Abstract
Intrauterine growth restriction (IUGR) due to placental insufficiency is a leading cause of perinatal complications for which there is no effective prenatal therapy. We have previously demonstrated that intraplacental injection of adenovirus-mediated insulin-like growth factor-1 (Ad-IGF-1) corrects fetal weight in a murine IUGR model induced by mesenteric uterine artery branch ligation. This study investigated the effect of intraplacental Ad-IGF-1 gene therapy in a rabbit model of naturally occurring IUGR (runt) due to placental insufficiency, which is similar to the human IUGR condition with onset in the early third trimester, brain sparing, and a reduction in liver weight. Laparotomy was performed on New Zealand White rabbits on day 21 of 30 days of gestation and litters were divided into five groups: Control (first position)+phosphate-buffered saline (PBS), control+Ad-IGF-1, runt (third position)+PBS, runt+Ad-IGF-1, and runt+Ad-LacZ. The effect of IGF-1 gene therapy on fetal, placental, liver, heart, lung, and musculoskeletal weights of the growth-restricted pups was examined. Protein expression after gene transfer was seen along the maternal–fetal placenta interface (n=12) 48 hr after gene therapy. There was minimal gene transfer detected in the pups or maternal organs. At term, compared with the normally grown first-position control, the runted third-position pups demonstrated significantly lower fetal, placental, liver, lung, and musculoskeletal weights. The fetal, liver, and musculoskeletal weights were restored to normal by intraplacental Ad-IGF-1 gene therapy (p<0.01), with no change in the placental weight. Intraplacental gene therapy is a novel strategy for the treatment of IUGR caused by placental insufficiency that takes advantage of an organ that will be discarded at birth. Development of nonviral IGF-1 gene delivery using placenta-specific promoters can potentially minimize toxicity to the mother and fetus and facilitate clinical translation of this novel therapy.
Introduction
Fetal growth restriction (FGR, also called intrauterine growth restriction [IUGR]) is a challenging complication of pregnancy for which there is no effective treatment. It is a term used to designate a fetus that has failed to reach its growth potential in utero because of genetic or environmental factors. FGR results in the birth of an infant who is small for gestational age (SGA), most commonly resulting in birth weight below the 10th percentile. Mortality and morbidity are increased in SGA infants compared with those who are appropriate for gestational age. It is the second leading cause of perinatal morbidity and mortality and affects about 5% of the general obstetric population.1,2 Using the 10th percentile as a standard results in overdiagnosis of IUGR, but enables aggressive screening, as birth weight is probably the single most important factor affecting neonatal morbidity and mortality. About one-fourth of infants who are below the 10th percentile have a normalized birth weight when it is corrected for low maternal weight, paternal phenotype, or residence at higher altitudes. However, some authors suggest using the 5th percentile to define severe IUGR infants.3 Although IUGR is a heterogeneous disease caused by maternal (chronic hypertension), fetal (chromosomal anomalies), and/or placental factors, placental insufficiency accounts for 75% of all cases of IUGR. Placental insufficiency is a complication of pregnancy, in which the placenta, its membranes, or the umbilical cord develop abnormally and cannot bring enough blood supply, oxygen, and nutrients to a fetus growing in the womb, thereby affecting the growth of the fetus. Compelling epidemiologic and clinical studies have shown a strong association between IUGR and the subsequent development of adult diseases such as obesity, type 2 diabetes, and cardiovascular diseases in later life.4–7 Importantly, the incidence of intrauterine growth restriction has increased, presumably due to an increased incidence of placental insufficiency, preterm labor, and/or increased number of multiple pregnancies with the use of in vitro fertilization.8 Furthermore, the survival of IUGR has improved, increasing the incidence of insulin resistance9–12 and metabolic syndrome,13 and exacerbating cardiovascular and renal disease in later life,6,14–16 thereby increasing the health care burden.17 At present, there is no effective prenatal treatment for IUGR or placental insufficiency except a safe premature delivery. Intervention during intrauterine life to prevent the effects of IUGR and postnatal growth perturbations may be the optimal strategy. Maternal supplementation with protein2 or oxygen,18 or infusion therapy (dextrose, vitamin C, vasodilators) with continuous bed rest, may improve intrauterine development, but none of these treatments have been successful at improving the growth of the fetus.
In IUGR, fetal as well as maternal growth factors, including insulin-like growth factor (IGF)-1, are reduced.18,19 The IGF axis has a major regulatory role in placental and fetal growth.20 In transgenic mouse models3,21 IGF-1, IGF-2, and IGF-1/IGF-2 deletion results in 60% weight reduction and reduced placental growth. Human studies also show that growth-restricted fetuses have a reduction in IGF-1, IGF-2, and IGF-binding protein-3 (IGFBP3) and an increase in IGFBP-1.22,23 Administration of recombinant IGF-1 to the mother has had little success in improving fetal growth in animal models,24,25 although studies by Sferruzzi-Perri and colleagues26 did demonstrate an increase in fetal growth and placental nutrient transport in guinea pigs after long-term maternal infusion. Although these studies demonstrate that IGF-1 can rescue the phenotype, this was done in early to mid-pregnancy, typically a point in human gestation before diagnosis of late-onset idiopathic IUGR. Similarly, studies by Darp and colleagues26,27 have demonstrated improved fetal growth after fetal intraamniotic and intravascular delivery of IGF-1 in pregnant sheep; however, multiple weekly injections were required to have an effect. Despite encouraging evidence, these studies also suggest that the use of exogenous protein supplementation of recombinant IGF-1 does not significantly change the primary IUGR end points in a clinically applicable regimen of treatment administration.
Gene transfer is an attractive alternative compared with exogenous protein supplementation for achieving highly efficient expression of growth factors. Placental gene transfer is appealing because it offers the advantage of site-directed gene transfer with reduced risk of either maternal or fetal gene transfer. We have demonstrated that adenovirus-mediated overexpression of growth factors, for example, with Ad-IGF-1, via site-specific intraplacental gene transfer in a murine model was efficient in increasing placental growth and transport, with minimal maternal or fetal dissemination.22,28 Although gene transfer has inherent potential risks of insertional mutagenesis and germ-line gene transfer, the use of adenovirus and the disposable nature of the placenta may mitigate many of these risks.
Taken together, we examined the effect of intraplacental injection of Ad-IGF-1 on fetal and placental growth in a naturally occurring rabbit model of placental insufficiency.29,30 The rabbit has a bicornuate uterus, with four to six fetuses in each horn. In both horns, the presence of a uterine vascular watershed area at position 3 from the ovarian end makes the fetus at this position naturally growth restricted (Runt), with a term birth weight ratio of 0.85 relative to other fetuses.29 The timing of this vascular insult parallels the human IUGR situation with the onset of the condition in the third trimester of the gestation and characterized by brain-sparing and reduction in liver weight. It has been previously established that growth restriction successfully balances reduced oxygen delivery and consumption. Chronic hypoxia in IUGR causes fetal circulatory redistribution during gestation to cardinal organs (brain, myocardium, and adrenal glands),31 the “brain-sparing” effect, which is a fetal adaptive reaction to placental insufficiency. Fetal liver receives approximately 70–75% of the blood from the nutrient- and oxygen-rich umbilical venous (UV) blood, and shunting UV blood through the ductus venosus (DV), thereby bypassing the liver and delivering nutrient-rich blood to the systemic circulation, is one mechanism of redistributing cardiac output, which contributes to the brain sparing seen in IUGR fetuses and results in reduced adaptation and function of the liver. We hypothesize that intraplacental gene therapy with Ad-IGF-1 will restore fetal weight in a naturally occurring rabbit model of intrauterine fetal growth restriction due to placental insufficiency. We report the successful application of gene therapy, using direct intraplacental injection of a replication-incompetent recombinant adenoviral vector, as proof of concept for the ability of intraplacental gene therapy to correct liver and fetal weight due to placental insufficiency.
Materials and Methods
Ethics statement
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia (Philadelphia, PA). Time-mated, pregnant New Zealand White rabbits were obtained from Covance (Denver, PA). Animals were anesthetized with intravenous ketamine and isoflurane inhalation for all procedures. After any procedure, animals were allowed to recover in incubators overnight and all efforts were made to minimize suffering.
Adenoviral constructs
All constructs used in this study were first-generation recombinant replication-defective, serotype 5 adenoviral vectors. All adenoviral genomes had either E1 or both E1 and E3 regions deleted. All transgenes were driven by the cytomegalovirus (CMV) promoter. The parental adenovirus for Ad-IGF-1 was Ad-Easy and the parental adenovirus for Ad-LacZ was dl7001. Both viruses were prepared with 293 cells, purified by double cesium chloride density centrifugation,29 desalted by passage through a DG-10 column (Bio-Rad Laboratories, Hercules, CA), and stored in 50% glycerol at −80°C. Viral titers were determined by measuring absorbance at 260 nm, using a DU-640 spectrophotometer (Beckman Instruments, Fullerton, CA), and by plaque assay. Viral preparations were diluted at least 1:10 with phosphate-buffered saline (PBS) before use in animals in order to prevent glycerol toxicity. The adenoviral construct for human IGF (hIGF)-1 was generously supplied by L. Sweeney (University of Pennsylvania, Philadelphia, PA). The recombinant adenovirus carrying the β-galactosidase transgene (Ad-LacZ) was obtained from J. Wilson (University of Pennsylvania).
Study groups
For establishment of the naturally occurring rabbit runt model in our laboratory, n=6 time-mated pregnant rabbits were used. To randomize, position 1 and position 3 pups (pup position relative to ovarian end) from either the left or the right horn (only one horn per dam) were preassigned to the control group or runt group, respectively. We did not account for pup sex in this study. Although male fetuses tend to be larger than female fetuses, our ongoing work suggests in mouse and rat models that male fetuses are also more severely affected by IUGR in terms of birth weight, catchup growth, and adult onset of obesity and cardiovascular diseases (our unpublished data). In the current experiments, because of the relatively modest numbers of animals in each group and because the pups were randomly preassigned to the various treatment or control groups, we chose not to segregate by sex. At term (30 days), pups and placentas were delivered by caesarian section (C-section). We controlled for the number of pups in the litter by using only dams that had 8–12 pups in the litter with only 4–6 pups on each horn. Placental, fetal, lung, liver, heart, and musculoskeletal weights of the preassigned pups were measured by a blinded investigator. Position 3 pups were compared with position 1 pups, using a t test assuming equal variances. Power analysis of preliminary fetal weight data from n=6 animals demonstrated that n=1 animal is required to achieve a test power >0.8. However, n=5 or 6 pups, each from a different dam, were included in each of the experimental groups in this study.
After the runt model was reproducibly established in our laboratory, position 1 and position 3 pups of pregnant rabbits were randomly preassigned into the various treatment or control groups based on their position relative to ovarian end and the treatments administered.
1. Control (n=6 pups, each randomized to right or left horn from n=6 dams): Fetuses at position 1, exposed to the stress of surgery by transuterine intraplacental injection of PBS.
2. Runt (n=6 pups, each randomized to the same right or left horn from n=6 dams as the control group): Naturally occurring runts at position 3, treated by transuterine intraplacental injection of PBS.
3. Runt+Ad-IGF-1 (n=5 pups, each randomized to right or left horn from n=5 dams): Naturally occurring runts at position 3, treated by transuterine intraplacental injection of Ad-IGF-1 (1×109 plaque-forming units [PFU]).
4. Runt+Ad-LacZ (n=5 pups, each randomized to right or left horn from n=5 dams): Naturally occurring runts at position 3, treated by transuterine intraplacental injection of adenoviral vector carrying the β-galactosidase reporter gene (Ad-LacZ) (1×109 PFU).
5. Control+Ad-IGF-1 (n=5 pups, each randomized to right or left horn from n=5 dams): Normally growing fetuses at position 1, treated by transuterine intraplacental injection of Ad-IGF-1(1×109 PFU).
Experimental design
Under sterile conditions, pregnant rabbits on day 21 of 30 days of gestation were anesthetized with intravenous ketamine (20 mg/kg) and isoflurane inhalation (1–3% in oxygen). Anesthetic depth was gauged by animal's respiratory rate and muscle tone. A midline laparotomy was performed to expose the bicornuate uterus. Fetuses were identified by their position relative to the ovary in their respective horn of the uterus. Intraplacental treatments were delivered to each of the placentas via direct injection with a 30-gauge needle in a volume of 40 μl (two injections of 20 μl each into the placenta) by a trained researcher. Once in the placenta, the syringe was withdrawn to feel negative pressure and to make sure the treatment was not injected into vessels or blood spaces. (See Supplementary Fig. S1 for additional description of the position of pups 1 and 3; supplementary data are available online at www.liebertpub.com/hum. Also, refer to Supplementary Video S1 file for the injection procedure.) After placental injection, the uterus was returned to the abdomen, and the incision was closed in two layers. Respiratory rate, level of activity, and vocalized distress from pain were monitored continuously during surgery and recovery. On gestational day 30 (term) the previous laparotomy incisions were opened under anesthesia, and pups and placentas were delivered, weighed, and fixed or snap frozen in liquid nitrogen for further analysis. Viability and survival of each fetus were recorded to examine survival rates. A sham group of animals that underwent only the stress of the surgery, including midline laparotomy, exposure of the uterine horns, and closure, was included in the study. No intraplacental injections were administered to the pups in these animals. Fetal organs (liver, heart, and lung) were weighed and fixed or snap frozen in liquid nitrogen for further analysis. The musculoskeletal weight was recorded as the weight of the carcass after all the organs in the chest, abdominal cavity, and peritoneal cavity were removed.
Dose–response study
We have previously validated that Ad-IGF-1 transduces various cells in the placenta (trophoblasts and endothelial cells) in a dose-dependent manner.22,28,32 Therefore, in this study, we first determined the intraplacental gene transfer efficiency of the adenoviral vectors in the rabbit model. The β-galactosidase transgene (Ad-LacZ) was used as a reporter to assess gene transfer efficiency and tropism. In a subset of pregnant rabbits, we tested intraplacental injections of two concentrations of Ad-LacZ (1×108 or 1×109 PFU, n=3–6 does in each group, position 1 and 3 placentas on both sides of the uterine horn receiving injections). The does and pups were harvested 48 hr postinjection on gestation day 23 after sufficient time had passed for viral transfection and transgenic protein expression. Vector dissemination/transgenic protein expression in the placenta, fetuses (whole mount), and major maternal organs was histologically determined. Tissues were fixed and embedded in paraffin, and 5-μm sections were collected. Sections were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal)29 and counterstained with nuclear fast red. Efficiency of gene transfer and distribution of the transgenic protein expression were determined by intensity of staining, indicating successful gene transfer.
Detection of adenoviral genomic DNA by PCR
Fetal tissues sampled included gonads, liver, heart, kidneys, and lung. Placentas were split into maternal side and fetal side (labyrinth) and adenoviral E4 DNA was used as a positive control. After harvest, tissues were snap frozen in liquid nitrogen. DNA was extracted with a DNeasy tissue kit (Qiagen, Valencia, CA). Primers were obtained from the DNA Core Facility (Cincinnati Children's Research Foundation, Cincinnati, OH). E4D (5′-GCA ATC ATG ATT CGC TGC TTG AG-3′) and E4G (5′-AGA ATA AGC CAC ACC CAG CCA AC-3′) primers were used to amplify a 440-bp fragment from the E4 region of the recombinant adenovirus.13 The annealing temperature was 55°C. Approximately 1 μg of genomic DNA was used as template with 300 nmol of each primer in a 50-μl PCR under the following thermal cycling conditions: initial denaturation at 94°C for 3 min; 35 cycles of 94°C (40 sec), 58°C (40 sec), 72°C (40 sec); final extension at 72°C for 10 min. PCR products were loaded into a 2% agarose–TAE gel (GenePure LE agarose; ISC BioExpress, Kaysville, UT) and run at 80 mV. Assay conditions validated detection of adenoviral DNA spiked into 1 μg of genomic DNA down to 10 copies. The positive control was adenoviral E4 DNA and the negative control was water. Expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5′-ACC ACA GTC CAT GCC ATC AC-3′; reverse, 5′-TCC ACC ACC CTG TTG CTG TA-3′).
Immunohistochemistry
Formalin-fixed placentas were paraffin embedded and sectioned at 5 μm. These sections were deparaffinized in xylenes and rehydrated through an ethanol series. Antigen retrieval was performed with target retrieval solution (Dako, Carpinteria, CA). Immunohistochemistry was performed; briefly, samples were quenched for endogenous peroxidase, using 3% hydrogen peroxide in water. Tissues were blocked with 5% normal goat serum in PBS–Tween (0.1% Tween). Tissue sections were incubated in anti-CD45 antibody (diluted 1:200; Santa Cruz Biotechnology, Santa Cruz, CA). After washes, samples were incubated with biotinylated goat anti-mouse IgG antibody (diluted 1:200; Vector Laboratories, Burlingame, CA). Chromogen detection was performed with peroxidase-conjugated avidin–biotin complex (VECTASTAIN Elite ABC kit; Vector Laboratories) and then developed with 3′,3′-diaminobenzidine (DAB+; Dako) and counterstained with hematoxylin (Dako). After staining, slides were dehydrated and mounted (PROTOCOL xylene mounting media; Fisher Scientific, Pittsburg, PA). Rabbit spleen was used as a positive control for CD45 staining.
Statistical analysis
Statistical comparisons between experimental groups were performed by either Student t test or analysis of variance (ANOVA; SPSS, Chicago, IL) and post hoc tests with Bonferroni corrections as appropriate. Results were considered statistically significant at p<0.05. Data are presented as averages±SEM.
Results
Study-related survival rates
Survival rate in the sham group
Four dams were used as sham controls, where the animals were exposed to the stress of laparotomy on day 21. All of these animals carried their litter to term and were delivered by C-section on day 30. Five of the 38 total pups (13.16%) were resorbed on day 30. This spontaneous resorption is commonly observed in rabbit litters.
Survival rate of animals in the dose escalation group
Three dams were used for the dose escalation study at lower vector dose. On day 21 of gestation, a midline laparotomy was performed and the uterine horns were exposed. Pups at positions 1 and 3 on both uterine horns in these animals received transuterine intraplacental injection of Ad-LacZ at a concentration of 1×108 PFU per placenta. Forty-eight hours later, the laparotomy incisions were opened and the pups that had been injected were harvested. All three dams carried their litter to 48 hr postsurgery and injection. Of the 12 pups that received transuterine intraplacental injection of 1×108 PFU Ad-LacZ, 1 was resorbed at 48 hr. Six dams were used for the dose escalation study at higher vector dose. These dams similarly underwent a midline laparotomy on day 21 to expose their uterine horn. Pups at positions 1 and 3 on both sides of the uterine horn in these animals received transuterine intraplacental injection of Ad-LacZ at a concentration of 1×109 PFU per placenta. Forty-eight hours later, the laparotomy incisions were opened and the pups that had been injected were harvested. One dam aborted all of her pups and was therefore excluded from the study. The other five dams carried pups to 48 hr postsurgery and injection. All 20 pups that received Ad-LacZ injections at 1×109 PFU survived to 48 hr postsurgery.
Survival rate of animals in the various control and treatment groups
Six dams were used as controls for the study (used in group 1 [control] and group 2 [runt] described in Materials and Methods), where the dams were exposed to the stress of the surgery on day 21 and the pups received transuterine intraplacental injection of PBS at position 1 and position 3 as described. All of these animals carried their litter to term and were delivered by C-section on day 30. Spontaneous resorption in these dams was similar to the sham. Of the 12 pups (6 at position 1 [control] and 6 at position 3 [runt]) that received transuterine intraplacental injection of PBS, 1 was aborted on day 30.
Eight dams were used for the Ad-IGF-1 treatment. These dams similarly underwent a midline laparotomy on day 21 to expose their uterine horn. Pups at positions 1 and 3 on one of the uterine horns in these animals received transuterine intraplacental injection of Ad-IGF-1 at a concentration of 1×109 PFU per placenta. Two of these dams aborted all their pups and were excluded from the study. The remaining dams carried their litter to term and were delivered by C-section on day 30. One of them had all stillborn pups and was excluded from the study. In the remaining 5 dams there was a total of 52 pups, of which 8 were resorbed (15.38%). In particular, of the 10 pups (5 at position 1 [control] and 5 at position 3 [runt]) that received intraplacental injection of Ad-IGF-1, 1 of them (at position 3) was resorbed.
Six dams were used for the Ad-LacZ treatment. These dams similarly underwent a midline laparotomy on day 21 to expose their uterine horn. Pups at positions 1 and 3 on one of the uterine horns in these animals received transuterine intraplacental injection of Ad-LacZ. One of these dams aborted all of her pups and was excluded from the study. The remaining dams carried their litter to term and were delivered by C-section on day 30. Of the 10 pups (5 at position 1 [control] and 5 at position 3 [runt]) that received intraplacental injection of Ad-LacZ-1, 2 of them (1 each at positions 1 and 3) were resorbed.
Dose escalation: 1×109 PFU of Ad-IGF-1 is the most efficient dose
In the dose escalation study, we tested intraplacental injections of two concentrations of Ad-LacZ (1×108 or 1×109 PFU) to determine the efficiency of gene transfer in the placentas. In all experiments doe survival was 100%. There was no significant difference in the position 1 and position 3 fetal survival rates between different doses 48 hr after intraplacental administration of the viral particles. Ad-LacZ injection at 1×108 PFU resulted in minimal gene transfer to the placenta 48 hr after the treatment. β-galactosidase (β-Gal) expression was observed in 0 of 12 placentas treated with 1×108 PFU of Ad-LacZ (data not shown). In contrast, efficient gene transfer and significant β-galactosidase expression were seen in placentas injected with 1×109 PFU of Ad-LacZ. Eleven of 12 placentas injected with 1×109 PFU of Ad-LacZ showed high levels of X-Gal staining in the labyrinth zone, as seen in the representative micrograph in Fig. 1. There were no histological changes in injected placentas.
FIG. 1.
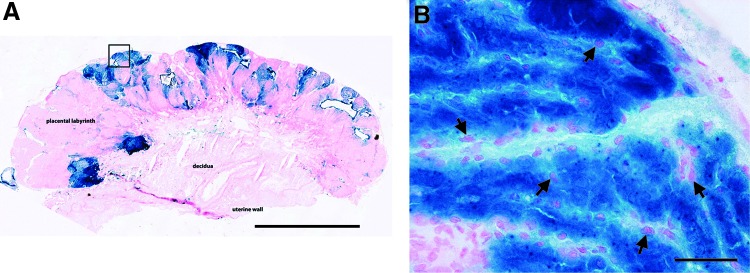
Evaluation of site-specific placental gene transfer and transgenic protein expression. 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining was performed to evaluate β-galactosidase expression in placentas injected with 1×109 plaque-forming units (PFU) of Ad-LacZ. (A) High levels of X-Gal staining were observed in the labyrinth zone and along the maternal–fetal placenta interface (original magnification,×5; scale bar, 2000 μm). (B) Higher magnification (original magnification,×40; scale bars, 50 μm) of the boxed area in (A) demonstrates almost 100% staining of trophoblast cells in the labyrinth zone. Arrows indicate representative cells.
Maternal and fetal dissemination of Ad-LacZ
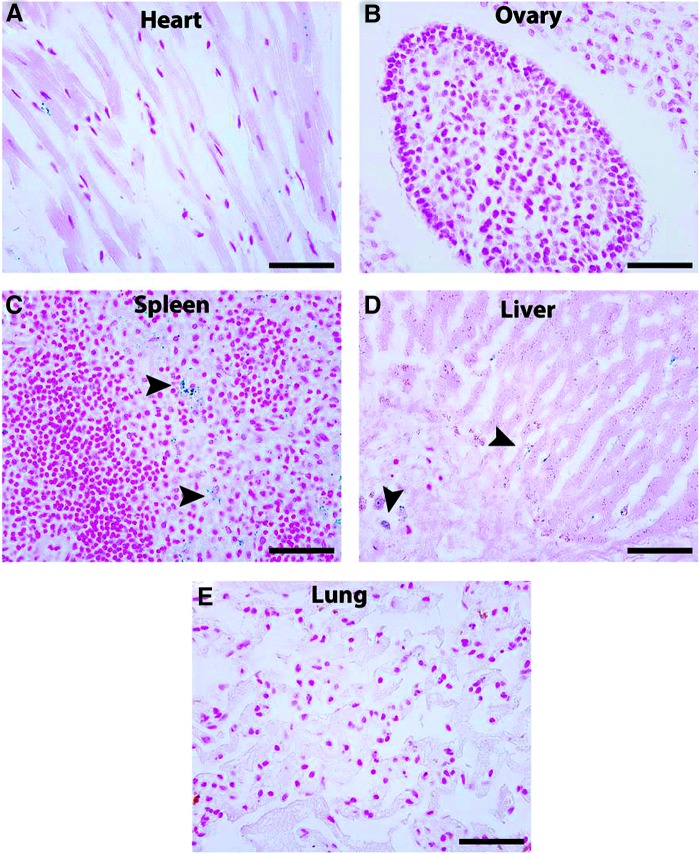
Maternal dissemination was studied in all major organs in does whose placentas were injected with 1×109 PFU of Ad-LacZ. X-Gal staining demonstrated no positive cells in the heart, ovary, or lung, but sparse positive cells were seen in the liver and spleen. Representative images are shown in Fig. 2.
FIG. 2.
Maternal dissemination of adenoviral vectors after placental gene transfer. Maternal dissemination was examined in does whose placentas were injected with 1×109 PFU of Ad-LacZ. X-Gal staining was performed on all major organs to evaluate β-galactosidase expression. No staining was seen in the heart (A), ovary (B), or lung (E). Minimal staining was seen (arrowheads) on higher power examination in the spleen (C) and liver (D). Scale bars, 50 μm.
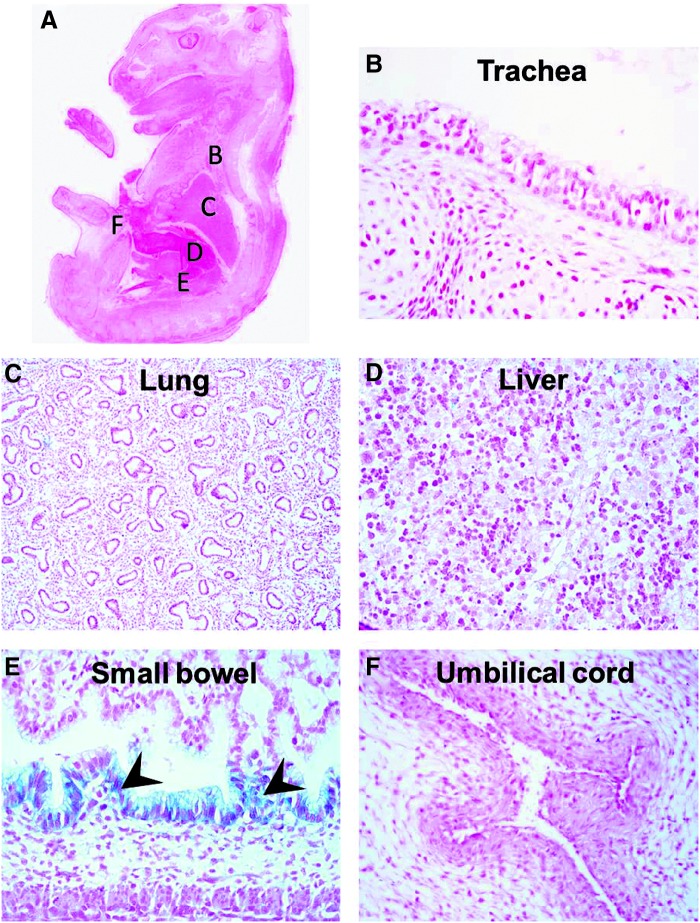
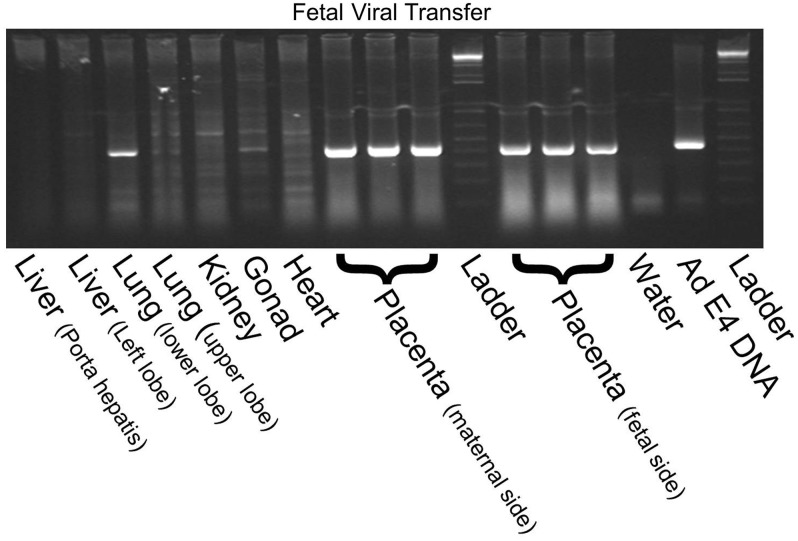
Fetal dissemination was examined by X-Gal staining of whole-mount fetuses whose placentas were injected with 1×109 PFU of Ad-LacZ. There was no significant dissemination of the transgenic protein (X-Gal staining) in any of the fetuses. Only the small bowel and umbilical cord had a few spots of moderate β-Gal expression (which was likely endogenous nonspecific β-Gal activity, as it was seen in untreated controls). Representative images are shown in Fig. 3. As a more sensitive test for viral transfer to the pups, we performed PCR for the adenoviral E4 region in various fetal organs after intraplacental Ad-LacZ injection. We confirmed our result of no adenoviral DNA in the fetal liver, heart, kidney, and gonad. In contrast to β-Gal staining data, we detected some adenovirus in the lung. As expected, we found intense bands for the placenta from both maternal and fetal sides. Representative PCR is shown in Fig. 4.
FIG. 3.
Fetal dissemination of adenoviral vector after placental gene transfer. β-Galactosidase expression was examined in fetuses by X-Gal staining of whole-mount fetuses whose placentas were injected with 1×109 PFU of Ad-LacZ. There was no significant dissemination of the transgenic protein in the fetuses (A). No positive staining was seen in the major organs including the trachea (B), lung (C), and liver (D). Only the small bowel (E; arrowheads) and umbilical cord (F) had a few spots of moderate LacZ expression.
FIG. 4.
PCR evaluation of fetal dissemination of adenoviral vector after placental gene transfer. PCR for the adenoviral E4 region of the viral vectors was performed in various fetal organs of fetuses whose placentas were injected with 1×109 PFU of Ad-LacZ. No adenoviral DNA was found in the fetal liver, kidney, gonad, or heart. Some adenovirus was detected in the fetal lung. Intense bands were detected in the placenta from both maternal and fetal sides. Water sample served as a negative control and adenovirus E4 was included as a positive control.
Viral inflammatory response on gestation day 30
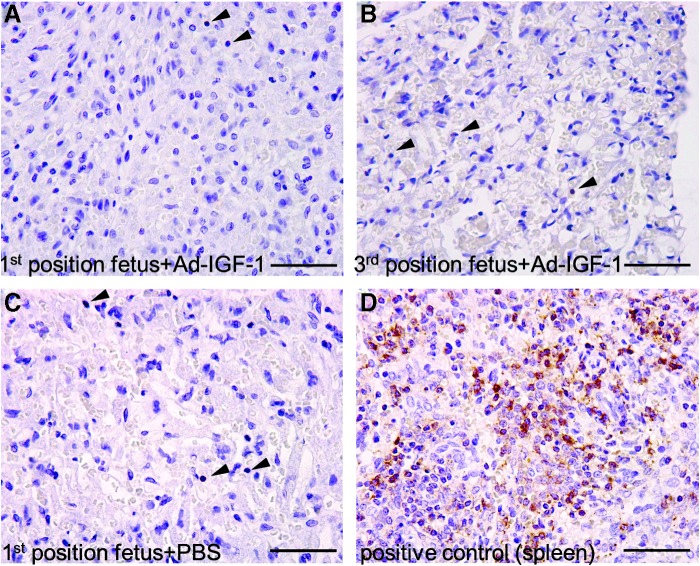
We then determined the effect of adenovirus-mediated IGF-1 therapy on placental inflammation, using anti-CD45 immunohistochemistry. There was no inflammatory response above normal after intraplacental Ad-IGF1 injection (1×109 PFU). Scattered CD45-positive cells were seen in the labyrinth zone with no significant differences between the treatment groups in the runted or normal positions. Representative images are shown (Fig. 5A–C; arrowheads indicate positive staining). Spleen from an untreated rabbit was used as positive control for the staining (Fig. 5D).
FIG. 5.
Evaluation of the effect of adenovirus-mediated IGF-1 therapy on placental inflammation. Anti-CD45 immunohistochemistry was performed on placentas that were injected with 1×109 PFU of Ad-IGF-1. There was no significant difference between adenovirus-treated (A and B) and PBS-treated (C) placentas, in terms of the number of CD45-positive cells in the labyrinth zone. Rabbit spleen (D) was used as a positive control. Arrowheads indicate positive cells. Images were taken at an original magnification of×40; scale bars, 50 μm.
Naturally occurring runts at third position from the ovarian end of the uterine horn
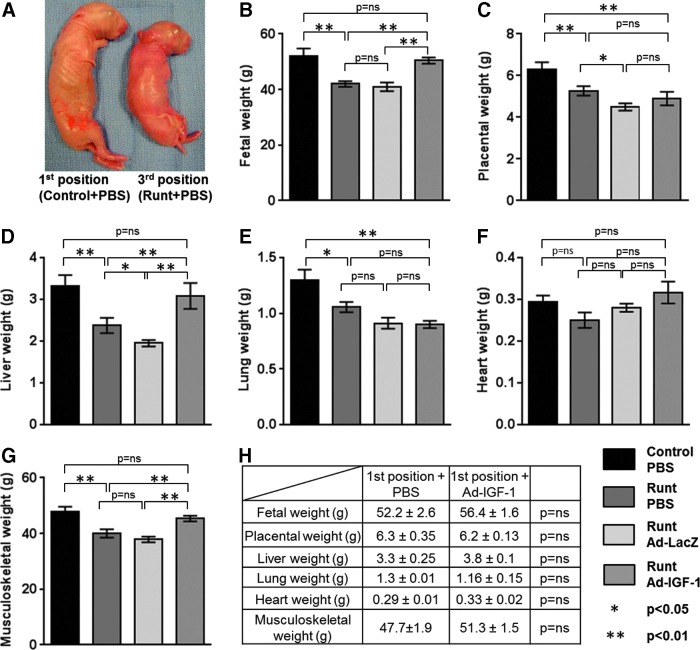
In all experiments doe survival was 100%. There was no significant difference in the position 1 and position 3 fetal survival rates between different treatments on gestational day 30. The runt group demonstrated significantly lower birth weight compared with the normally growing control group at first position (42.0±1.8 vs. 52.2±2.6 g; p<0.01; n=6). Similarly, placental weight (5.3±0.2 vs. 6.3±0.4 g; p<0.01; n=6), liver weight (2.4±0.2 vs. 3.3±0.3 g; p<0.01; n=6), lung weight (1.1±0.05 vs. 1.3±0.09 g; p<0.05; n=6), and musculoskeletal weight (40.0±1.5 vs. 47.7±1.9 g; p<0.01; n=6) were significantly lower in the runt group as compared with the control group. However, there was no significant difference in weights of the hearts in the runt group compared with hearts in control pups (0.25±0.02 vs. 0.29±0.02 g; p=not significant [NS]; n=6). These results demonstrate that the fetus at the third position from the ovarian end is naturally intrauterine growth restricted, with birth weight at ∼81% of the normal offspring and reduced liver weight, confirming this as an appropriate model to study the effects of Ad-IGF-1 on rescuing the naturally occurring IUGR phenotype due to placental insufficiency (Fig. 6).
FIG. 6.
Validation of the rabbit IUGR model and the effect of Ad-IGF-1 gene therapy. (A and B) The pups at the third position from the ovarian end were growth restricted, with birth weight at 81% of the normal offspring, confirming this as an appropriate model to study the effects of Ad-IGF-1 therapy on rescuing the naturally occurring IUGR phenotype in the rabbit model. Similarly, placental (C), liver (D), lung (E), and musculoskeletal weights (G) were significantly lower at the third position compared with the first position. However, there was no significant difference in the weights of the heart (F) in the third-position fetuses compared with first-position fetuses. Ad-IGF-1 treatment resulted in a significant increase in fetal weight, fetal liver weight, and musculoskeletal weight of the runts, to a level similar to that of the normally growing control group at the first position. Ad-IGF-1 treatment did not change placental or fetal lung weight. (H) An additional comparison of the fetal parameters and placental weights between Ad-IGF-1-treated and PBS-treated normally growing pups (at the first position) demonstrated that IGF-1 gene therapy has no effect on normally growing fetuses.
Intraplacental Ad-IGF-1 injection rescues birth weight in the naturally occurring rabbit runt model
Ad-IGF-1-treated runts demonstrated a significant increase in fetal weight compared with the runt and Ad-LacZ treatments (50.45±1.1 vs. 42.0±1.8 and 41.48±1.62 g; p<0.01). There was no significant difference in fetal weight in the runts treated with Ad-IGF-1 compared with the control group (50.45±1.1 vs. 52.2±2.6 g; p=NS) (Fig. 6B). While Ad-IGF-1 treatment restored the fetal weight, it did not affect the placental weight. There was no significant difference in placental weights between the Ad-IGF-1-treated runts compared with the runt and Ad-LacZ treatments (4.9±0.3 vs. 5.3±0.2 and 4.5±0.2 g; p=NS). The placental weight of the Ad-IGF-1-treated runts remained significantly lower than that of the control group (4.9±0.3 vs. 6.3±0.35 g; p<0.01) (Fig. 6C).
Similarly, there was a significant increase in liver weight of the Ad-IGF-1-treated runts compared with the runt and Ad-LacZ treatments (3.1±0.3 vs. 2.4±0.2 and 1.95±0.08 g; p<0.01); furthermore, Ad-IGF-1 treatment rescued the liver weight of runts with no significant difference when compared with liver weight in the control group (3.1±0.3 vs. 3.3±0.25 g; p=NS) (Fig. 6D). There was no significant increase in lung weight in the Ad-IGF-1-treated runts compared with the runt or Ad-LacZ treatment (0.93±0.03 vs. 1.05±0.05 and 0.91±0.05 g; p=NS), and the Ad-IGF-1-treated runts demonstrated significantly lower lung weight when compared with the control group (0.93±0.3 vs. 1.3±0.09 g; p<0.01) (Fig. 6E). There were no significant differences in heart weight between any of the groups (Fig. 6F). Finally, there was a significant increase in the musculoskeletal weight of the Ad-IGF-1-treated runts compared with the runt and Ad-LacZ treatments (45.4±1.03 vs. 40.0±1.5 and 37.9±1.08 g; p<0.01); furthermore, musculoskeletal weight of Ad-IGF-1-treated runts was rescued with no significant difference when compared with the control group (45.4±1.03 vs. 47.7±1.9 g; p=NS) (Fig. 6G).
To identify any potential detrimental effects of Ad-IGF-1 gene therapy, we determined the effects of Ad-IGF-1 treatment on the normally growing control group. Interestingly, we did not see a significant difference in any of measured fetal parameters or placental weights between the first-position controls or first-position pups treated with Ad-IGF-1 (Fig. 6H).
Discussion
This study demonstrates that intraplacental adenovirus-mediated IGF-1 gene therapy can restore birth weight in the naturally occurring fetal growth-restricted rabbit model of placental insufficiency. In addition, liver weight was also restored to normal with Ad-IGF-1 treatment. It has been previously established that IGF-1 can stimulate growth and increase muscle mass in postnatal animals.33,34 Our results suggest this effect of IGF-1 also exists in utero in developing fetuses. Interestingly, placental weight did not increase with Ad-IGF-1 treatment, but we found the transgenic protein to be expressed throughout the rabbit placental labyrinth, the area of active nutrient and oxygen delivery to the placenta, and we believe that IGF-1 may play a role in enhancing placental exchange area, function, or metabolism, leading to improved fetal growth.35
Many studies have replicated IUGR models in rodents or larger animal species,36 by altering maternal nutrition during gestation37 or by administration of glucocorticoids38; however, the majority of these models are not ideal and fail to fully recapitulate placental insufficiency, a major cause of human IUGR.39 Even in studies that induced IUGR by uterine artery ligation to create placental insufficiency, pups were not selectively ligated and resulted in a wide range of birth weights.40 The sheep has been used extensively as a large animal model of IUGR induced via maternal hyperthermia, ligation of an umbilical artery, or embolization of the placenta in late gestation and maternal overnutrition in the pregnant adolescent ewe. However, fetal cardiovascular responses vary according to the method used to induce placental dysfunction.30 We have developed a novel murine model of IUGR that recapitulates the characteristics of human placental vascular insufficiency induced via mesenteric uterine artery branch ligation.22 Although this novel mouse model of fetal growth restriction is the first nongenetic mouse model of placental insufficiency that offers its application in long-term follow-up studies and the development of transgenic mice to better study the underlying mechanisms in placental insufficiency, the onset of IUGR is on gestational day 18 of a 20-day murine gestation period, which may be too late for human-translational work. The naturally occurring intrauterine growth-restricted (runted) rabbit model is appealing because it reflects human IUGR in developed countries where placental insufficiency is the main cause of IUGR.29 Furthermore, the timing of the vascular insult in this model parallels human IUGR in its onset in the early third trimester with brain sparing at the cost of the liver. The longer gestation and 10-day trimesters in the rabbit allow better understanding of the ramifications for clinical translation of intraplacental gene therapy administered at the beginning of the third trimester to correct IUGR caused by placental insufficiency. Using the normally growing first-position litter of the same horn as internal control further allows controlling for the variability in growth restriction and weights due to different numbers of litters. The degree of growth restriction we observed in this study is similar to that which has been previously reported in the rabbit runt model,30 with almost 20% reduction in fetal weight in the third position compared with the normally grown first-position pups. The easy identification of the runted litter may allow further follow-up for long-term studies.
IGF-1 has been previously implicated as playing a major role in fetal and placental development. There is a positive correlation between fetal size, birth weight and length, placental weight, and postnatal growth rate with IGF-1 levels.19,21 Previous work has also shown that a deficiency in IGF-1 is correlated with IUGR.18,19,21 In addition, IGF-1 knockout mice also exhibit fetal growth restriction, without altering actual placental growth.3 Fetal programming in the liver, as seen associated with a rodent model of IUGR, in which there is a decrease in fetal liver growth and an upregulation in the expression of hepatic gluconeogenic enzymes, leads to impaired glucose tolerance in postnatal life.41 In our model, intraplacental Ad-IGF-1 treatment that leads to the restoration of fetal liver growth may prevent these effects, and further studies are needed to investigate fetal programming effects of intraplacental IGF-1 treatment. Work in our laboratory demonstrated that intraplacental Ad-IGF-1 injection corrects fetal weight in a murine mouse model induced by mesenteric uterine artery branch ligation and increases both vascular area and labyrinth volume in the mouse IUGR model, maintaining area and volume at control levels. However, it should be noted in this context that IGF1 is only one, albeit important, part of the complex etiology of placental insufficiency. Fetal–placental vascular development is a continuum. It begins soon after implantation and evolves throughout pregnancy. Numerous angiogenic growth factors regulate this process, including vascular endothelial growth factor (VEGF), placental growth factor (PGF), platelet-derived growth factor (PDGF), and transforming growth factor (TGF-B).41,42 Carr and colleagues demonstrated that Ad-VEGF gene therapy improves fetal growth in a sheep model of FGR.24 Epidermal growth factor infusion has been shown to normalize fetal weight and small intestinal development in fetal rabbit intrauterine growth retardation.43 IUGR is also regulated by apoptotic genes, making the fetus more susceptible to hypoxic–ischemic insults.44
Overall, there have been major improvements in all aspects of gene delivery vector and targeting of gene expression during the past few decades. There is no universally ideal viral vector system. The type of disease to be treated needs to be defined before decisions are made as to which vector type should be applied.45 Adenoviral vector is appealing for intraplacental gene therapy because it is replication deficient, it demonstrates early onset of transgenic protein expression (within 24 hr), wide transduction when driven by a CMV promoter,46,47 and short-term transgenic protein expression (typically ranging from 10 to 14 days).48 Although early versions of adenoviruses showed toxic side effects and strong immune responses, newer second- and third-generation vectors with many of the viral genes deleted have demonstrated significant improvements. There are, however, serious concerns about the application of gene therapy in utero. Direct intraplacental gene transfer offers the advantage of site-directed gene transfer with decreased risk of either maternal or fetal somatic cell gene transfer, and even less likelihood of fetal germ cell gene transfer. As we observed in both the mouse and rabbit models, intraplacental gene transfer resulted in minimal gene transfer to the fetus, except in the lung, and we believe that contamination in the lung can be abrogated by improved injection technique. In addition, once the fetus is born, the transduced placenta is discarded, eliminating the risk of continued fetal and maternal exposure to virally transduced cells or transgenic protein expression. The use of gene therapy via direct placental injection may be a promising technique for future studies in the treatment of IUGR. Although adenovirus-mediated gene transfer in this study shows proof of concept in achieving highly efficient local expression of a transgenic protein and fetal growth correction, nonviral delivery mechanisms with placenta-specific promoters will eventually be the more clinically translatable form of in utero placental gene therapy.
In future studies we plan to examine the mechanisms by which IGF-1 exert these effects in the placenta. It will be necessary to examine more thoroughly the dissemination of transgenic proteins in the fetus and the mother; placental structure, function, and metabolism; along with signaling pathways and also the role of IGF-binding proteins in the setting of IGF-1 expression in IUGR. Subsequently, following up the treated animals long term postnatally is necessary to assess not only the efficacy of placental delivery of IGF-1 in correcting growth restriction, but also to demonstrate that it can attenuate the risk of IUGR-associated adult-onset diseases.
In conclusion, we report the usefulness of the rabbit model of IUGR, the transduction efficiency and safety of using adenoviral gene therapy in this model, and the successful application of Ad-IGF-1 gene therapy as a novel approach to modulate placental growth and provide a treatment strategy for IUGR due to placental insufficiency. The results of these preliminary studies demonstrate that placental gene therapy may be an effective therapy for IUGR for which no treatment is currently available.
Supplementary Material
Acknowledgments
The authors sincerely thank Dr. Helen N. Jones for scientific discussions and editorial help, and Stephanie A. Lang for technical help with immunohistochemistry. This research was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK59242, R01-DK074055, and R01-DK072446), Juvenile Diabetes Research Foundation, and American Diabetes Association to T.M. Crombleholme.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cerdeira AS, Kopcow HD, and Karumanchi SA. Regulatory T cells in preeclampsia: some answers, more questions? Am J Pathol 2012;181:1900–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer MS, and Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev 2003:CD000032. [DOI] [PubMed] [Google Scholar]
- 3.Faraci M, Renda E, Monte S, et al. Fetal growth restriction: current perspectives. J Prenat Med 2011;5:31–33 [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Bagby SP, and Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2006;2:700–707 [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Gelow J, Thornburg K, et al. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. Eur J Heart Fail 2010;12:819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, Osmond C, Golding J, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J 1989;298:564–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Painter RC, Roseboom TJ, Van Montfrans GA, et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol 2005;16:189–194 [DOI] [PubMed] [Google Scholar]
- 8.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010;88:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn SE, Hull RL, and Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 10.Ozanne SE, Fernandez-Twinn D, and Hales CN. Fetal growth and adult diseases. Semin Perinatol 2004;28:81–87 [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Stoffers DA, Nicholls RD, et al. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 2008;118:2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons RA. Developmental origins of beta-cell failure in type 2 diabetes: the role of epigenetic mechanisms. Pediatr Res 2007;61:64R–67R [DOI] [PubMed] [Google Scholar]
- 13.Cottrell EC, and Ozanne SE. Early life programming of obesity and metabolic disease. Physiol Behav 2008;94:17–28 [DOI] [PubMed] [Google Scholar]
- 14.Myrie SB, Mcknight LL, Van Vliet BN, et al. Low birth weight is associated with reduced nephron number and increased blood pressure in adulthood in a novel spontaneous intrauterine growth-restricted model in Yucatan miniature swine. Neonatology 2011;100:380–386 [DOI] [PubMed] [Google Scholar]
- 15.Owens JA, Gatford KL, De Blasio MJ, et al. Restriction of placental growth in sheep impairs insulin secretion but not sensitivity before birth. J Physiol 2007;584:935–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Painter RC, Roseboom TJ, and Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 2005;20:345–352 [DOI] [PubMed] [Google Scholar]
- 17.Vidal AC, Murphy SK, Murtha AP, et al. Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int J Obes (Lond) 2013;37:907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battaglia C, Artini PG, D'Ambrogio G, et al. Maternal hyperoxygenation in the treatment of intrauterine growth retardation. Am J Obstet Gynecol 1992;167:430–435 [DOI] [PubMed] [Google Scholar]
- 19.Vidal AC, Murtha AP, Murphy SK, et al. Maternal BMI, IGF-I levels, and birth weight in African American and white infants. Int J Pediatr 2013;2013:191472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidal AC, Henry NM, Murphy SK, et al. PEG1/MEST and IGF2 DNA methylation in CIN and in cervical cancer. Clin Transl Oncol 2014;16:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fall CH, Pandit AN, Law CM, et al. Size at birth and plasma insulin-like growth factor-1 concentrations. Arch Dis Child 1995;73:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habli M, Jones H, Aronow B, et al. Recapitulation of characteristics of human placental vascular insufficiency in a novel mouse model. Placenta 2013;34:1150–1158 [DOI] [PubMed] [Google Scholar]
- 23.Nishimura Y, Ii M, Qin G, et al. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol 2012;132:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr DJ, Wallace JM, Aitken RP, et al. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies. Hum Gene Ther 2014;25:375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rana S, Schnettler WT, Powe C, et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens Pregnancy 2013;32:189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sferruzi-Perri AN, Owens JA, Standen P, et al. Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrient partitioning near term. Am J Physiol Endocrinol Metabol 2007;292:E668–E676 [DOI] [PubMed] [Google Scholar]
- 27.Darp RA, De Boo HA, Phua HH, et al. Differential regulation of igf1 and igf1r mRNA levels in the two hepatic lobes following intrauterine growth restriction and its treatment with intra-amniotic insulin-like growth factor-1 in ovine fetuses. Reprod Fertil Dev 2010;22:1188–1197 [DOI] [PubMed] [Google Scholar]
- 28.Jones HN, Crombleholme T, and Habli M. Adenoviral-mediated placental gene transfer of IGF-1 corrects placental insufficiency via enhanced placental glucose transport mechanisms. PLoS One 2013;8:e74632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchmiller-Crair TL, Gregg JP, Rivera FA Jr., et al. Delayed disaccharidase development in a rabbit model of intrauterine growth retardation. Pediatr Res 2001;50:520–524 [DOI] [PubMed] [Google Scholar]
- 30.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 2008;35:730–743 [DOI] [PubMed] [Google Scholar]
- 31.Thornburg KL. Fetal response to intrauterine stress. Ciba Found Symp 1991;156:17–29; discussion 29–37 [DOI] [PubMed] [Google Scholar]
- 32.Jones H, Crombleholme T, and Habli M. Regulation of amino acid transporters by adenoviral-mediated human insulin-like growth factor-1 in a mouse model of placental insufficiency in vivo and the human trophoblast line BeWo in vitro. Placenta 2014;35:132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiaffino S, and Mammucari C. Regulation of skeletal muscle growth by the IGF1–Akt/PKB pathway: insights from genetic models. Skelet Muscle 2011;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 2008;154:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerdeira AS, Rajakumar A, Royle CM, et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol 2013;190:3939–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mühlhäusler B, Adam CL, Marrocco E, et al. Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. J Physiol 2005;565:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumortier O, Blondeau B, Duvillie B, et al. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia 2007;50:2495–2503 [DOI] [PubMed] [Google Scholar]
- 38.Nyirenda MJ, Lindsay RS, Kenyon CJ, et al. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 1998;101:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMillen IC, and Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 2005;85:571–633 [DOI] [PubMed] [Google Scholar]
- 40.Simmons RA, Templeton LJ, and Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 2001;50:2279–2286 [DOI] [PubMed] [Google Scholar]
- 41.Arroyo JA, and Winn VD. Vasculogenesis and angiogenesis in the IUGR placenta. Semin Perinatol 2008;32:172–177 [DOI] [PubMed] [Google Scholar]
- 42.Cerdeira AS, and Karumanchi SA. Angiogenic factors in preeclampsia and related disorders. Cold Spring Harb Perspect Med 2012;2:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cellini C, Xu J, Arriaga A, et al. Effect of epidermal growth factor infusion on fetal rabbit intrauterine growth retardation and small intestinal development. J Pediatr Surg 2004;39:891–897; discussion 891–897. [DOI] [PubMed] [Google Scholar]
- 44.Borzsonyi B, Demendi C, Rigo J Jr., et al. The regulation of apoptosis in intrauterine growth restriction: a study of Bcl-2 and Bax gene expression in human placenta. J Matern Fetal Neonatal Med 2013;26:347–350 [DOI] [PubMed] [Google Scholar]
- 45.Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol 2003;21:117–122 [DOI] [PubMed] [Google Scholar]
- 46.Dumasius V, Jameel M, Burhop J, et al. In vivo timing of onset of transgene expression following adenoviral-mediated gene transfer. Virology 2003;308:243–249 [DOI] [PubMed] [Google Scholar]
- 47.Fisher KD, Stallwood Y, Green NK, et al. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther 2001;8:341–348 [DOI] [PubMed] [Google Scholar]
- 48.Rana S, Cerdeira AS, Wenger J, et al. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS One 2012;7:e48259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.