Abstract
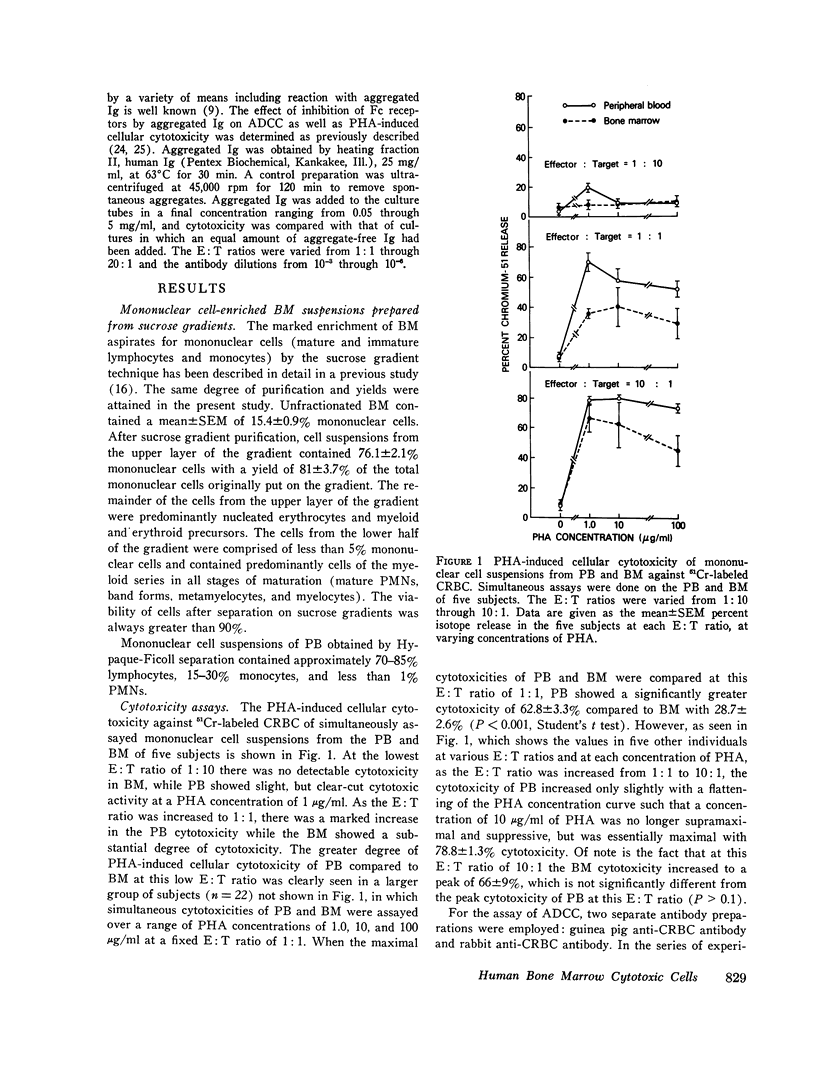
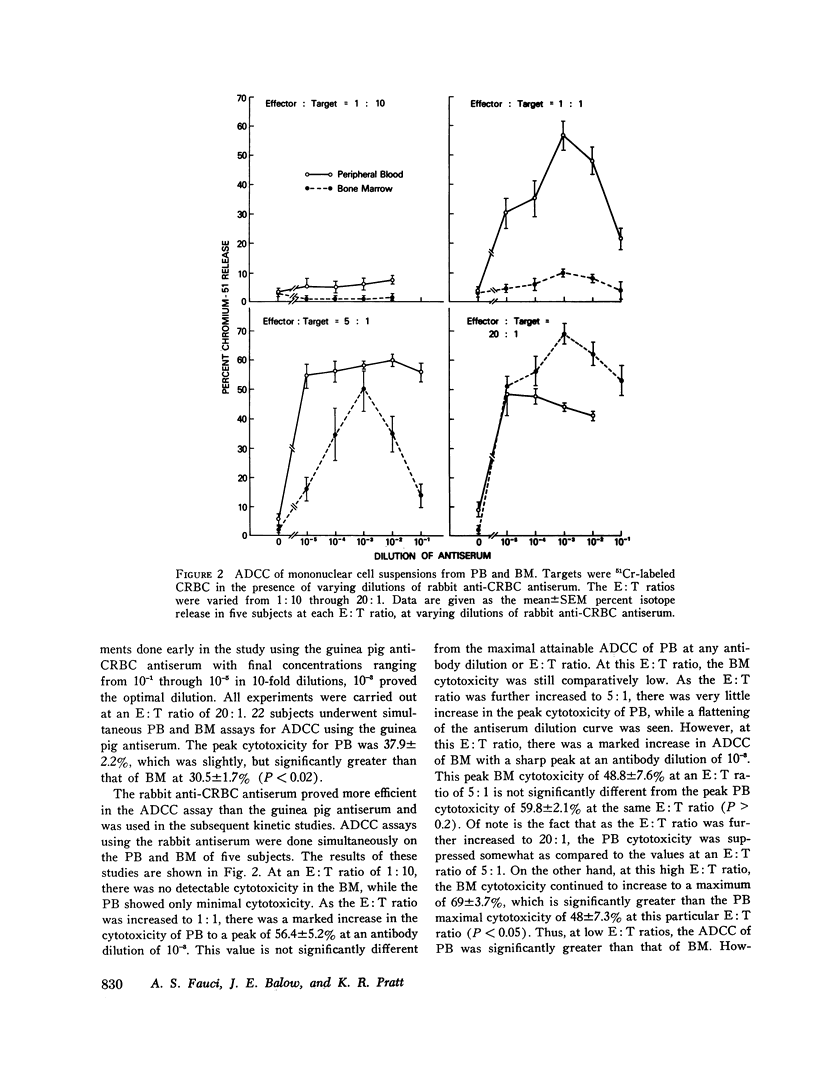
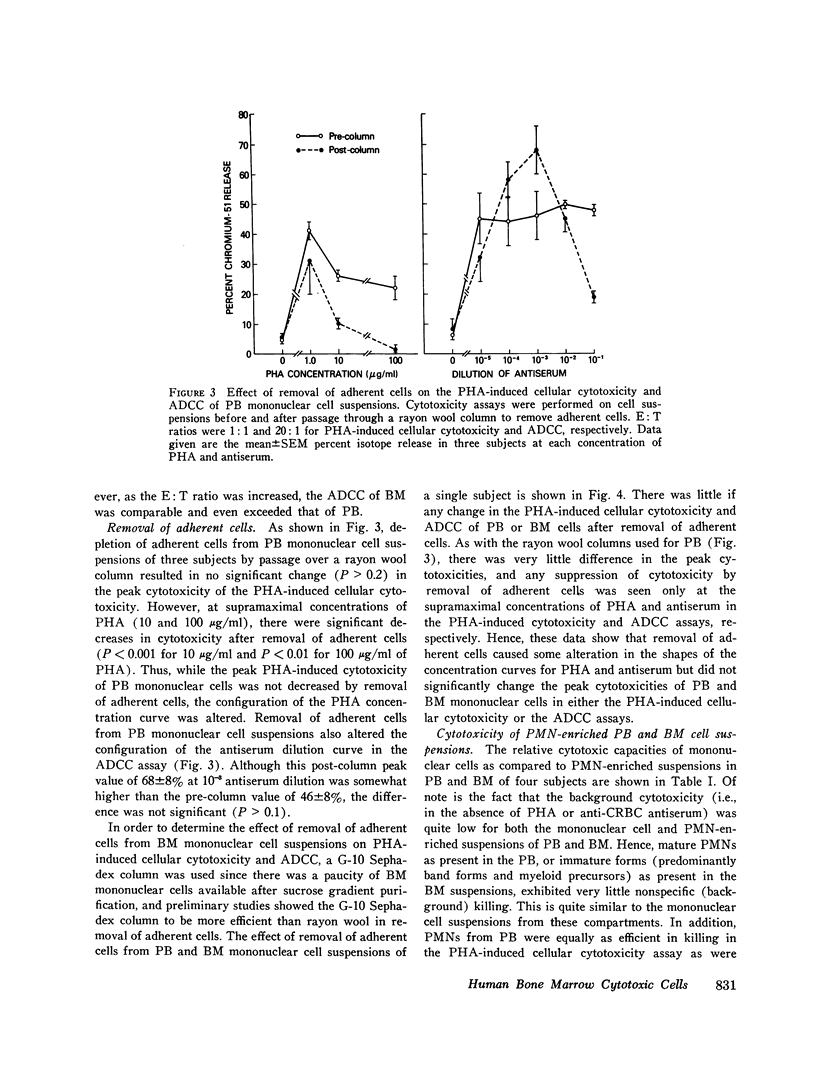
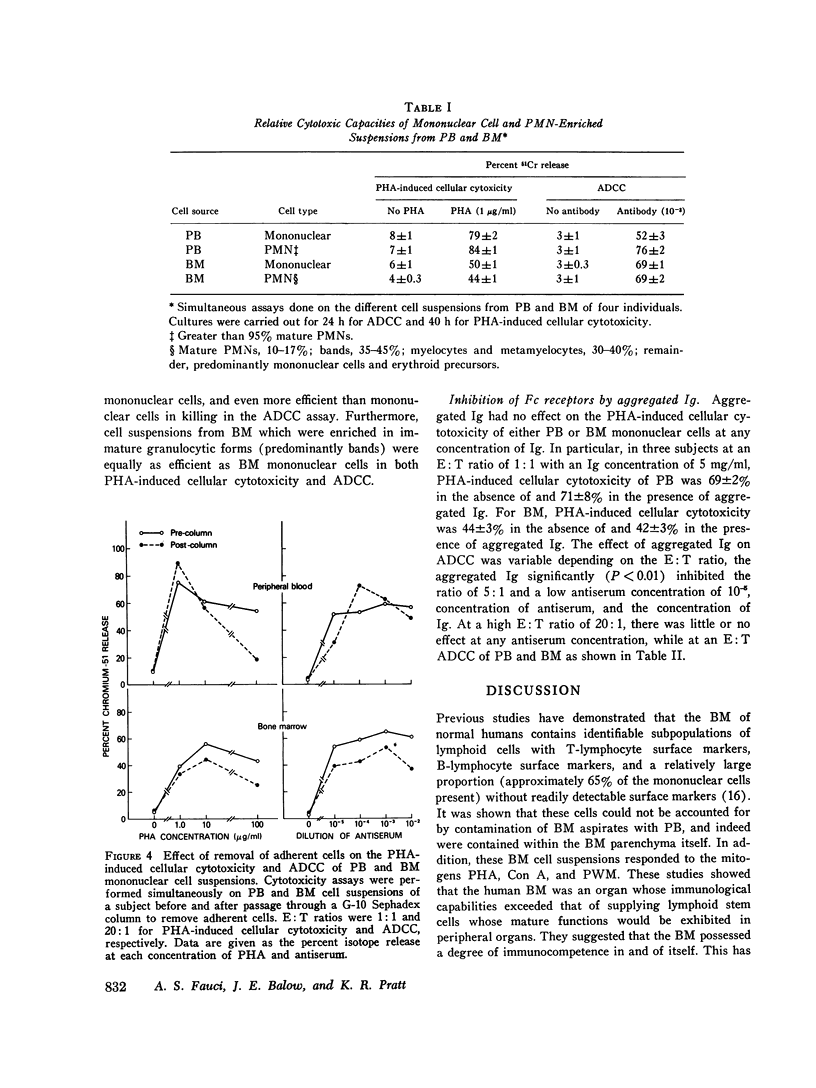
This study was undertaken to determine the capability of lymphocytes in the bone marrow of normal individuals to mediate nonspecific killer cell functions in assays of phytohemagglutinin (PHA)-induced cellular cytotoxicity, and antibody-dependent cellular cytotoxicity (ADCC) against 51Cr-labeled chicken erythrocyte target cells. Relatively pure mononuclear cell suspensions were obtained from bone marrow aspirates in 30 normal volunteers by sucrose gradient centrifugations and from the peripheral blood of the same individuals by Hypaque-Ficoll density centrifugations. At an effector: target ratio of 10:1, the PHA-induced cellular cytotoxicity of peripheral blood was 78.8 +/- 1.3%, while that of bone marrow was not significantly less at 66 +/- 9% (P greater than 0.1). At low effector:target ratios, the ADCC of bone marrow was negligible, while at higher effector:target ratios (20:1) bone marrow ADCC was 69 +/- 3.7%, which was comparable to that of peripheral blood. The lymphocytes themselves in the mononuclear cell suspensions of both peripheral blood and bone marrow were capable of cytotoxicity activity since depletion of monocytes from the suspensions by adherence to rayon wool and G-10 Sephadex columns did not remove the cytotoxic activity. Blocking of the Fc receptor on the effector cells by the addition of aggregated gamma globulin to the cultures suppressed the ADCC but not the PHA-induced cellular cytotoxicity of both peripheral blood and bone marrow, indicating that ADCC is dependent on an Fc receptor on the effector cell in both compartments. These studies demonstrate that the bone marrow of normal humans contains populations of lymphoid cells which have highly efficient killer cell capacities. It is uncertain what portion of these cells arise in the bone marrow and what portion enter the bone marrow parenchyma as part of the recirculating lymphocyte pool. These findings have relevance in the clearer understanding of the killer cell potential of grafted human marrow, as well as the bone marrow sequestration of functionally capable lymphocyte subpopulations in disease states and during chemotherapy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Richter M. The role of bone marrow in the immune response. Adv Immunol. 1970;12:201–270. doi: 10.1016/s0065-2776(08)60170-4. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Mayhew B. Passive transfer of contact sensitivity by bone marrow cells and evidence for their origin from immunized lymph nodes. Int Arch Allergy Appl Immunol. 1974;46(2):256–260. doi: 10.1159/000231128. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Sprent J., Pye J. A receptor for antibody on B lymphocytes. I. Method of detection and functional significance. J Exp Med. 1972 Mar 1;135(3):610–626. doi: 10.1084/jem.135.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borella L., Green A. A. Sequestration of PHA-responsive cells (T-lymphocytes) in the bone marrow of leukemic children undergoing long-term immunosuppressive therapy. J Immunol. 1972 Nov;109(5):927–932. [PubMed] [Google Scholar]
- Calder E. A., Urbaniak S. J., Penhale W. J., Irvine W. J. Characterization of human lymphoid cell-mediated antibody-dependent cytotoxicity (LDAC). Clin Exp Immunol. 1974 Dec;18(4):579–593. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. In vitro cytotoxic activity of thymus cells sensitized to alloantigens. Nature. 1970 Jul 4;227(5253):72–73. doi: 10.1038/227072a0. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- Chen M. G., Price G. B., Makinodan T. Incidence of delayed mortality (secondary disease) in allogeneic radiation chimeras receiving bone marrow from aged mice. J Immunol. 1972 May;108(5):1370–1378. [PubMed] [Google Scholar]
- Claman H. N. Bone marrow T cells. I. Response to the T cell mitogens, phytohemagglutinin and concanavalin A. J Immunol. 1974 Mar;112(3):960–964. [PubMed] [Google Scholar]
- Cohen J. J. Hydrocortisone resistance of activated initiator cells in graft versus host reactions. Nature. 1971 Jan 22;229(5282):274–275. doi: 10.1038/229274a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Thymus-derived lymphocytes sequestered in the bone marrow of hydrocortisone-treated mice. J Immunol. 1972 Mar;108(3):841–844. [PubMed] [Google Scholar]
- Fauci A. S. Corticosteroids and circulating lymphocytes. Transplant Proc. 1975 Mar;7(1):37–40. [PubMed] [Google Scholar]
- Fauci A. S. Human bone marrow lymphocytes. I. Distribution of lymphocyte subpopulations in the bone marrow of normal individuals. J Clin Invest. 1975 Jul;56(1):98–110. doi: 10.1172/JCI108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. I. Redistribution of circulating T and b lymphocytes to the bone marrow. Immunology. 1975 Apr;28(4):669–680. [PMC free article] [PubMed] [Google Scholar]
- Ford W. L., Gowans J. L. The traffic of lymphocytes. Semin Hematol. 1969 Jan;6(1):67–83. [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J. Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J Immunol. 1975 Mar;114(3):1047–1051. [PubMed] [Google Scholar]
- Henney C. S. On the mechanism of T-cell mediated cytolysis. Transplant Rev. 1973;17(0):37–70. doi: 10.1111/j.1600-065x.1973.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Henson P. M. The adherence of leucocytes and platelets induced by fixed IgG antibody or complement. Immunology. 1969 Jan;16(1):107–121. [PMC free article] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- Jondal M., Wigzell H., Aiuti F. Human lymphocyte subpopulations: classification according to surface markers and-or functional characteristics. Transplant Rev. 1973;16:163–195. doi: 10.1111/j.1600-065x.1973.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Blaese R. M. Pokeweed mitogen-, concanavalin A-, and phytohemagglutinin-induced development of cytotoxic effector lymphocytes. An evaluation of the mechanisms of T cell-mediated cytotoxicity. J Exp Med. 1973 Oct 1;138(4):812–824. doi: 10.1084/jem.138.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Antibody in the induction and inhibition of lymphocyte cytotoxicity. Transplant Rev. 1972;13:67–90. doi: 10.1111/j.1600-065x.1972.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Muchmore A. V., Nelson D. L., Kirchner H., Blaese R. M. A reappraisal of the effector cells mediating mitogen induced cellular cytotoxicity. Cell Immunol. 1975 Sep;19(1):78–90. doi: 10.1016/0008-8749(75)90293-2. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H. Contactual lysis of antibody-coated chicken erythrocytes by purified lymphocytes. Cell Immunol. 1970 Sep;1(3):300–315. doi: 10.1016/0008-8749(70)90051-1. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Holm G. Cytotoxic action of stimulated lymphocytes on allogenic and autologous erythrocytes. Science. 1968 Apr 19;160(3825):306–309. doi: 10.1126/science.160.3825.306. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Wigzell H. Lymphocyte mediated cytotoxicity in vitro. Induction and inhibition by humoral antibody and nature of effector cells. Transplant Rev. 1972;13:91–114. doi: 10.1111/j.1600-065x.1972.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Blake J. T., Rosenthal A. S. The peritoneal exudate lymphocyte. I. Differences in antigen responsiveness between peritoneal exudate and lymph node lymphocytes from immunized guinea pigs. J Exp Med. 1971 Nov 1;134(5):1170–1186. doi: 10.1084/jem.134.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse C. Migration of long-lived lymphocytes to the bone marrow and to other lymphomyeloid tissues in normal parabiotic guinea pigs. Blood. 1972 Jul;40(1):90–97. [PubMed] [Google Scholar]
- Röpke C., Everett N. B. Migration of small lymphocytes in adult mice demonstrated by parabiosis. Cell Tissue Kinet. 1974 Mar;7(2):137–150. doi: 10.1111/j.1365-2184.1974.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Bianco A. R., Handwerger B. S., Kahn C. R. Demonstration that monocytes rather than lymphocytes are the insulin-binding cells in preparations of humah peripheral blood mononuclear leukocytes: implications for studies of insulin-resistant states in man. Proc Natl Acad Sci U S A. 1975 Feb;72(2):474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood G., Blaese R. M. Phytohaemagglutinin-induced cytotoxic effector lymphocyte function in patients with the Wiskott-Aldrich syndrome (WAS). Clin Exp Immunol. 1973 Apr;13(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Singhal S. K., Richter M. Cells involved in the immune response. IV. The response of normal and immune rabbit bone marrow and lymphoid tissue lymphocytes to antigens in vitro. J Exp Med. 1968 Nov 1;128(5):1099–1128. doi: 10.1084/jem.128.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. D., Herzenberg L. A. The Fc receptor on thymus-derived lymphocytes. I. Detection of a subpopulation of murine T lymphocytes bearing the Fc receptor. J Exp Med. 1975 Sep 1;142(3):611–621. doi: 10.1084/jem.142.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxel J. A., Stobo J. D., Paul W. E., Green I. Antibody-dependent lymphoid cell-mediated cytotoxicity: no requirement for thymus-derived lymphocytes. Science. 1972 Jan 14;175(4018):194–196. doi: 10.1126/science.175.4018.194. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Osmond D. G. Blastogenic response of lymphocytes separated from bone marrow to allogeneic lymphoid cells in vitro. Immunology. 1971 Nov;21(5):767–779. [PMC free article] [PubMed] [Google Scholar]
- Zighelboim J., Gale R. P., Chiu A., Bonavida B., Ossorio R. C., Fahey J. L. Antibody dependent cellular cytotoxicity: cytotoxicity mediated by non t-lymphocytes. Clin Immunol Immunopathol. 1974 Nov;3(2):193–200. doi: 10.1016/0090-1229(74)90005-1. [DOI] [PubMed] [Google Scholar]
- van Bekkum D. W. Use and abuse of hemopoietic cell grafts in immune deficiency diseases. Transplant Rev. 1972;9:3–53. doi: 10.1111/j.1600-065x.1972.tb01560.x. [DOI] [PubMed] [Google Scholar]



