1 proteins from MORs to NR1 subunits (Fig. 10A and Supplementary Fig. S7), and S1RA given before morphine greatly increased this translocation. Thus, when S1RA was given from 1 to 24 h before morphine, the transfer of HINT1 proteins increased. Under these circumstances, PKC still acts on NR1 C1 S890/896/897 (Fig. 6A); however, the kinase is now with HINT1 at the NMDAR side and hardly reaches the MOR to promote those changes that are responsible for acute analgesic tolerance. Thus, the MOR-NMDAR physical and functional uncoupling was observed for HINT1−/− mice (48) and for σ1R−/− mice in this study.
FIG. 10.
The σ1R antagonist S1RA transfers HINT1 proteins from morphine-activated MORs to NMDAR NR1 subunits. (A) Groups of 48 mice each received an icv injection of only morphine or 3 nmol S1RA at 10, 30 min, 1, 3, or 24 h before treatment with 10 nmol morphine. A total of eight mice from each group were sacrificed at 90 min, 6 or 24 h after opioid treatment. Control mice received saline instead of S1RA or morphine. The MORs were IP from PAG synaptosomes, and the co-precipitated NR1 subunits were immunodetected by WB. *Significantly different from the respective control group that received saline instead of morphine, p<0.05. Association of HINT1 with MORs and NR1 subunits. In the experimental groups described earlier, the association of HINT1 proteins with MORs and NR1 subunits was determined for control, morphine- and S1RA-morphine-treated groups at 90 min and 24 h postmorphine treatment. The circle shows that morphine alone induces the transfer of HINT1 to NR1 subunits. The S1RA-morphine intervals that did not enhance analgesia but conferred protection from tolerance are framed. Controls of MOR and NR1 immunoprecipitation are shown in Supplementary Figure S7. The diagrams represent the framed S1RA-morphine intervals. S1RA administered 1 or 24 h before morphine did not enhance analgesia or alter the MOR-promoted NMDAR activity at 24 h, as indicated by NMDAR (****) (Figs. 5A and 6A). However, some protection against analgesic tolerance was observed (Fig. 6B). On the S1RA-mediated removal of σ1Rs, the NR1 subunit binds tightly to HINT1 at the MOR C terminus. HINT1 carries the activated PKCγ that acts on NR1 C1 S890/896, promoting the separation of the MOR C terminus from NR1-HINT1. Under these circumstances, CaMKII and PKC barely reach the MOR, and then analgesic tolerance to morphine develops at a slower rate (6, 9, 48). (B) Native or mutated NR1 T879A (100 nM) binds similarly to HINT1 (200 nM). While PKC-phosphorylated native NR1 displayed poor affinity for HINT1, PKC-phosphorylated NR1 T879A bound to the HINT1 protein. The phosphorylation of either form of NR1 greatly augmented its association with σ1Rs. Amino acid charge, see key in Fig. 3C. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In the absence of morphine, σ1R antagonists disrupt σ1R-NR1 binding, which facilitates the association of NR1 subunits with HINT1 (Fig. 7A). Notably, several hours after S1RA administration and in the absence of morphine challenge, the basal association between MORs and NR1s increased (Supplementary Fig. S8). It is under these circumstances that morphine promotes the long-term transfer of HINT1 proteins to NMDARs (Fig. 10A). Regarding the NR1-HINT1 interaction, in vitro PKC acts on NR1 C1 T879 and Ser 890, 896 (60), thereby abolishing HINT1 binding (Fig. 7A). However, ex vivo data suggest that HINT1 binds to NR1 subunits in the presence of the serine phosphorylation of C1 segment, such as S890 (Figs. 5C and 10A). Within the S1RA-induced MOR-NMDAR complex and before morphine activates PKCγ, HINT1 could bind to the NR1 region of T879. Thus, we evaluated whether HINT1 binds to PKCγ-phosphorylated T879A NR1 subunits, and the results indicate that this binding is indeed possible (Fig. 10B). Interestingly, PKCγ increased the binding of NR1 subunits to σ1Rs, indicating that in the absence of σ1R antagonists, the phosphorylation of C1 segment could increase the ability of σ1Rs to remove MOR-HINT1 binding to NR1 subunits.
Discussion
The antinociceptive effects of opioids are related to their capacity to diminish calcium-dependent neurotransmitter release. Thus, to prevent opioids from producing an excessive reduction of neuronal excitability, NMDARs are recruited to the MOR environment, where they become activated to restrain opioid signaling (45, 61). For this control to be effective, the negative feedback must be proportional to the power of MOR signaling. The disruption of this balance could provoke a disproportionate NMDAR function, leading to cell damage or to an excessive MOR activation, which negatively affects cell homeostasis. The results of this study indicate that σ1Rs are necessary to establish the NMDAR control on MOR signaling by facilitating the MOR-HINT1-σ1R-NMDAR protein assembly. In this context, the σ1R cooperates with the HINT1 protein to equilibrate the negative influence of NMDARs to the strength of the MOR signals. This process is redox regulated, and NOS inhibition or the targeted deletion of either HINT1 or σ1R increases the MOR-mediated analgesic effects that elude regulation through NMDAR activity (20, 48, 57; and the present study). Interestingly, σ1R antagonists affect MOR function comparable to the effect of the removal of σ1R/HINT1 proteins, that is, the impairment of the capacity of MORs to trigger NMDAR-mediated restrain on opioid signaling. Notwithstanding, the experimental use of σ1R antagonists in wild-type mice was successful in increasing MOR-mediated analgesic effects without promoting tolerance. Therefore, this cellular mechanism can be exploited without triggering an excessive MOR function, which could recruit alternative systems to ultimately induce MOR hypofunction.
The σ1R as a ligand-regulated chaperone in its interaction with different proteins could adopt different conformations. Thus, in its particular binding to NMDAR NR1 subunits at the cell membrane, the cellular and in vitro assays suggest that the σ1R loop faces the cytosolic side (4, 44, 55). The NR1 C terminal sequence is a target of the calcium-binding protein CaM, which applies negative feedback to down-regulate the gating of the NMDAR calcium channel in response to high levels of cytosolic Ca2+ (11). The NR1 C1 amino-acid sequence is essentially a positively charged domain that includes the binding site for the σ1R loop that overlaps with that of the negatively charged HINT1 protein (34) and with the Ca2+-CaM binding domain. The σ1R loop binds specific regions on NR1 C0-C1 segments, while the σ1Rcd interacts with the NR1 C0 region. The σ1R has an SIM that could potentially bind a series of sumoylated proteins in the MOR environment, such as the RGSZ2 (16). The relevance of these SUMO-SIM regulatory interactions warrants further study.
In response to opioids, such as morphine, the σ1R is essential to bring the NMDARs under the control of MOR-HINT1 complexes. This process is exquisitely regulated, begins with the activation of MORs, and requires the redox-regulated HINT1 protein, the redox zinc switch RGSZ2 protein, and redox sensor proteins, such as PKC and Raf-1 (15, 47, 50, 51, 54). In the absence of MOR activation, the HINT1 protein carries the RGSZ2-nNOS assemblage that blocks MOR interaction with NMDARs. Opioids release this barrier and enable the arrival of the NMDAR to the MOR-HINT1 complex through NO and the zinc-dependent binding of PKCγ to the HINT1 protein (47, 50, 54). Within the MOR-NMDAR interaction, HINT1 binds the Ca2+-CaM site in the NR1 C1 segment and inhibits NMDAR activity, whereas the σ1R covers the C0 and the upper region of the C1 segment and reduces the overall affinity of NR1 subunits toward HINT1 proteins (60). To build up the negative feedback on opioid signaling, PKCγ phosphorylates the NR1 C1 Ca2+-CaM site to release the NMDAR from the HINT1 inhibitory influence. The NMDAR is now ready to collaborate with the MOR to increase PKCγ and CaMKII activities and make operative the control on opioid signaling (14). Whether the activity of the MORs reaches a certain threshold, PKCγ increases the formation of reactive oxygen species (ROS) by acting on NOX/NADPH, consolidating the long-term PKCγ activation that is required to regulate the Raf-1/MAPK cascade and enhancing NMDAR function. Thus, NADPH/ROS production and the sustained activation of PKCγ triggered by opioids are essential to develop and maintain NMDAR-mediated tolerance to the analgesic effects of morphine (10, 23).
In the absence of HINT1, the MOR-NMDAR cross-regulation is disrupted, and PKCγ cannot reach specific residues in the MOR C-terminal or the third internal loop, as required to desensitize responses to opioids and, consequently, the capacity of morphine to produce antinociception increases (6, 9, 48). In σ1R−/− mice, MORs and NMDARs are also molecularly and functionally disconnected because the HINT1 protein swaps MORs with NMDAR NR1 subunits. The cytosolic calcium levels regulate the permeation of Ca2+ ions through the NMDAR pore; thus, σ1R binds to the NR1 subunit in a calcium-dependent manner, blocking the entrance of Ca2+-CaM and also weakening that of HINT1 proteins (55). In the presence of Ca2+-CaM, the removal of σ1Rs facilitates its inhibitory binding to the NR1 subunit to reduce NMDAR calcium fluxes. However, reductions in the cytosolic calcium levels diminish the interaction of NR1 subunits with σ1Rs and the presence of Ca2+-CaM, thereby favoring NR1 binding to HINT1 proteins. Under these circumstances, MOR-coupled HINT1 binds tightly to Ca2+-CaM domains on NR1 subunits, producing some inhibition of NMDAR function (20, 63). Then, opioid-activated PKCγ binds to HINT1 and, acting on NR1 S890/896, separates NR1-HINT1-PKCγ from the MOR. The transfer of the PKCγ scaffold, the HINT1 protein, to NR1 subunits situates this kinase activity apart from MORs. Accordingly, the in vivo administration of recombinant σ1Rs to σ1R−/− mice removed the HINT1 binding from NR1 subunits and restored the negative influence of NMDARs on MOR-mediated antinociception.
The role of σ1Rs in the function of at NMDAR suggests that σ1R ligands could regulate the strength of MOR signaling. Indeed, it has been consistently reported that σ1R agonists reduce and σ1R antagonists enhance morphine analgesia (25, 39). In in vitro assays, agonists enhanced and antagonists reduced the association of σ1Rs with NR1 subunits; however, the reducing effects of σ1R agonists on opioid analgesia are not so evident. The release of endogenous σ1R agonists, most likely neurosteroids, could mask the effect of the exogenous. The selective σ1R antagonist S1RA induces a two-fold reduction in the analgesic ED50s of different opioids against thermal stimuli (64, 70). The effect of icv S1RA on morphine supraspinal analgesia depended on the interval between the administration of these drugs, showing an optimal effect at ∼10–30 min and nearly no effect after 1 h. When S1RA was administered shortly before morphine, the σ1R antagonist reduced the capacity of the opioid to activate the NMDAR/CaMKII pathway in the MOR environment. Consequently, the negative feedback of NMDARs on MOR signaling was impaired, and morphine analgesia increased from the initial intervals postopioid with almost no development of acute tolerance.
Because the NMDAR contributes to the recruitment and activation of PKCγ in the postsynapse (5), when used at intervals that reduced the morphine-induced activation of NMDARs, antagonists of σ1Rs also weakened that of PKCγ. The extension of the S1RA-morphine interval for 3 or 24 h did not potentiate morphine analgesia, but some protection against acute tolerance was still observed. The molecular data suggest that the administration of S1RA long before morphine treatment increases the capacity of the opioid to swap the HINT1 proteins from MORs to NR1 subunits. In σ1R−/− mice, the HINT1 protein, to the detriment of its association with MORs, was mostly found at NR1 subunits. This observation suggests that S1RA alters the conformation of the σ1R (7, 33) and then removes its regulatory binding to the NR1 subunit. At this point, two scenarios could account for the influence of the S1RA-morphine interval on morphine analgesia and NMDAR function: In the first scenario, Ca2+ levels are provided by morphine-activated PLCβ, and S1RA, by disrupting σ1R-NR1 binding, facilitates the access of Ca2+-CaM to reduce the activation that MOR-PKCγ promotes on NMDARs. The reduction of NMDAR function diminishes the cellular influx of Ca2+ and the local formation of Ca2+-CaM, and on clearance of the σ1R antagonist the σ1Rs bind again to NR1 subunits, enabling in the absence of HINT1-bound RGSZ2 the inhibitory re-association of NMDARs with MOR-HINT1 complexes. Thus, S1RA removes the negative influence of NMDARs on MOR function; enhances morphine analgesia in naïve mice; as well as recovers the effects of morphine in mice chronically treated with the opioid. The other scenario considers the absence of MOR activation, low Ca2+, and, consequently, low Ca2+-CaM levels; in these circumstances S1RA, by removing the σ1R, promotes the tight binding of RGSZ2-free MOR-HINT1 complexes to NR1 subunits. When morphine recruits PKCγ to the HINT1 protein, the absence of σ1Rs facilitates the swap of HINT1 from MORs to NR1 subunits. The activation of MORs increases, via PLCβ, calcium levels and that of Ca2+-CaM; however, PKCγ acting on NR1 S890 prevents Ca2+-CaM and HINT1 inhibitory binding to CaM binding site on the NR1 subunit while not abrogating that of HINT1 to the upstream region of the NR1 C1 segment. In these circumstances, when bound to HINT1-NR1, PKCγ cannot transmit its negative influence on the MOR cytosolic residues, and the morphine analgesic tolerance develops at a slower rate. The clearance of the σ1R antagonist S1RA, together with the increases in local calcium, allows σ1R to disrupt NR1-HINT1 interaction and re-establish σ1R-NR1 and MOR-NR1 complexes. Thus, the S1RA- and calcium-dependent status of the σ1R-NR1 association determines whether HINT1 swaps partners between MOR and NR1 subunits.
Morphine alone also promoted the transfer of HINT1 proteins, but this effect was limited in extent and reversible in the short term. In the absence of the σ1R antagonist, the presence of calcium rapidly restored σ1R-NR1 binding and the regulation of HINT1 through MORs. Thus, HINT1 swapping is under physiological regulation by endogenous agonists of the σ1R (19). Notably, the neuroactive steroid pregnenolone is released in response to drugs that act at GPCR-NMDAR complexes, such as cannabinoids and opioids (62). Pregnenolone displays agonist activity at σ1Rs, whereas its metabolite progesterone acts as an antagonist (37). However, pregnenolone is rapidly converted into progesterone, and its effects are mostly associated with the antagonism of σ1Rs (65). To delay this metabolism, pregnenolone is sulfated by pregnenolone sulfotransferase (SULT2B1a), and this enzyme is induced in response to events that are associated with NMDAR activity, such as AMPA receptors and NO (30).
Thus, σ1R agonists promote and antagonists reduce the activity of NMDARs, and these effects have been documented in electrophysiological studies as changes in NMDAR currents (36, 68) and variations in the PKC-mediated phosphorylation of NR1 subunits (26, 27). These profiles of neurosteroids on NMDAR activity when coupled with the opioid-mediated activation of MOR most likely contribute to the regulation of their analgesic effects. Thus, pregnenolone sulfate would promote NMDAR control of MOR activity, and if the strength of MOR signaling produces an NMDAR activity that compromises cell homeostasis, then pregnenolone could be converted into the σ1R antagonist progesterone to uncouple NMDARs from the activating influence of MORs.
In addition, in neuropathic pain where NMDARs become over activated, the antagonists of σ1Rs could disconnect the origin of such activation and/or promote the binding of negative regulators of NMDAR function, thereby demonstrating a therapeutic potential (8, 52, 53, 70).
Materials and Methods
In vitro interactions between recombinant proteins: pull-down of recombinant proteins and phosphorylation assays
A series of recombinant proteins were obtained (see Supplementary Materials and Methods section), and having demonstrated that the σ1R, NR1 C0-C1-C2, or HINT1 proteins did not bind to GST (100 nM, Z02039; GenScript Co., Piscataway, NJ) (55, 56), we determined the association of σ1R with NMDAR NR1 subunits. The NR1 C-terminal sequence C0-C1-C2 or mutated NR1 T879A was immobilized through covalent attachment to NHS-activated sepharose 4 fast flow (#17-0906-01; GE Healthcare, Barcelona, Spain) according to the manufacturer's instructions. The HINT1 protein (200 nM) and σ1R variants (100 nM) were incubated either alone (negative control) or together with the immobilized proteins in 400 μl of a buffer containing 50 mM Tris-HCl, pH 7.4 and 0.2% CHAPS and mixed by rotation for 30 min at RT. The influence of Ca2+ on this association was evaluated after the addition of 2.5 mM CaCl2 to the media. After incubation, the pellets were obtained by centrifugation, washed thrice, solubilized in 2×Laemmli buffer, and analyzed by Western blotting.
The regions involved in the interaction of NR1 C0-C1 with σ1R, HINT1, or Calmodulin were investigated through peptide interference of binding using 13 peptides (overlapping five residues) covering C0-C1 region of NR1. The interaction σ1R-NR1 was also analyzed with peptides mapping regions in the σ1R loop and cd (GenScript Co.). The purity of these peptides was higher than 95%.
PKCγ-mediated phosphorylation of the HINT1-RGSZ2 assembly. The Gα subunits were previously incubated with 10 μM GTPγS in 50 μl of 10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA buffer (Gi2GTPγS) and the unbound GTPγS was removed (centrifugal filter devices; 10 kDa nominal MW limit, Amicon Microcon YM-10#42407; Merck-Millipore, Barcelona, Spain). After 20 min, the HINT1 and RGSZ2 proteins were incorporated into HINT1-RGSZ2 complexes, then Gαi2GTPγS subunits were added to a final 100 nM, and incubation was continued for an additional period of 15 min. Subsequently, 30 nM PKCγ in 100 μl of kinase buffer (60 mM HEPES-NaOH [pH 7.5]; 3 mM MgCl2; 3 mM MnCl2; 3 μM Na-orthovanadate; 1 mM DTT and 250 μM ATP) was added to the mixture; the reaction was conducted at room temperature, and it was terminated after 20 min by the addition of the PKC inhibitor Gö7874 (Calbiochem; #365252) at a concentration of 5 μM.
The influence of SUMO1 on σ1R's association with the NR1 C0-C1-C2 subunits was determined through the preincubation of recombinant σ1R (100 nM) with agarose-SUMO1 for 30 min with rotation at room temperature in 150 μl of 50 mM Tris-HCl, pH 7.5, 2.5 mM CaCl2, and 0.2% CHAPS. After the removal of free σ1R, NR1 C0-C1-C2 (100 nM) was added to these protein mixtures and incubated for an additional 30 min. In a set of assays, free SUMO1 was added to preformed agarose-NR1-σ1R complexes (100 nM) for 30 min at RT. Agarose-SUMO1 pellets containing the bound proteins were obtained by centrifugation, washed thrice, solubilized in 2×Laemmli buffer, and analyzed by Western blotting.
Animals and evaluation of antinociception
Wild-type and homozygous (σ1R−/−) male sigma receptor knockout mice, backcrossed (N10 generation) onto a CD1 albino genetic background (Harlan Iberica, Barcelona, Spain), and homozygous (HINT1−/−), generously supplied by I.B. Weinstein/J.B. Wang, were used in this study (32, 51). The mice were housed and used in strict accordance with the European Community guidelines for the Care and Use of Laboratory Animals (Council Directive 86/609/EEC). All the procedures for handling and sacrificing the animals were approved by the Committee on Animal Care at CSIC. The animals were housed at 22°C under a 12 h light/dark cycle (lights on from 8 a.m. to 8 p.m.). Food and water were provided ad libitum. The response of the animals to nociceptive stimuli was assessed using the warm water (52°C) tail-flick test. The tail-flick analgesic test applies a thermal noxious stimulus to promote flicking of the mouse's tail, and opioids given by icv route increase the time elapsed between application of the stimulus and the flick. This response comprises a spinal reflex that is under facilitator drive by the brain stem nociceptive modulating network. After icv administration of opioids, the MORs in the ventral region of PAG play an important role in the supraspinal pathways that modulate spinal nociceptive processing (66, 67). Thus, icv morphine modulates descending serotoninergic and adrenergic systems and inhibits responses to nociceptive stimuli, including nociceptive withdrawal reflexes that are organized segmentally, such as the hind limb withdrawal and tail flick reflexes (18). In this analgesic test, the baseline latencies ranged from 1.7 to 2.0 s, and this parameter was not significantly affected by the σ1R ligands or the solvent used ethanol/Cremophor EL/physiological saline (1:1:18), 1.9±0.2 s (n=10). A cut-off time of 10 s was used to minimize the risk of tissue damage. Antinociception is expressed as a percentage of the maximum possible effect (MPE=100×[test latency−baseline latency]/[cut−off time-baseline latency]). Groups of 8 to 10 mice received a dose of morphine, and antinociception was assessed at different time intervals thereafter.
Production of acute and chronic tolerance to the antinociceptive effect of morphine
The development of morphine-induced acute opioid tolerance was monitored as described (15). Briefly, the animals received an icv priming dose of 10 nmol morphine in the right lateral ventricle. A 10 nmol dose produced 70%–80% of the MPE in the “tail-flick” test for analgesia. Controls were injected only with the opioid priming dose, whereas the experimental groups received the drug under study before the morphine priming dose or 24 h later for a few minutes (typically 30 min) before the morphine test dose of 10 nmol morphine. At this point, the analgesic effect of the 10 nmol priming dose had dissipated as evidenced by the restoration of baseline latencies in the tail-flick test. In some assays, the desensitizing effect of icv administration of a priming dose of 10 nmol morphine was addressed by an identical test dose of the opioid administered 24 and 48 h later.
For the study of the time interval required to recover analgesic response from acute tolerance, all the mice were icv-injected with 10 nmol morphine and divided into sub-groups of eight mice each. At increasing intervals postopioid priming dose, a different group was icv-injected with the morphine test dose of 10 nmol morphine. The antinociceptive effect of morphine was evaluated at 30 min postinjection, allowing time for the compound to reach its peak analgesic effect. Development of acute tolerance was ascertained through the comparison of the effects promoted by the morphine priming and test doses. Thus, data are expressed as the mean±SEM from groups of eight mice.
Mice were also pretreated with a schedule of chronic morphine administration (29). The mice were anesthetized by isoflurane inhalation before stereotaxically implanting a sterile cannula in the lateral ventricle (coordinates: 0.3 mm caudal, 1 mm lateral from bregma, and depth 2.3 mm) and fixing it to the skull with dental cement. The animals were allowed to recover for 4 days before the experiments commenced, and icv injections of 10 nmol morphine per mouse were administered daily for 7 consecutive days. The placement of the cannula was verified for each mouse, and only the data obtained from mice with a correctly inserted cannula were included in the statistical analysis.
Immunohistochemistry
A rabbit IgG labeling kit (Zenon Tricolor Rabbit IgG labeling Kit #1; Molecular Probes, Eugene, OR) was used for triple-labeling with the rabbit polyclonal IgGs against: σ1R (internal region 139–157, Invitrogen, Madrid, Spain, 42-3300; Alexa Fluor 488), NMDAR NR1 subunit (C-terminus, Merck-Millipore, Chemicon AB9864; Alexa Fluor 555), and MOR (C-terminal region, Abcam, Cambridge, United Kingdom, ab134054; Alexa Fluor 647). Mouse coronal brain sections with the PAG were incubated with the labeling complex overnight and examined by confocal microscopy (Leica TCS SP-5/LAS AF Lite Software; Microsystems, GmbH, Hohenstein-Ernstthal, Germany). Controls for immunohistochemistry were performed according to standard protocols (for further see details in Supplementary Materials and Methods section and Supplementary Fig. S3).
Immunoprecipitation and Western blotting
After icv morphine, groups of eight mice were killed at various intervals postopioid; PAG were obtained and processed to obtain the synaptosomal pellet as previously described (49), and used for MOR and NR1 immunoprecipitation and co-precipitation of HINT1, NR1, and MOR. This procedure has been described elsewhere (13, 17). Further details are provided in Supplementary Materials and Methods section.
Statistical analysis
ANOVA, followed by the Student–Newman–Keuls test (SigmaStat; SPSS Science Software, Erkrath, Germany) was performed, and significance was defined as p<0.05.
Supplementary Material
Abbreviations Used
- Ca2+-CaM
calcium calmodulin
- CaMKII
calcium and calmodulin dependent kinase II
- cd
C-terminal domain
- CRD
cysteine-rich domain
- CRM
cholesterol-binding motif
- GPCR
G-protein-coupled receptor
- GS
glutathione-sepharose
- HINT1
histidine triad nucleotide-binding protein 1
- HR
hydrophobic region
- icv
intracerebroventricular
- MOR
mu-opioid receptor
- MPE
maximum possible effect
- NMDAR
glutamate N-methyl-d-aspartate acid receptor
- NO
nitric oxide
- nNOS
neural nitric oxide synthase
- NR1/2
subunit 1/2 of the glutamate NMDAR
- PAG
periaqueductal grey
- PG
progesterone
- PKC
protein kinase C
- PMAR
potential membrane attachment region
- PN
pregnenolone
- RGS
regulators of G protein signaling
- ROS
reactive oxygen species
- σ1R
sigma 1 receptor
- S1RA
4-[2-[[5-methyl-1-(2-naphthalenyl)-1H-pyrazol-3-yl]oxy]ethyl] morpholine
- SANR
sumo-associated negative region
- SBDL
steroid-binding-like domain
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SEM
standard error of the mean
- SIM
SUMO-interacting motif
- SNAP
(5)-nitroso-N-acetylpenicillamine
- SUMO
small ubiquitin-related modifier
- TM
transmembrane
- TPEN
N′,N′,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine
- WB
Western blotting
Acknowledgments
The authors would like to thank Concha Bailón and Gabriela de Alba for their excellent technical assistance. Cajal drawings were provided by the Legado Cajal, Cajal Institute, CSIC, Madrid, Spain. This research was supported by the “Ministerio de Sanidad y Consumo-Plan de Drogas 2011–2014” and the “Ministerio de Economía y Competividad (MINECO), SAF 2012-34991.”
Author Disclosure Statement
The authors declare that, excluding income received from their primary employer “Ministerio de Economía y Competitividad, MINECO,” no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional services and that there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. M.M. and J.M.V. are full-time employees at Esteve.
References
- 1.Abadias M, Escriche M, Vaque A, Sust M, and Encina G. Safety, tolerability and pharmacokinetics of single and multiple doses of a novel sigma-1 receptor antagonist in three randomized phase I studies. Br J Clin Pharmacol 75: 103–117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajit SK, Ramineni S, Edris W, Hunt RA, Hum WT, Hepler JR, and Young KH. RGSZ1 interacts with protein kinase C interacting protein PKCI-1 and modulates mu opioid receptor signaling. Cell Signal 19: 723–730, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Aydar E, Palmer CP, Klyachko VA, and Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34: 399–410, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Balasuriya D, Stewart AP, and Edwardson JM. The sigma-1 receptor interacts directly with GluN1 but not GluN2A in the GluN1/GluN2A NMDA receptor. J Neurosci 33: 18219–18224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravarthy B, Morley P, and Whitfield J. Ca2+-calmodulin and protein kinase Cs: a hypothetical synthesis of their conflicting convergences on shared substrate domains. Trends Neurosci 22: 12–16, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Chen YJ, Oldfield S, Butcher AJ, Tobin AB, Saxena K, Gurevich VV, Benovic JL, Henderson G, and Kelly E. Identification of phosphorylation sites in the COOH-terminal tail of the mu-opioid receptor. J Neurochem 124: 189–199, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu UB, Ramachandran S, Hajipour AR, and Ruoho AE. Photoaffinity labeling of the sigma-1 receptor with N-[3-(4-nitrophenyl)propyl]-N-dodecylamine: evidence of receptor dimers. Biochemistry 52: 859–868, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz JL, Zamanillo D, Corbera J, Baeyens JM, Maldonado R, Pericas MA, Vela JM, and Torrens A. Selective sigma-1 (σ1) receptor antagonists: emerging target for the treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 9: 172–183, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Doll C, Konietzko J, Poll F, Koch T, Höllt V, and Schulz S. Agonist-selective patterns of μ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol 164: 298–307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle T, Bryant L, Muscoli C, Cuzzocrea S, Esposito E, Chen Z, and Salvemini D. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett 483: 85–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers MD, Zhang S, Bernhadt JP, and Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84: 745–755, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Finn AK. and Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32: 829–839, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Garzón J, Rodríguez-Muñoz M, López-Fando A, and Sánchez-Blázquez P. Activation of μ-opioid receptors transfers control of Gα subunits to the regulator of G-protein signaling RGS9-2: role in receptor desensitization. J Biol Chem 280: 8951–8960, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Garzón J, Rodríguez-Muñoz M, and Sánchez-Blazquez P. Direct association of Mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance. Curr Drug Abuse Rev 5: 199–226, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Garzón J, Rodríguez-Muñoz M, Vicente-Sánchez A, Bailón C, Martínez-Murillo R, and Sánchez-Blázquez P. RGSZ2 binds to the neural nitric oxide synthase PDZ domain to regulate mu-opioid receptor-mediated potentiation of the N-methyl-D-aspartate receptor-calmodulin-dependent protein kinase II pathway. Antioxid Redox Signal 15: 873–887, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Garzón J, Rodríguez-Muñoz M, Vicente-Sánchez A, García-López MA, Martínez-Murillo R, Fischer T, and Sánchez-Blázquez P. SUMO-SIM Interactions Regulate the Activity of RGSZ2 Proteins. PLoS One 6: e28557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garzón J, Torre-Madrid E, Rodríguez-Muñoz M, Vicente-Sánchez A, and Sánchez-Blázquez P. Gz mediates the long-lasting desensitization of brain CB1 receptors and is essential for cross-tolerance with morphine. Mol Pain 5: 11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebhart GF. and Jones SL. Effects of morphine given in the brain stem on the activity of dorsal horn nociceptive neurons. Prog Brain Res 77: 229–243, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Gibbs TT. and Farb DH. Dueling enigmas: neurosteroids and sigma receptors in the limelight. Sci STKE 2000: e1, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Guang W, Wang H, Su T, Weinstein IB, and Wang JB. Role of mPKCI, a novel mu-opioid receptor interactive protein, in receptor desensitization, phosphorylation, and morphine-induced analgesia. Mol Pharmacol 66: 1285–1292, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, and Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A 93: 8072–8077, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi T. and Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs 18: 269–284, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Ibi M, Matsuno K, Matsumoto M, Sasaki M, Nakagawa T, Katsuyama M, Iwata K, Zhang J, Kaneko S, and Yabe-Nishimura C. Involvement of NOX1/NADPH oxidase in morphine-induced analgesia and tolerance. J Neurosci 31: 18094–18103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerscher O. SUMO junction-what's your function? New insights through SUMO-interacting motifs. EMBO Rep 8: 550–555, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim FJ, Kovalyshyn I, Burgman M, Neilan C, Chien CC, and Pasternak GW. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol Pharmacol 77: 695–703, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, Beitz AJ, and Lee JH. Intrathecal treatment with sigma1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br J Pharmacol 148: 490–498, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HW, Roh DH, Yoon SY, Seo HS, Kwon YB, Han HJ, Kim KW, Beitz AJ, and Lee JH. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol 154: 1125–1134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitaichi K, Chabot JG, Moebius FF, Flandorfer A, Glossmann H, and Quirion R. Expression of the purported sigma1 (σ1) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J Chem Neuroanat 20: 375–387, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Kitto KF. and Fairbanks CA. Supraspinally administered agmatine prevents the development of supraspinal morphine analgesic tolerance. Eur J Pharmacol 536: 133–137, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kohjitani A, Fuda H, Hanyu O, and Strott CA. Regulation of SULT2B1a (pregnenolone sulfotransferase) expression in rat C6 glioma cells: relevance of AMPA receptor-mediated NO signaling. Neurosci Lett 430: 75–80, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Kourrich S, Su TP, Fujimoto M, and Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci 35: 762–771, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, and Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci 18: 2188–2196, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Laurini E, Da Col V, Wunsch B, and Pricl S. Analysis of the molecular interactions of the potent analgesic S1RA with the sigma1 receptor. Bioorg Med Chem Lett 23: 2868–2871, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Lima CD, Klein MG, Weinstein IB, and Hendrickson WA. Three-dimensional structure of human protein kinase C interacting protein 1, a member of the HIT family of proteins. Proc Natl Acad Sci U S A 93: 5357–5362, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin WR, Eades CG, Thompson JA, Huppler RE, and Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther 197: 517–532, 1976 [PubMed] [Google Scholar]
- 36.Martina M, Turcotte ME, Halman S, and Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol 578: 143–157, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurice T. Neurosteroids and sigma1 receptors, biochemical and behavioral relevance. Pharmacopsychiatry 37Suppl 3: S171–S182, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Maurice T. and Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther 124: 195–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei J. and Pasternak GW. Sigma1 receptor modulation of opioid analgesia in the mouse. J Pharmacol Exp Ther 300: 1070–1074, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Merrill MA, Malik Z, Akyol Z, Bartos JA, Leonard AS, Hudmon A, Shea MA, and Hell JW. Displacement of alpha-actinin from the NMDA receptor NR1 C0 domain By Ca2+/calmodulin promotes CaMKII binding. Biochemistry 46: 8485–8497, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monnet FP, Debonnel G, Junien JL, and De MC. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur J Pharmacol 179: 441–445, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Okamoto T, Schlegel A, Scherer PE, and Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273: 5419–5422, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Ortega-Roldan JL, Ossa F, and Schnell JR. Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (BiP) interactions. J Biol Chem 288: 21448–21457, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pal A, Chu UB, Ramachandran S, Grawoig D, Guo LW, Hajipour AR, and Ruoho AE. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the sigma-1 receptor. J Biol Chem 283: 19646–19656, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasternak GW, Kolesnikov YA, and Babey AM. Perspectives on the N-methyl-D-aspartate/nitric oxide cascade and opioid tolerance. Neuropsychopharmacology 13: 309–313, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Robson MJ, Noorbakhsh B, Seminerio MJ, and Matsumoto RR. Sigma-1 receptors: potential targets for the treatment of substance abuse. Curr Pharm Des 18: 902–919, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez-Muñoz M. and Garzón J. Nitric oxide and zinc-mediated protein assemblies involved in mu opioid receptor signaling. Mol Neurobiol 48: 769–782, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez-Muñoz M, Sánchez-Blázquez P, Vicente-Sánchez A, Bailón C, Martín-Aznar B, and Garzón J. The histidine triad nucleotide-binding protein 1 supports mu-opioid receptor-glutamate NMDA receptor cross-regulation. Cell Mol Life Sci 68: 2933–2949, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodríguez-Muñoz M, Sánchez-Blázquez P, Vicente-Sánchez A, Berrocoso E, and Garzón J. The mu-opioid receptor and the nmda receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology 37: 338–349, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodríguez-Muñoz M, Torre-Madrid E, Sánchez-Blázquez P, and Garzón J. NO-released zinc supports the simultaneous binding of Raf-1 and PKCgamma cysteine-rich domains to HINT1 protein at the mu-opioid receptor. Antioxid Redox Signal 14: 2413–2425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez-Muñoz M, Torre-Madrid E, Sánchez-Blázquez P, Wang JB, and Garzón J. NMDAR-nNOS generated zinc recruits PKCgamma to the HINT1-RGS17 complex bound to the C terminus of Mu-opioid receptors. Cell Signal 20: 1855–1864, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Kim KW, Han HJ, Beitz AJ, Na HS, and Lee JH. Intrathecal injection of the sigma1 receptor antagonist BD1047 blocks both mechanical allodynia and increases in spinal NR1 expression during the induction phase of rodent neuropathic pain. Anesthesiology 109: 879–889, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Romero L, Zamanillo D, Nadal X, Sanchez-Arroyos R, Rivera-Arconada I, Dordal A, Montero A, Muro A, Bura A, Segales C, Laloya M, Hernandez E, Portillo-Salido E, Escriche M, Codony X, Encina G, Burgueno J, Merlos M, Baeyens JM, Giraldo J, Lopez-Garcia JA, Maldonado R, Plata-Salaman CR, and Vela JM. Pharmacological properties of S1RA, a new sigma-1 receptor antagonist that inhibits neuropathic pain and activity-induced spinal sensitization. Br J Pharmacol 166: 2289–2306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sánchez-Blázquez P, Rodríguez-Muñoz M, Bailón C, and Garzón J. GPCRs promote the release of zinc ions mediated by nNOS/NO and the Redox transducer RGSZ2 protein. Antioxid Redox Signal 17: 1163–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Sánchez-Blázquez P, Rodríguez-Muñoz M, Herrero-Labrador R, Burgueño J, Zamanillo D, and Garzón J. The calcium-sensitive Sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: implications in antinociception and psychotic diseases. Int J Neuropsychopharmacol 17: 1943–1955, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Sánchez-Blázquez P, Rodríguez-Muñoz M, Vicente-Sánchez A, and Garzón J. Cannabinoid receptors couple to NMDA receptors to reduce the production of NO and the mobilization of zinc induced by glutamate. Antioxid Redox Signal 19: 1766–1782, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sánchez-Fernández C, Nieto FR, González-Cano R, Artacho-Cordón A, Romero L, Montilla-García A, Zamanillo D, Baeyens JM, Entrena JM, and Cobos EJ. Potentiation of morphine-induced mechanical antinociception by sigma receptor inhibition: role of peripheral sigma receptors. Neuropharmacology 70: 348–358, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Shioda N, Ishikawa K, Tagashira H, Ishizuka T, Yawo H, and Fukunaga K. Expression of a truncated form of the endoplasmic reticulum chaperone protein, sigma1 receptor, promotes mitochondrial energy depletion and apoptosis. J Biol Chem 287: 23318–23331, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su TP, Hayashi T, Maurice T, Buch S, and Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci 31: 557–566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, and Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem 272: 5157–5166, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Trujillo KA. The neurobiology of opiate tolerance, dependence and sensitization: mechanisms of NMDA receptor-dependent synaptic plasticity. Neurotox Res 4: 373–391, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, Kasanetz F, Baillie GL, Panin F, Cathala A, Roullot-Lacarriere V, Fabre S, Hurst DP, Lynch DL, Shore DM, Deroche-Gamonet V, Spampinato U, Revest JM, Maldonado R, Reggio PH, Ross RA, Marsicano G, and Piazza PV. Pregnenolone can protect the brain from cannabis intoxication. Science 343: 94–98, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vicente-Sánchez A, Sánchez-Blázquez P, Rodríguez-Muñoz M, and Garzón J. HINT1 protein cooperates with cannabinoid 1 receptor to negatively regulate glutamate NMDA receptor activity. Mol Brain 6: 42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vidal-Torres A, de la Puente B, Rocasalbas M, Tourino C, Andreea BS, Fernandez-Pastor B, Romero L, Codony X, Zamanillo D, Buschmann H, Merlos M, Manuel BJ, Maldonado R, and Vela JM. Sigma-1 receptor antagonism as opioid adjuvant strategy: Enhancement of opioid antinociception without increasing adverse effects. Eur J Pharmacol 711: 63–72, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Wu FS, Gibbs TT, and Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol 40: 333–336, 1991 [PubMed] [Google Scholar]
- 66.Yaksh TL, Al-Rodhan NR, and Jensen TS. Sites of action of opiates in production of analgesia. Prog Brain Res 77: 371–394, 1988 [DOI] [PubMed] [Google Scholar]
- 67.Yaksh TL, Yeung JC, and Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res 114: 83–103, 1976 [DOI] [PubMed] [Google Scholar]
- 68.Yamazaki Y, Ishioka M, Matsubayashi H, Amano T, and Sasa M. Inhibition by sigma receptor ligand, MS-377, of N-methyl-D-aspartate-induced currents in dopamine neurons of the rat ventral tegmental area. Psychopharmacology (Berl) 161: 64–69, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Yoon SY, Roh DH, Seo HS, Kang SY, Moon JY, Song S, Beitz AJ, and Lee JH. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: involvement of the sigma-1 receptor. Neuropharmacology 59: 460–467, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Zamanillo D, Romero L, Merlos M, and Vela JM. Sigma 1 receptor: a new therapeutic target for pain. Eur J Pharmacol 716: 78–93, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.