Background: The role of the N-terminal ubiquitin-like domain of the E3 ligase parkin is not fully understood.
Results: Parkin is recruited to the 26 S proteasome through the interaction of its ubiquitin-like domain with the intrinsic proteasomal ubiquitin receptor Rpn13.
Conclusion: Parkin turnover and E3 ligase activity can be regulated by its recruitment to the 26 S proteasome via Rpn13.
Significance: Parkin-Rpn13 interaction might be exploited as a potential therapeutic strategy.
Keywords: E3 Ubiquitin Ligase, Parkin, Parkinson Disease, Proteasome, Ubiquitin, Ubiquitylation (Ubiquitination), Rpn13
Abstract
Mutations in the Park2 gene, encoding the RING-HECT hybrid E3 ubiquitin ligase parkin, are responsible for a common familial form of Parkinson disease. By mono- and polyubiquitinating target proteins, parkin regulates various cellular processes, including degradation of proteins within the 26 S proteasome, a large multimeric degradation machine. In our attempt to further elucidate the function of parkin, we have identified the proteasomal ubiquitin receptor Rpn13/ADRM1 as a parkin-interacting protein. We show that the N-terminal ubiquitin-like (Ubl) domain of parkin binds directly to the pleckstrin-like receptor for ubiquitin (Pru) domain within Rpn13. Using mutational analysis and NMR, we find that Pru binding involves the hydrophobic patch surrounding Ile-44 in the parkin Ubl, a region that is highly conserved between ubiquitin and Ubl domains. However, compared with ubiquitin, the parkin Ubl exhibits greater than 10-fold higher affinity for the Pru domain. Moreover, knockdown of Rpn13 in cells increases parkin levels and abrogates parkin recruitment to the 26 S proteasome, establishing Rpn13 as the major proteasomal receptor for parkin. In contrast, silencing Rpn13 did not impair parkin recruitment to mitochondria or parkin-mediated mitophagy upon carbonyl cyanide m-chlorophenyl hydrazone-induced mitochondrial depolarization. However, it did delay the clearance of mitochondrial proteins (TIM23, TIM44, and TOM20) and enhance parkin autoubiquitination. Taken together, these findings implicate Rpn13 in linking parkin to the 26 S proteasome and regulating the clearance of mitochondrial proteins during mitophagy.
Introduction
Parkinson disease (PD)7 is characterized by disabling motor and nonmotor symptoms caused by the degeneration of dopaminergic neurons in the substantia nigra, as well as other neuronal populations in the brain. Protein misfolding and aggregation, resulting in the formation of inclusion bodies and other aggregates within neurons, represent one of the hallmarks of neurodegenerative diseases, including PD. Such inclusions are likely to stem from an underlying cellular defect in protein quality control pathways, including the ubiquitin proteasome system. Whereas most cases of PD occur in a sporadic fashion, ∼5–10% cases are inherited in a typical Mendelian fashion. Many of these familial cases are caused by mutations in the PARK2 gene, which lead to an autosomal recessive juvenile onset form of PD (1–3). PARK2 encodes the parkin protein, which functions as an E3 ubiquitin ligase, a class of proteins that conjugates the small protein ubiquitin (Ub) onto substrate proteins (4). Numerous parkin substrates, including CDCrel-1 (5), Pael-R (6), cyclin E (7), Eps15 (8), PICK1 (9), endophilin-A (10), and synphilin-1 (11), have been identified; however, their precise role in PD still remains to be elucidated. Indeed, parkin-mediated ubiquitination appears to function in several distinct cellular pathways, depending on the number and type of Ub modifications involved. Substrate monoubiquitination by parkin plays a role in receptor trafficking (8), whereas polyubiquitination by Lys-6-, Lys-27-, and Lys-63-linked Ub chains has been suggested to play a role in inclusion formation and mitophagy (12–14). However, proteasomal degradation by parkin-mediated Lys-48-linked substrate polyubiquitination has been the most extensively studied function of parkin. In particular, AIPM2 (aminoacyl-tRNA synthetase-interacting multifunctional protein type 2; p38/JTV-1) (15), FBP1 (fuse-binding protein 1) (16), and PARIS (17) have been found to accumulate in parkin knock-out mice, suggesting that parkin-mediated ubiquitination is required for their targeting to the 26 S proteasome for degradation. Recently, parkin activity has been associated with the clearance of depolarized mitochondria by mitophagy (18), where parkin recruitment to mitochondria and its ubiquitin ligase activity are controlled by PINK1 (PTEN-induced putative kinase 1) phosphorylation of both parkin and Ub (19–21). According to the current model, parkin is recruited to the damaged mitochondria by PINK1 (18, 22), where it polyubiquitinates several mitochondrial proteins (13, 23–26). Importantly, turnover of mitochondrial proteins requires parkin and the 26 S proteasome function. Parkin contains an ubiquitin-like (Ubl) domain at its N terminus and a RING0-RING in between RING (RBR) domain at its C terminus. The RBR domain is involved in binding Ub-conjugating enzymes (E2), which is critical for parkin-mediated ubiquitination. Indeed, many PD-linked mutations occur in the RBR domain and impair the E3 activity of parkin (27). In contrast, less is known about the function of the parkin N-terminal Ubl domain. Mutations within the Ubl domain render parkin insoluble and unstable (28, 29). We have previously shown that the Ubl functions as a key interaction module allowing parkin to interact with proteins containing ubiquitin-binding domains, such as Eps15, Ataxin-3, and endophilin-A (8, 10, 30). Interestingly, Tsai et al. (31) showed that the Ubl domain of parkin is necessary for its direct interaction with purified 26 S proteasomes in vitro. Additionally, two independent groups found that parkin could interact with the 26 S proteasome by binding the 19 S proteasome subunit Rpn10/S5a (32) or the 20 S subunit α4/PSMA7 (33). Although Rpn10 was shown to interact with the parkin Ubl domain (32), the C-terminal IBR-RING domain of parkin was found to interact with the C-terminal part of α4/PSMA7 (33). However, the precise mode of interaction between parkin and the proteasome remains to be determined.
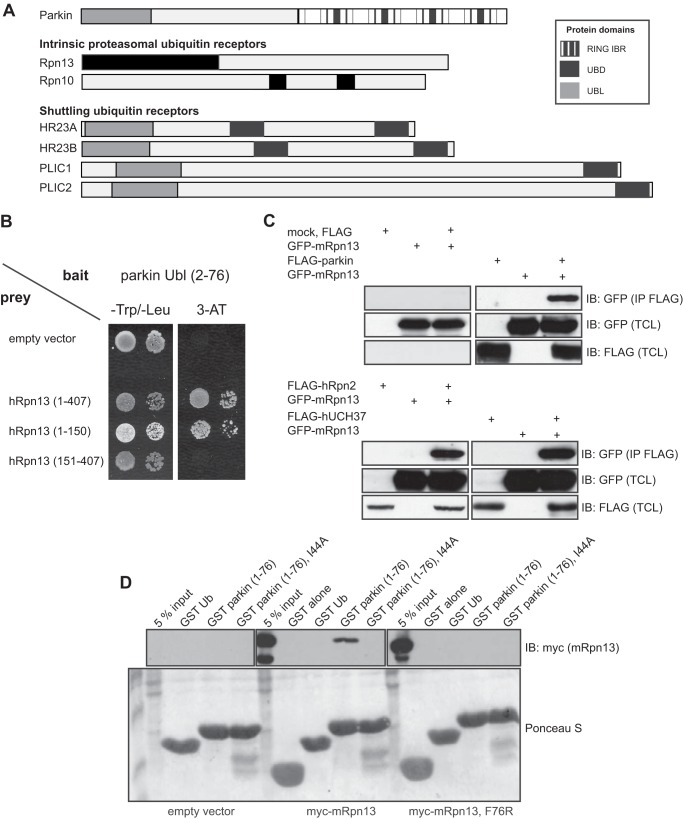
Efficient degradation of misfolded proteins requires their polyubiquitination and recruitment to the 26 S proteasome. The proteasomal ubiquitin receptors Rpn10 (34) and Rpn13/ADRM1 (35, 36) can directly bind ubiquitinated cargo at the 19 S proteasome (see Fig. 1A). Moreover, several Ub shuttling receptors, such as Rad23 (hHR23A/B in humans) and Dsk2 (hPLIC1/2 in humans), bind the 19 S proteasomal regulatory subunit via their N-terminal Ubl domains while recruiting ubiquitinated cargo to the proteasome with their C-terminal ubiquitin-associated domain(s) (see Fig. 1A) (37–40). Additionally, several E3 Ub ligases, such as Hul5 and Ufd4, have been associated with the proteasome in yeast (41, 42), which might help locally couple substrate ubiquitination with its subsequent degradation by the 26 S proteasome.
FIGURE 1.
The Ubl domain of parkin binds the Pru domain of Rpn13. A, schematic representation of the E3 ligase parkin and proteasome-related ubiquitin receptors. Specific domains and motifs are depicted. UBD, ubiquitin-binding domain; UBL, ubiquitin-like domain; IBR, in between ring fingers. B, mapping of the parkin Ubl and Rpn13 binding regions. Yeast strain Y190 was transformed with parkin Ubl-pYTH9 vector and various fragments of hRpn13 in pACT2 vector as preys. C, overexpressed mRpn13 and parkin bind, as shown by co-immunoprecipitation in HEK293T lysates. Plasmids for hRpn2 and hUCH37 were used as positive binding controls. D, residues involved in Rpn13-Ub binding also mediate the Rpn13-parkin interaction. Lysates from HEK293T cells transiently transfected with full-length Myc-mRpn13 (aa 1–407) or its corresponding F76R mutant were used for GST pulldown assays with GST alone, GST Ub (positive control), and either WT or I44A mutant of GST-tagged Ubl domain of parkin. Empty vector pcDNA 3.1 was used as a negative control. IB, immunoblotting; TCL, total cell lysate.
In this paper, we show that parkin is recruited to the 26 S proteasome via a direct interaction with the 19 S proteasomal subunit Rpn13. This recruitment requires the intact Ubl domain of parkin, which binds the pleckstrin-like receptor for ubiquitin (Pru) domain of Rpn13 with high affinity (Kd = 3 μm). No significant differences in mitochondrial recruitment of parkin and mitophagy were observed upon Rpn13 knockdown. However, mitochondrial membrane depolarization in the Rpn13 knockdown cells caused a significant delay in the clearance of both outer and inner mitochondrial proteins (TIM23, TIM44, and TOM20), accompanied by an initial increase in the levels of ubiquitinated parkin. The rate of clearance for parkin substrates that are normally destined to the proteasome is thus delayed. However, knockdown of Rpn13 does not affect the stability of all parkin substrates (MFN1, MFN2, Eps15, PICK1, or p38/JTV1). Additionally, endogenous levels of parkin increase upon Rpn13 knockdown in cells. Furthermore, loss of Rpn13 abolishes parkin binding to the proteasome, thus establishing Rpn13 as the major parkin receptor within the 26 S proteasome. We also found that parkin activity is increased upon Rpn13 binding, possibly because of the loss of the Ubl-mediated autoinhibition of parkin.
EXPERIMENTAL PROCEDURES
Plasmids and Antibodies
The Ubl domain of human parkin (aa 2–76) was subcloned into the pYTH9 vector and used in the yeast two-hybrid screen as bait. Different fragments of human Rpn13 (hRpn13; aa 1–407, 1–150, and 151–407), human Rpn10 (hRpn10; aa 1–377, 1–195, and 196–377), human Rpn2 (hRpn2)/PSMD1 (aa 1–400, 301–700, and 601–953), and human Rpn1 (hRpn1)/PSMD2 (aa 1–908 and 409–551) were cloned into the prey vector pACT2 and used in 1:1 interaction studies. In all the assays, Saccharomyces cerevisiae strain Y190 was used. Different fragments of human parkin (aa 1–465, 77–465, and 1–76) were subcloned into the pMAL-C2X, pcDNA3.1, and pGEX-4T-1 plasmids. Human parkin in pCMV-2b vector was a kind gift of T. Dawson. Human UCH37 and hRpn2 were cloned into the NpFLAG-CMV2 vector, whereas murine Rpn13 (mRpn13) constructs (aa 1–407, 1–150, 101–407, and 151–407) were subcloned into the pGEX-4T1, prSET-His, pGEX-6P1, and pEGFP-C1 vectors. Fragments of human Rpn10 (aa 1–377, 1–195, and 196–306) and PSMA7 (aa 1–248) were cloned into the pGEX-4T1 vector. The Ubl domain (aa 27–111) of human PLIC2 (hPLIC2) and the C-terminal portion of hRpn2 (aa 797–953) were cloned into the pGEX-4T1 vector. The GST Ub construct was a kind gift of Dr. L. Hicke. Tandem repetition of four ubiquitin moieties (tetraUb) in pGEX-4T2 was kindly provided by Dr. C. Guo and Dr. E. Friedberg. Point mutants of mRpn13 (F76R), shRNAmiR-resistant Myc-mRpn13, human parkin (I44A, I44R, R42P, K48A, and C431F) and deletion mutants (lacking aa 214–296) of hRpn10 were generated by in vitro mutagenesis (Stratagene). All the constructs were verified by sequencing before use. The following antibodies were used: GFP (Living Colors; Clontech), M2 FLAG and vinculin (both Sigma-Aldrich), parkin (PRK8), MBP, ubiquitin (P4D1), pUb-R2 (Rpn10) and Myc (9E10), TOM20, TIM23, TIM44, Mitofusins 1 and 2 (MFN1 and MFN2) (all Santa Cruz Biotechnology), His (New England Biolabs), Rhot1/Miro (Novus Biologicals), Rpn13, Rpt1, and Rpn2 (all Biomol), actin (Millipore), p38 (kindly provided by Dr. O. Corti, Paris, France), Eps15 (BD Transduction Laboratories), and PICK1 (Affinity).
Yeast Two-hybrid System
The S. cerevisiae strain Y190 was transformed with various bait and prey vectors as described previously (43) and indicated in the text and figure legends. A human cDNA library (Clontech) in the pACT2 vector was used as prey for the large scale screen. Yeast was grown on a synthetic dropout (SD) medium lacking Leu and Trp (SD/−Trp/−Leu). Colonies appearing after 2–4 days on the SD/−Trp/−Leu plates were grown overnight in 5 ml of the same liquid selection medium. 0.5 ml of overnight culture was transferred to 4.5 ml of fresh SD/−Trp/−Leu medium for 1–2 h, and the concentration of cells was estimated by measuring the A600. The amount of cells per ml was adjusted by diluting with fresh medium. 3 μl of cell suspension containing the same amount of cells was spotted on SD/−Trp/−Leu agar plates, as well as on selective 3-AT (3-amino-1,2,4-triazole) agar plates (SD/−Trp/−Leu/−His, 25 mm 3-AT). Two days after spotting, cells were transferred to a Whatman filter paper and assayed for β-galactosidase activity with X-β-Gal (Carl Roth GmbH). All of the experiments were repeated three times.
Fusion Proteins and GST Pulldown Assay
GST and MBP fusion proteins were expressed in Escherichia coli strains BL21 or BL21 DE3 using 0.5 mm isopropyl β-d-thiogalactopyranoside for protein induction and incubated at 16 °C overnight. The harvested cells were lysed by sonication in lysis buffer (20 mm Tris-HCl, pH 7.5, 10 mm EDTA, 5 mm EGTA, 150 mm NaCl, 0.5% Triton X-100), followed by centrifugation (20 min, 4 °C, 10,000 × g). Supernatant containing GST fusion proteins was affinity-purified using glutathione-Sepharose 4B beads (GE Healthcare), whereas MBP-tagged proteins were bound to amylose resins (New England Biolabs). Purified MBP-tagged proteins were eluted from the amylose resins with 10 mm maltose and subsequently used for direct protein binding analysis with GST-fused proteins bound to beads. Alternatively, GST-fused proteins were cleaved by thrombin (GE Healthcare) at 24 °C overnight in 1× cleavage buffer (20 mm Tris-HCl, pH 8.4, 150 mm NaCl, 2.5 mm CaCl2, 1 mm DTT), followed by centrifugation and thrombin inactivation by 1 mm PMSF. Supernatant containing protein cleaved from GST was used for subsequent GST pulldown assay with GST proteins bound to beads. GST pulldown assays for recombinant protein-protein interactions were performed in 1× incubation buffer (20 mm Tris-HCl, pH 8.4, 150 mm NaCl, 2.5 mm CaCl2, 10% glycerol, 1% Triton X-100, 1 mm DTT) for 4 h at 4 °C.
Co-immunoprecipitation
Plasmids encoding indicated cDNAs were transfected into HEK293T cells using Genejuice (Merck) according to the manufacturer's instructions. After 24 h, cells were lysed in ice-cold lysis buffer (50 mm HEPES, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 10% glycerol, 1% Triton X-100, 25 mm NaF, 10 μm ZnCl2) freshly complemented with various inhibitors (10 μg/ml aprotinin, 2 μg/ml leupeptin, 1 mm sodium orthovanadate, 1 mm PMSF, 10 mm N-ethylmaleimide) and centrifuged (20 min, 13,000 rpm, 4 °C). The Triton X-100-soluble fraction was incubated with indicated primary antibody at 4 °C overnight with rotation. Protein G-Sepharose (Roche) was added for 1 h, after which the beads were washed three times in ice-cold lysis buffer and subsequently denaturated in Laemmli buffer containing 5% β-mercaptoethanol.
Nuclear Magnetic Resonance
The mRpn13 Pru (aa 1–130) and 15N-labeled parkin Ubl (aa 1–76) were purified by size exclusion chromatography in NMR buffer (30 mm sodium phosphate, pH 7.0, 200 mm NaCl, 0.5 mm DTT, 0.1 mm EDTA). Proteins were concentrated by using ultrafiltration devices (molecular weight cutoff, 3000) and 5% D2O was added. 1H-15N heteronuclear single quantum coherence spectra were recorded at a temperature of 303 K by using proteins at various concentrations: [15N-Ubl]: 800, 410, 270, and 207 μm; and [Pru]: 0, 180, 240, and 260 μm (molar ratios: 0, 0.44, 0.89, and 1.25). Weighted average chemical shift perturbations were measured as the difference between the first and last spectra using the following formula.
Surface Plasmon Resonance
SPR was performed using a nickel-nitrilotriacetic acid chip on a BIAcore T100 instrument (GE Healthcare) at 25 °C, by using the HBS-P buffer (10 mm HEPES, 150 mm NaCl, 0.05% P20, pH 7.4). To activate the surface, 100 mm NiSO4 was injected, followed by 125 nm His6-Pru injection until 1000 response units were obtained. Afterward, concentration series of parkin Ubl (0.05–25 μm) or Ub (0.2–50 μm) were injected for 1 min. Affinities were determined by fitting the steady-state equilibrium responses (50–60 s postinjection) as a function of concentration using a simple 1:1 binding isotherm.
RNAi-based Knockdown of Proteasome Subunits
For Rpn13 knockdown, a lentivirus-based shRNAmiR approach was used. The following oligonucleotides encoding the anti-hRpn13 shRNAs were designed by using the BLOCK-iT software from Invitrogen: oligonucleotide 356 sense, 5′-TGCTGTTGAACTTCAGCACGTAGACCGTTTTGGCCACTGACTGACGGTCTACGCTGAAGTTCAA-3′; oligonucleotide 356 antisense, 5′-CCTGTTGAACTTCAGCGTAGACCGTCAGTCAGTGGCCAAAACGGTCTACGTGCTGAAGTTCAAC-3′; oligonucleotide 1048 sense, 5′-TGCTGTATTCTGGATCTCATCCGCGGGTTTTGGCCACTGACTGACCCGCGGATGATCCAGAATA-3′; and oligonucleotide 1048 antisense, 5′-CCTGTATTCTGGATCATCCGCGGGTCAGTCAGTGGCCAAAACCCGCGGATGAGATCCAGAATAC-3′.
Oligonucleotides were resuspended to a final concentration of 1 μg/μl, and 2.5 μl of each was combined and annealed in a buffer containing 20 mm Tris-HCl, pH 7.8, 100 mm NaCl, and 0.2 mm EDTA; heated to 95 °C; and then slowly cooled down to 40 °C. The annealed oligonucleotides were phosphorylated using T4 polynucleotide kinase according to the manufacturer's instructions (Invitrogen) and cloned into the pcDNA6.2/GW-EmGFP.miR vector (Invitrogen). All clones were confirmed by sequencing and transiently transfected into HEK293T cells to determine the efficiency of hRpn13 knockdown. For hRpn10 knockdown, a siRNA approach was used. HEK293T cells were transiently transfected with 10 nm siGENOME SMARTpool siRNA (M-011365; Dharmacon, Lafayette, Colorado) by using Lipofectamine RNAiMAX transfection reagent (Invitrogen).
Lentiviral miRNA Production
The expression cassette from the above mentioned constructs were PCR-amplified using the following primers: miRGFP BglII sense, 5′-GCGCAGATCTACCGGTCGCCACCATGGTGAGCAAGGGCGAGGAGC-3′;and miRXFP XhoI sense, 5′-GCGCCTCGAGTGCGGCCGGATCTGGGCCATTTGTTCCATGTGAGTGC-3′. The PCR products were subcloned into the BglII and XhoI sites of the vector for viral production (pRRLsinPPTeGFP; kindly provided by Dr. B. Ritter, McGill University). All clones were confirmed by sequencing. For the production of viral particles, the Miami System (Addgene) was used. This HIV-based system consists of the expression vector described above (pRRLsinPPTeGFP-356 or -1048) and three packaging vectors: pRSV-Rev, pMD2.g, and pMDLg/pRRE. The viral particles were produced in HEK293T cells following the standard Miami System procedures and the security regulations of McGill University.
In Vitro Ubiquitination Assay
To test the effect of Rpn13 on parkin activity, 1.5 μg of purified recombinant mRpn13 proteins (aa 1–407, 1–150, and 151–407), obtained by cleavage of GST Rpn13 fusions bound to glutathione-Sepharose 4B beads (GE Healthcare) with Prescission Protease (GE Healthcare) and subsequent removal of GST-fused Prescission Protease by centrifugation were mixed with 50 nm His-E1 (Boston Biochem), 500 nm UbcH7 (Millipore), 3 μg of purified His-parkin (wild-type or C431F mutant) in 1× in vitro ubiquitination buffer (25 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 5% glycerol, 2 mm DTT, 2 mm ATP). The reaction was carried out for 2 h at 37 °C and stopped by addition of Laemmli buffer containing 5% β-mercaptoethanol. Samples were separated by 10% SDS-PAGE, transferred to 0.22 μm nitrocellulose membrane, and immunoblotted by anti-ubiquitin antibody.
siRNA Transfections, Western Blot Analyses, and Fixed Cell Immunofluorescence
All siRNA transfections, Western blot analyses, and fixed cell immunofluorescence in U2OS cells stably expressing GFP-parkin were performed as previously described (14). Nontargeting or Rpn13 smartPool siRNA (Qiagen) were used at a final concentration of 10 nm. At 60 h post-transfection, cells were either left untreated or were treated with CCCP (final concentration, 20 μm; Sigma) for the indicated time.
RESULTS
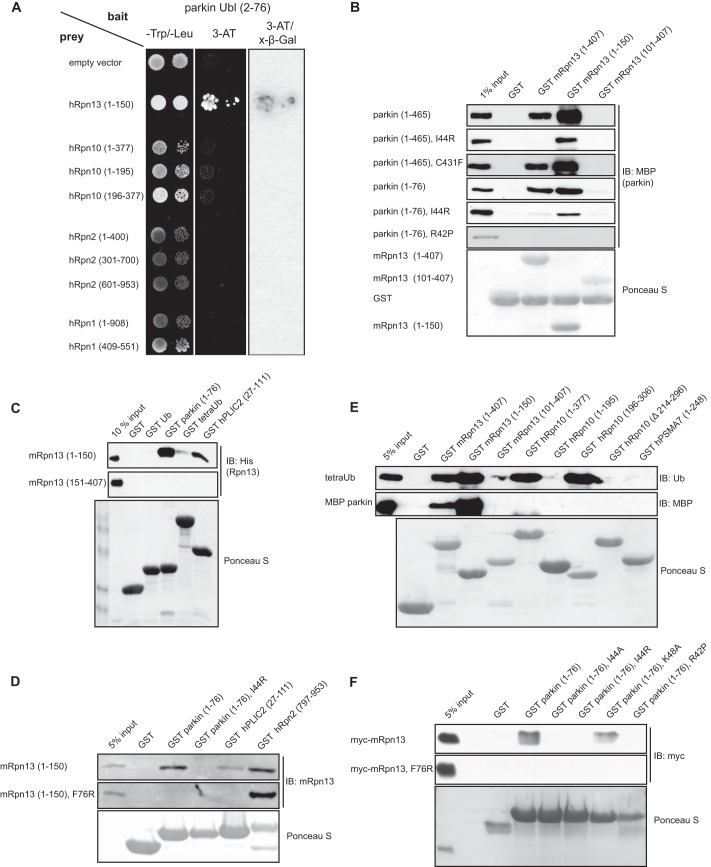
One of the major aims of our research in recent years has been to identify novel Ub- and Ubl-binding proteins/domains and to determine their physiological role in the cell (35, 43, 44). We were specifically interested in parkin because the function of its N-terminal Ubl domain had not been completely characterized. To that aim, we expressed the Ubl domain of human parkin in a yeast two-hybrid bait vector and, by using the pACT2-based library as a prey, identified several putative parkin-binding proteins, including multiple clones of the proteasome ubiquitin receptor Rpn13 (data not shown). We have previously characterized the Pru domain at the N terminus of Rpn13 as a novel ubiquitin-binding domain, establishing Rpn13 as one of the major Ub receptors within the proteasome (Fig. 1A) (35, 36). By using 1:1 interaction studies in yeast (Figs. 1B and 2A), co-immunoprecipitation of overexpressed FLAG-tagged parkin and GFP-tagged Rpn13 in HEK293T cells (Fig. 1C), GST pulldown of transiently overexpressed Myc-tagged mRpn13 with GST-fused Ubl domain of parkin (Fig. 1D), as well as by direct in vitro binding assays with purified parkin and Rpn13 proteins (Fig. 2, B and C), we confirmed that the parkin-Rpn13 interaction was direct. Moreover, we showed that the interaction is mediated through the Rpn13 Pru (aa 1–150) and the parkin Ubl domain (aa 1–76) (Figs. 1D and 2, A–E). By generating point mutations within the respective domains, we could additionally show that the interaction is centered around the hydrophobic region containing the Ile-44 residue in the parkin Ubl (Figs. 1D and 2, B, D, and F), which is structurally well conserved among many Ubl domains and Ub and is critical for binding to several other ubiquitin-binding domains. Conversely, the Phe-76 residue in the Pru domain of Rpn13, which is critical for Ub binding, was also essential for the interaction with parkin (Figs. 1D and 2, D and F). We also tested several Parkinson disease causing mutations for binding to Rpn13. Whereas the R42P mutant (described as poorly folded and unstable) (45, 46) could not bind Rpn13 (Fig. 2, B, D, and F), mutation K48A (which retains a similar three-dimensional fold) did not abolish the interaction with Rpn13 (45, 46) (Fig. 2F).
FIGURE 2.
The Ubl domain of parkin interacts with the 19 S proteasomal subunit Rpn13. A, Ubl domain of parkin binds Pru domain of Rpn13. Yeast strain Y190 transformed with parkin Ubl-pYTH9 plasmid was additionally transformed with plasmids encoding various parts of mRpn13, hRpn10, hRpn2, and hRpn1 cDNA, and their binding was assayed by selective growth and X-β-Gal assay. B, Ubl domain of parkin directly binds Pru domain of Rpn13 via its Ile-44 hydrophobic patch. Using in vitro binding assay, various point and deletion mutants of parkin, expressed as MBP fusion proteins eluted from amylose resins, were tested for direct binding to different domains of Rpn13, which were expressed as GST fusion proteins and coupled to glutathione-Sepharose 4B beads. Ponceau S indicates levels of GST-coupled beads used in GST pulldown assay. Prior SDS-PAGE washed GST-beads were cleaved with thrombin for better visualization. C, Ubl domain of parkin, similar to ubiquitin and Ubl domain of hPLIC2 (aa 27–111), directly binds Pru domain of Rpn13. Using in vitro binding assay, ubiquitin and Ubl domains, expressed as GST fusion proteins, were tested for direct binding to different domains of Rpn13, which were expressed as His fusion proteins and eluted from nickel-nitrilotriacetic acid resins before GST pulldown assay. Ponceau S indicates levels of GST-coupled beads used in GST pulldown assay. D, loop mutation (F76R) in Rpn13 and hydrophobic patch mutation (I44R) in parkin abolish their direct binding. Purified WT (aa 1–150) and F76R Pru domain (aa 1–150), obtained by removal of GST moiety from GST fusion proteins by thrombin cleavage, were tested for binding to WT and I44R Ubl domain (aa 1–76) of parkin. Parkin constructs were expressed as GST-fused proteins coupled to glutathione-Sepharose 4B beads. As positive binding controls, GST-fused fragments of hRpn2 (aa 797–953) and hPLIC2 (aa 27–111) were used. E, purified MBP-fused parkin, eluted from amylose resins, was tested for direct binding to fragments of different proteasomal subunits, including mRpn13, hRpn10, and hPSMA7 as GST-tagged proteins. TetraUb cleaved with thrombin from GST beads served as a positive binding control. F, GST pulldown assay with lysates from HEK293T cells transiently transfected with full-length Myc-mRpn13 (upper panel) or F76R Myc-mRpn13 (lower panel) plasmids and GST alone (negative control) and various GST-fused mutants of parkin Ubl domain bound to beads. IB, immunoblotting.
Because both ubiquitinated and Ubl-containing proteins can be recruited to the proteasome by binding to various subunits within the 19 S proteasome subcomplex, including Rpn10 (34), Rpn2 (47), Rpn1 (48), and Rpn13 (35, 36), we performed additional 1:1 interaction studies in yeast to address that issue (Fig. 2A). To our surprise, we found that parkin binds Rpn13, but not Rpn10, Rpn2, or Rpn1 under the same experimental conditions (Fig. 2A). These results were confirmed by using in vitro binding assays (Fig. 2E), in which purified MBP-tagged parkin was tested for direct binding to various fragments of Rpn13, Rpn10, and PSMA7.
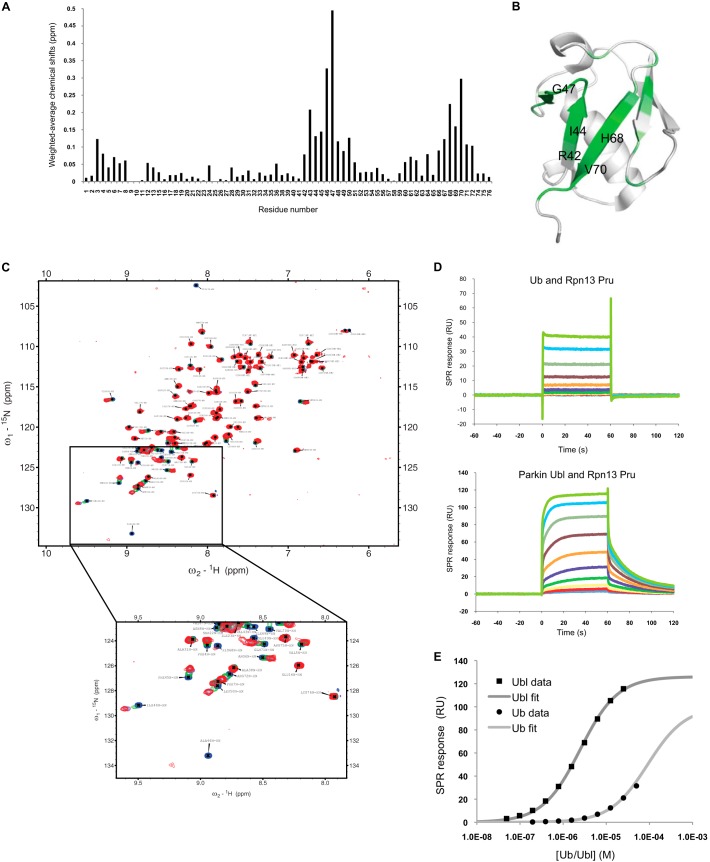
To further characterize the interaction between parkin and Rpn13, we used NMR to measure the chemical shift perturbations in the 15N-labeled parkin Ubl domain upon addition of the Rpn13 Pru domain. The data confirmed that the parkin Ubl mediates its interaction with the Rpn13 Pru by using the hydrophobic patch positioned around Ile-44 (Fig. 3, A and B). The Ubl residues that displayed the largest chemical shifts upon Rpn13 Pru binding are localized on the five-strand β-sheet face of the Ubl with little perturbation elsewhere (Fig. 3B). Moreover, the Ubl residues that experience NMR perturbations upon Rpn13 binding are similar to those induced by the UIM from Eps15 and Rpn10, as well as Ub residues binding either Rpn13 or UIMs. The chemical shift changes in the titration are in the fast-intermediate exchange regime, and the perturbations saturate just over a stoichiometric ratio of 1:1 (Fig. 3C), suggesting that the equilibrium dissociation constant is ≪100 μm. Because affinity constants cannot be measured precisely by NMR when the protein concentrations are much greater than the dissociation constants, we resorted to SPR to quantify the binding between Rpn13 Pru and parkin Ubl or Ub (Fig. 3, D and E). Interestingly, results showed a higher Kd value between Ub and Pru domain (65 ± 25 μm) than between parkin Ubl and Pru domain (3 ± 2 μm). Furthermore, the shape of the dissociation phase after the injection of the Ubl strongly suggests a higher affinity between Pru and Ubl than between Pru and Ub (Fig. 3, D and E). The low micromolar affinity constant measured between the Ubl and Rpn13 is in qualitative agreement with the NMR results. Moreover, the calculated Kd between parkin Ubl and Rpn13 Pru domain is ∼2 orders of magnitude lower than the one reported for parkin Ubl binding to the Rpn10 UIMs (217 ± 51 μm) (49), likely explaining the difficulty in confirming the parkin-Rpn10 interaction by pulldown assays (32).
FIGURE 3.
NMR and SPR characterization of parkin Ubl binding to the Rpn13 Pru. A, weighted average NMR chemical shift perturbations in 15N-labeled parkin Ubl upon binding to Rpn13 Pru domain. B, NMR chemical shift perturbations mapped on the crystal structure of the murine parkin Ubl domain (Protein Data Bank code 2ZEQ (60)). Perturbations are colored in dark green (>0.15 ppm), medium green (0.1–0.15 ppm), or pale green (0.05–0.1 ppm). Important residues involved in the interaction are labeled in black. C, NMR 15N-1H heteronuclear single quantum coherence spectra of 15N-labeled parkin Ubl in the presence of Rpn13 Pru domain. Molar ratios were 0 (blue), 0.44 (green), 0.89 (purple), and 1.25 (red). Spectra are plotted at equivalent contour levels, and thresholds were adjusted to account for differences in protein concentrations and number of scans. The boxed area is enlarged below. Notice that there is significant broadening at 0.44 and 0.89 molar ratios for peaks that shift most (green and purple spectra). D, SPR sensorgrams of Ub or parkin Ubl analyte solution injected at different concentrations over immobilized ligand His6-tagged Rpn13 Pru. Notice the slower association and dissociation kinetics of the parkin Ubl compared with Ub. E, fitting of the SPR steady-state equilibrium responses as a function of analyte concentration to obtain equilibrium dissociation constants of 65 ± 25 and 3 ± 2 μm for Ub and Ubl for binding to His6-Rpn13 Pru.
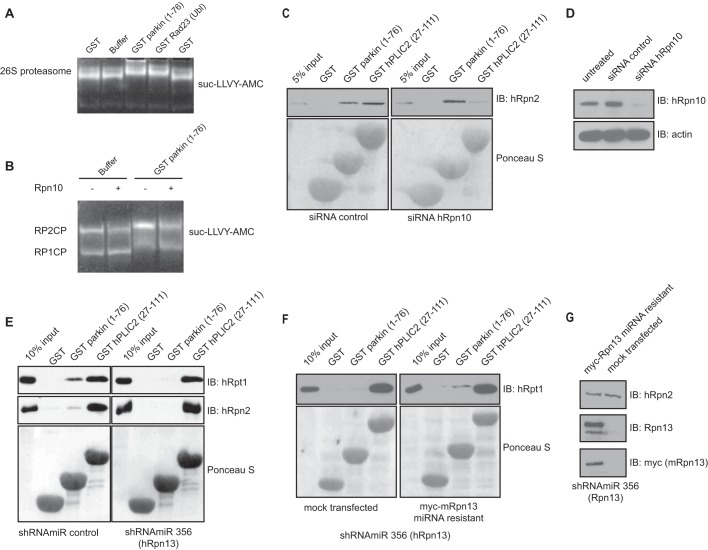
We could also show that the Ubl domain of parkin directly interacts with the active purified bovine proteasome (Fig. 4A) by using gel mobility shift assay. Additionally, we could demonstrate that the binding of GST parkin to purified yeast proteasome occurs irrespective of the presence of Rpn10 in proteasomes purified from yeast (Fig. 4B), which is in accordance with our protein binding assays, showing no interaction between parkin and Rpn10 (Fig. 2, A and E). In accordance with that, silencing of Rpn10 did not affect parkin Ubl binding to the proteasome but interfered with hPLIC2 binding, which is mediated through both Rpn10 and Rpn13 (Fig. 4, C and D).
FIGURE 4.
Parkin interacts with the proteasome specifically via Rpn13. A, gel mobility shift assay of the interaction between the Ubl domain of parkin and the bovine proteasome. Gel mobility shift upon addition of GST (negative control), GST Rad23 Ubl (positive control), or GST parkin Ubl was monitored by nondenaturing PAGE and visualized with fluorogenic substrate suc-LLVY-AMC. Binding of the Ubl domain of parkin decreases the mobility of the proteasomes. B, gel mobility shift assay of the interaction between the Ubl domain of parkin and the proteasome purified from wild-type or Rpn10-deleted (Rpn10Δ) mutant of S. cerevisiae. The mobility of the proteasome following nondenaturing PAGE was visualized with fluorogenic substrate suc-LLVY-AMC. Binding of the Ubl domain of parkin decreased the mobility of the proteasomes irrespective of Rpn10 presence. RP1CP and RP2CP refer to singly or doubly 19 S capped 20 S proteasomes, respectively. Active proteasome was purified and analyzed by nondenaturating PAGE as described (61). C, a siRNA-mediated knockdown of hRpn10 does not affect parkin binding to proteasome. Lysates from HEK293T cells transfected with control nontargeting or anti hRpn10 siRNAs were incubated with GST, GST-parkin Ubl, and GST-hPLIC2 Ubl bound to glutathione-Sepharose 4B. Proteasome binding was assessed by immunoblotting against the 19 S proteasome subunit hRpn2. D, the efficiency of hRpn10 silencing in HEK293T cells (controls for C) was assessed by immunoblotting for hRpn10 and actin (as a loading control). E, silencing of Rpn13 abolishes parkin binding to the proteasome. Lysates from HEK293T cells infected with the indicated shRNAmiR were incubated with GST, GST-parkin Ubl, and GST-hPLIC2 Ubl bound to glutathione-Sepharose 4B. Proteasome binding was assessed by immunoblotting against the 19 S proteasome subunits hRpt1 and hRpn2. F, a miRNA-resistant form of Rpn13 rescues the interaction between parkin and the proteasome. shRNAmiR 356-infected HEK293T cells were transiently transfected with a miRNA-resistant Myc-Rpn13 construct or mock transfected. Binding of the parkin Ubl to the proteasome was detected by hRpt1 immunoblotting in lysates from cells transiently transfected with Myc-Rpn13, unlike in the mock-transfected cells. G, the efficiency of Rpn13 silencing and rescue with miRNA-resistant Myc-Rpn13 were assessed by immunoblotting for Rpn13, Myc, and hRpn2 (controls for A and B). IB, immunoblotting.
To further elucidate the functional implications of the parkin-Rpn13 interaction, we used a lentivirus-delivered miRNA (shRNAmiR) approach to knockdown the expression of Rpn13 in HEK293T cells. Silencing of Rpn13 abolished the interaction between the parkin Ubl and endogenous proteasome in HEK293T cells, when probing for the 19 S subunits Rpt1 and Rpn2 (Fig. 4E). In contrast, binding of hPLIC2 to 19 S proteasome was not affected by the Rpn13 knockdown (Fig. 4, E and F). Overexpression of a miRNA-resistant form of Rpn13 in HEK293T cells efficiently rescued parkin Ubl binding to the proteasome, demonstrating that our findings were not due to an off target effect (Fig. 4, F and G). Taken together, our findings establish Rpn13 as the major receptor for parkin in the proteasome.
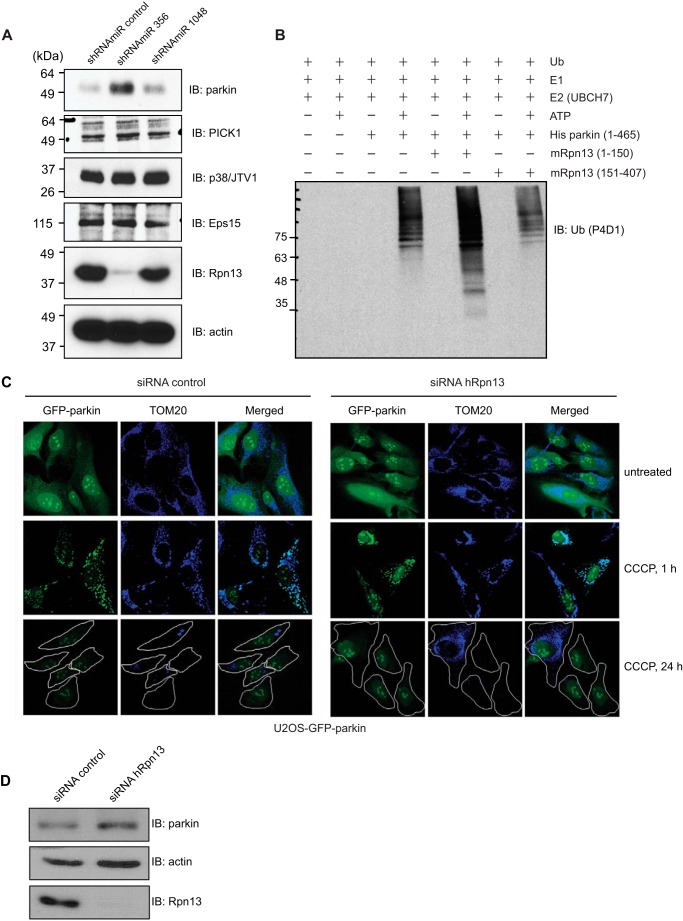
Surprisingly, we found that endogenous parkin levels increased dramatically in cells in which Rpn13 had been efficiently silenced compared with cells in which Rpn13 silencing was either inefficient or in those infected with control nontargeting miRNA (Fig. 5A). In contrast, we observed no effect of Rpn13 silencing on the endogenous levels of parkin substrates PICK1, Eps15, and p38/JTV1 (Fig. 5A).
FIGURE 5.
Interaction with Rpn13 increases the E3 ligase activity of parkin, whereas Rpn13 knockdown leads to increased level of parkin protein levels and has no effect on mitochondrial recruitment of parkin. A, parkin levels increase upon knockdown of Rpn13. Parkin protein levels only increase when shRNAmiR 356 and not the knockdown-ineffective shRNAmiR 1048 or control shRNAmiR are used. None of the analyzed parkin substrates (PICK1, p38/JTV1, and Eps15) were affected by the Rpn13 knockdown. Efficiency of Rpn13 silencing in HEK293T cells using lentivirus-delivered shRNAmiR against Rpn13 (shRNAmiR 356 and shRNAmiR 1048) and a control shRNAmiR was assessed by immunoblotting for Rpn13 and actin (as a loading control). B, in vitro ubiquitination reaction with purified His-parkin as E3 ligase. Reaction mixture (E1, E2 UbcH7, ATP, and ubiquitin) was complemented with equal amounts of various fragments of purified Rpn13. C, knockdown of Rpn13 has no effect on the mitochondrial recruitment of parkin or on the autophagic clearance of mitochondria. U2OS-GFP-parkin cells were transfected with nontargeting or Rpn13 siRNA (10 nm) for 60 h. Untreated cells or cells treated with CCCP for 1 or 24 h were fixed, and images were acquired after staining for the mitochondrial protein, TOM20. D, validation of Rpn13 siRNA knockdown. U2OS-GFP-parkin cells were transfected with nontargeting or Rpn13 siRNA oligonucleotides (10 nm) for 60 h. Cells were lysed and analyzed by immunoblotting for Rpn13, parkin, and actin. IB, immunoblotting.
To verify whether the interaction with Rpn13 further activates the E3 ligase activity of parkin, as already demonstrated for several parkin Ubl-interacting proteins (50), we performed an in vitro ubiquitination reaction with purified parkin in the presence of N-terminal Pru domain of Rpn13 or Rpn13 lacking its Pru domain. (Fig. 5B). We could observe an increase in polyubiquitination smear, indicative of increased parkin E3 ligase activity, when the Pru domain was added to the reaction (51).
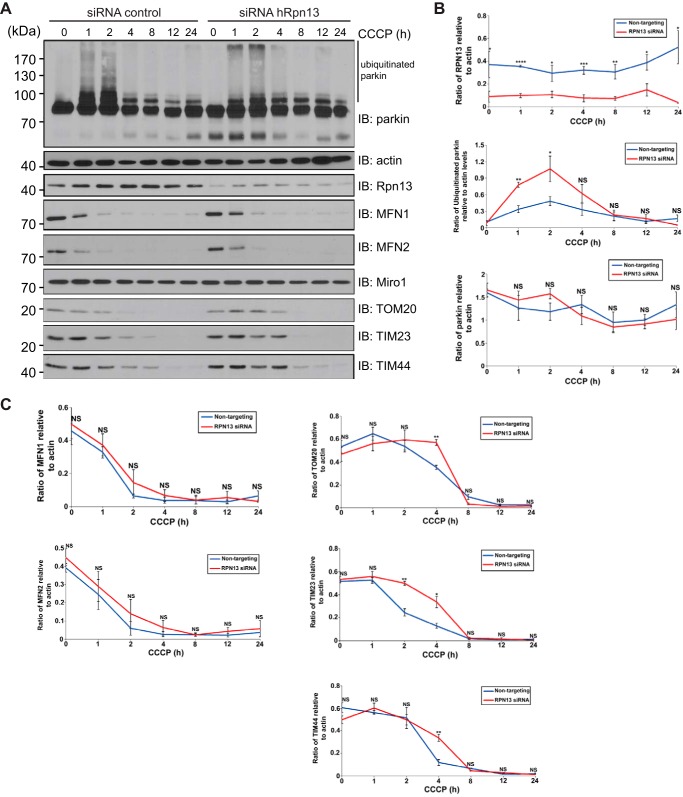
To further characterize the functional significance of Rpn13-parkin interaction, we studied the effect of Rpn13 silencing on the mitochondrial recruitment of parkin, degradation of its mitochondrial substrates, and parkin-mediated mitophagy upon CCCP treatment in U2OS cells stably expressing GFP-parkin (Figs. 5, C and D, and 6). No discernible differences in either the mitochondrial recruitment of parkin, the levels of MFN1 or MFN2, or mitophagy were observed following knockdown of Rpn13. Interestingly, after 4 h of treatment with CCCP, an absence of Rpn13 caused a significant delay in the clearance of both outer and inner mitochondrial proteins (TIM23, TIM44, and TOM20) (Fig. 6). Moreover, an increase in ubiquitinated parkin was observed after 1–2 h (Fig. 6A). Thus, in the absence of Rpn13, the rate of clearance of parkin substrates that are normally destined to the proteasome is delayed.
FIGURE 6.
Knockdown of Rpn13 delays the clearance of parkin substrates upon mitochondrial membrane depolarization. A, knockdown of Rpn13 delayed the clearance of TIM and TOM proteins. U2OS-GFP-parkin cells were transiently transfected with nontargeting or Rpn13 siRNA (10 nm) for 60 h. Cells were either left untreated or were treated with CCCP for the indicated intervals. Cells were lysed and analyzed by immunoblotting for parkin, Rpn13, actin, Mitofusins 1 and 2, Miro1, TIM23, TIM44, and TOM20 (quantified in Figs. 6B and 6C). B, quantification of Rpn13 and parkin protein levels upon CCCP treatment of Rpn13 knockdown U2OS-GFP-parkin cells. U2OS-GFP-parkin cells were transiently transfected with nontargeting or Rpn13 siRNA (10 nm) for 60 h and either left untreated or treated with CCCP for the indicated intervals (0–24 h). Protein levels of Rpn13 and ubiquitinated (parkin smear excluding unmodified parkin) and unmodified parkin upon CCCP treatment were compared between nontargeting and Rpn13 knockdown U2OS-GFP-parkin cells. The optical densities of the indicated proteins and total actin (used for normalization) were quantified using ImageJ (National Institutes of Health). The data represent the mean S.E. for three independent experiments. For statistical analysis, a two-way analysis of variance with Tukey post-test was performed. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; NS, not significant. C, quantification of mitochondrial protein levels upon CCCP treatment of Rpn13 knockdown U2OS-GFP-parkin cells. U2OS-GFP-parkin cells were transiently transfected with nontargeting or Rpn13 siRNA (10 nm) for 60 h and either left untreated or treated with CCCP for the indicated intervals (0–24 h). Decrease in protein levels of MFN1, MFN2, TOM20, TIM23, and TIM44 upon CCCP treatment was compared between nontargeting and Rpn13 knockdown U2OS-GFP-parkin cells. Data analysis was performed as in Fig. 6B. IB, immunoblotting.
DISCUSSION
In this study, we present evidence that parkin binds the proteasome through the ubiquitin receptor Rpn13. This interaction involves the hydrophobic region within the Ubl domain of parkin positioned around Ile-44 and the Pru domain at the N terminus of Rpn13. Indeed, mutations in either the Ubl or the Pru domain dramatically decrease the interaction between parkin and Rpn13. Moreover, silencing of Rpn13 in mammalian cells abolishes the binding between parkin Ubl and the proteasome. The interaction with Rpn13 is specific, because knocking down Rpn10 has no effect on parkin binding to the proteasome. Together, our findings establish Rpn13 as the major parkin receptor in the proteasome.
The ubiquitin proteasome system is the major degradation pathway for intracellular proteins, many of which are of particular interest in the context of neurodegenerative disease. The 26 S proteasome, which is at the center of the pathway, has thus been widely studied. Although initially regarded as a discrete protein complex, an increasing number of nonstoichiometric proteasome-interacting proteins have been discovered, including chaperones, deubiquitinating enzymes, as well as E3 and E4 Ub ligases (52). The diverse range of functions of these proteasome-interacting proteins underscores the high degree of regulation involved in ubiquitin-dependent protein degradation (52). In addition to parkin, a number of Ub ligases have been shown to function at the proteasome. In yeast, UBR1 and UFD4 interact with the proteasome via the 19 S subunits Rpt6 and Rpt4 (42). Interestingly, an UFD4 mutant, which is unable to bind the proteasome, nonetheless retains the ability to ubiquitinate its substrates. However, proteasomal degradation of these substrates is dramatically impaired, suggesting that the interaction between UFD4 and the proteasome is crucial for function (42).
The deubiquitinating enzyme Ubp6 (Usp14 in mammals) binds the proteasome via Rpn2. The interaction involves the Ubl domain of Ubp6 and results in a 300-fold enhancement of Ubp6 deubiquitinating enzyme activity (41, 53). Furthermore, Ubp6 activity is opposed by the Hul5 E4 ligase (41). In this model, poorly ubiquitinated substrates (or those modified by atypical Ub linkages such as Lys-6, Lys-11, Lys-27, Lys-29, and Lys-33) are first deubiquitinated by Ubp6 and then reubiquitinated by Hul5, using canonical Lys-48 linkages. This dynamic coupling of ubiquitination and deubiquitination appears to ensure the proper recognition and degradation of substrates at the 26 S proteasome. Similarly, through its C-terminal domain, Rpn13 binds and activates a deubiquitinating enzyme Uch37 (54, 55). A possible consequence of parkin binding to the 26 S proteasome via Rpn13 might be to oppose the activity of Uch37, as shown for Hul5, Ubp6, and Rpn2. Knockdown of Rpn13 in HEK293T cells did not affect the steady-state levels of parkin substrates p38/JTV1, PICK1, or Eps15. Thus, at least for these substrates, parkin binding to the proteasome may not be essential for turnover. However, the role of parkin in turnover of other substrates could not be excluded, and we therefore tested the effect of HEK293T knockdown in the context of mitophagy, where the role of parkin has been well established (13, 22, 24, 56). Indeed, Rpn13 knockdown caused a delay in the clearance of the outer mitochondrial protein TOM20 upon CCCP treatment. Additionally, we could also observe a consistent delay in the clearance of inner mitochondrial proteins TIM23 and TIM44 following CCCP exposure. TIM23 forms contacts between the outer and inner mitochondrial membranes and forms complex with TIM44 (57), which might, at least in part, explain why their levels are affected by Rpn13 knockdown similar to the levels of TOM20. Additionally, Chan et al. (23) also observed a slight reduction in the short isoforms of inner mitochondrial membrane protein Opa1, whereas levels of Mfn1, Mfn2, and Tom70 were significantly reduced in a parkin-dependent manner.
We also observed a marked increase in parkin levels in Rpn13 knockdown cells, suggesting that Rpn13 may regulate parkin turnover per se by recruiting it to the proteasome for degradation. Furthermore, we have also observed an increase in parkin levels in the livers of adult Rpn13 knock-out mice.8 This is in agreement with the observed increase in parkin levels upon Rpn13 silencing (Fig. 5A), increased parkin stability upon removal of its Ubl domain (58), and lack of strong increase in proteasomal binding to parkin lacking Ubl domain upon mitochondrial depolarization (56). Rpn13 might also regulate the amount of parkin depending on the availability of parkin substrates and might recruit parkin for its subsequent degradation when its substrates are not available or are present at low amounts. On the other hand, it might recruit parkin together with its ubiquitinated substrates, thus regulating the turnover of these proteins as well.
Similarly to Ubp6, parkin requires its Ubl domain to interact with the 26 S proteasomes, at least in vitro (31). However, the exact protein(s) within the proteasome involved in parkin binding, as well as the physiological relevance of this interaction, have not been completely elucidated. Sakata et al. (32) determined the three-dimensional structure of the Ubl domain of parkin and found by NMR that it interacted with Rpn10. However, they could not confirm the interaction by co-immunoprecipitation or by in vitro binding experiments, suggesting either a very weak interaction or technical limitations of their assays. Interestingly, our NMR results showed the same pattern of chemical shifts perturbations in the parkin Ubl domain with the Rpn13 Pru domain as previously described with the UIM domains of Rpn10 (49). These involve a contiguous surface containing residues Phe-13, Arg-42, Ile-44, Ala-46, Lys-48, Glu-49, Arg-51, Leu-61, Ile-66, Val-67, and Gln-71. However, the magnitude of the chemical shifts we observed was much higher when using the Rpn13 Pru domain compared with those obtained with the Rpn10 UIMs. In line with these observations, the calculated Kd value between the parkin Ubl and the Rpn13 Pru domain was more than 1 order of magnitude lower than the one reported for parkin Ubl and Rpn10 UIMs (217 ± 51 μm) (49), further arguing in favor of our model in which Rpn13 acts as the major receptor for parkin within the proteasome.
Several groups have shown that parkin recruitment to the 26 S proteasome via its Ubl domain increases the 26 S proteasomal activity (23, 59). Recently Chaugule et al. (50) found that the parkin Ubl domain inhibits its self-ubiquitination by binding to the intrinsic RING1 IBR and RING2 domains, thus keeping parkin in an inhibited state. Binding of the Eps15 UIMs (8) or the endophilin A1 SH3 domain (10) to the parkin Ubl releases the autoinhibition and activates its E3 ligase activity (50). Our in vitro ubiquitination data suggest that the interaction between Rpn13 and parkin not only positions parkin at the 19 S proteasome, but it also locally increases its E3 ligase activity at the proteasome, most probably by releasing its autoinhibition. However, the extent of parkin activation at the proteasome still remains to be determined. Our work clearly defines the molecular basis for the interaction between parkin and the proteasome and provides an attractive potential target for intervention in Parkinson disease.
Acknowledgments
We thank Dr. Olga Corti for kindly providing us with the antibody against p38. We also thank Grzegorz Zapart for the initial yeast two-hybrid screening.
This work was supported by Canadian Institutes of Health Research Operating Grants MOP-62714 and MOP-125924, as well as the LOEWE program of the State of Hesse (Germany).
K. Husnjak and I. Dikic, manuscript in preparation.
- PD
- Parkinson disease
- Ub
- ubiquitin
- Ubl
- ubiquitin-like
- Pru
- pleckstrin-like receptor for ubiquitin
- CCCP
- carbonyl cyanide m-chlorophenyl hydrazone
- UIM
- ubiquitin interaction motif
- PTEN
- phosphatase and tensin homolog
- RBR
- RING0-RING in between RING
- aa
- amino acid(s)
- SPR
- surface plasmon resonance.
REFERENCES
- 1. Giasson B. I., Lee V. M. (2001) Parkin and the molecular pathways of Parkinson's disease. Neuron 31, 885–888 [DOI] [PubMed] [Google Scholar]
- 2. Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 [DOI] [PubMed] [Google Scholar]
- 3. Gasser T. (2009) Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev. Mol. Med. 11, e22. [DOI] [PubMed] [Google Scholar]
- 4. Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., Suzuki T. (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 25, 302–305 [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y., Gao J., Chung K. K., Huang H., Dawson V. L., Dawson T. M. (2000) Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc. Natl. Acad. Sci. U.S.A. 97, 13354–13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imai Y., Soda M., Inoue H., Hattori N., Mizuno Y., Takahashi R. (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105, 891–902 [DOI] [PubMed] [Google Scholar]
- 7. Staropoli J. F., McDermott C., Martinat C., Schulman B., Demireva E., Abeliovich A. (2003) Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron 37, 735–749 [DOI] [PubMed] [Google Scholar]
- 8. Fallon L., Bélanger C. M., Corera A. T., Kontogiannea M., Regan-Klapisz E., Moreau F., Voortman J., Haber M., Rouleau G., Thorarinsdottir T., Brice A., van Bergen En Henegouwen P. M., Fon E. A. (2006) A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat. Cell Biol. 8, 834–842 [DOI] [PubMed] [Google Scholar]
- 9. Joch M., Ase A. R., Chen C. X., MacDonald P. A., Kontogiannea M., Corera A. T., Brice A., Séguéla P., Fon E. A. (2007) Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol. Biol. Cell 18, 3105–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trempe J. F., Chen C. X., Grenier K., Camacho E. M., Kozlov G., McPherson P. S., Gehring K., Fon E. A. (2009) SH3 domains from a subset of BAR proteins define a Ubl-binding domain and implicate parkin in synaptic ubiquitination. Mol. Cell 36, 1034–1047 [DOI] [PubMed] [Google Scholar]
- 11. Chung K. K., Zhang Y., Lim K. L., Tanaka Y., Huang H., Gao J., Ross C. A., Dawson V. L., Dawson T. M. (2001) Parkin ubiquitinates the α-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat. Med. 7, 1144–1150 [DOI] [PubMed] [Google Scholar]
- 12. Olzmann J. A., Li L., Chudaev M. V., Chen J., Perez F. A., Palmiter R. D., Chin L. S. (2007) Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6. J. Cell Biol. 178, 1025–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geisler S., Holmström K. M., Skujat D., Fiesel F. C., Rothfuss O. C., Kahle P. J., Springer W. (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119–131 [DOI] [PubMed] [Google Scholar]
- 14. Durcan T. M., Tang M. Y., Pérusse J. R., Dashti E. A., Aguileta M. A., McLelland G. L., Gros P., Shaler T. A., Faubert D., Coulombe B., Fon E. A. (2014) USP8 regulates mitophagy by removing K6-linked ubiquitin conjugates from parkin. EMBO J. 33, 2473–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ko H. S., von Coelln R., Sriram S. R., Kim S. W., Chung K. K., Pletnikova O., Troncoso J., Johnson B., Saffary R., Goh E. L., Song H., Park B. J., Kim M. J., Kim S., Dawson V. L., Dawson T. M. (2005) Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J. Neurosci. 25, 7968–7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imam S. Z., Zhou Q., Yamamoto A., Valente A. J., Ali S. F., Bains M., Roberts J. L., Kahle P. J., Clark R. A., Li S. (2011) Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson's disease. J. Neurosci. 31, 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shin J. H., Ko H. S., Kang H., Lee Y., Lee Y. I., Pletinkova O., Troconso J. C., Dawson V. L., Dawson T. M. (2011) PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson's disease. Cell 144, 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C. A., Sou Y. S., Saiki S., Kawajiri S., Sato F., Kimura M., Komatsu M., Hattori N., Tanaka K. (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kane L. A., Lazarou M., Fogel A. I., Li Y., Yamano K., Sarraf S. A., Banerjee S., Youle R. J. (2014) PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kazlauskaite A., Kondapalli C., Gourlay R., Campbell D. G., Ritorto M. S., Hofmann K., Alessi D. R., Knebel A., Trost M., Muqit M. M. (2014) Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 460, 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koyano F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M., Kimura Y., Tsuchiya H., Yoshihara H., Hirokawa T., Endo T., Fon E. A., Trempe J. F., Saeki Y., Tanaka K., Matsuda N. (2014) Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510, 162–166 [DOI] [PubMed] [Google Scholar]
- 22. Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan N. C., Salazar A. M., Pham A. H., Sweredoski M. J., Kolawa N. J., Graham R. L., Hess S., Chan D. C. (2011) Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 20, 1726–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka A., Cleland M. M., Xu S., Narendra D. P., Suen D. F., Karbowski M., Youle R. J. (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ziviani E., Tao R. N., Whitworth A. J. (2010) Drosophila Parkin requires PINK1 for mitochondrial translocation and ubiquitinates Mitofusin. Proc. Natl. Acad. Sci. U.S.A. 107, 5018–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshii S. R., Kishi C., Ishihara N., Mizushima N. (2011) Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J. Biol. Chem. 286, 19630–19640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moore D. J., West A. B., Dawson V. L., Dawson T. M. (2005) Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 28, 57–87 [DOI] [PubMed] [Google Scholar]
- 28. Henn I. H., Gostner J. M., Lackner P., Tatzelt J., Winklhofer K. F. (2005) Pathogenic mutations inactivate parkin by distinct mechanisms. J. Neurochem. 92, 114–122 [DOI] [PubMed] [Google Scholar]
- 29. Hampe C., Ardila-Osorio H., Fournier M., Brice A., Corti O. (2006) Biochemical analysis of Parkinson's disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum Mol. Genet. 15, 2059–2075 [DOI] [PubMed] [Google Scholar]
- 30. Durcan T. M., Kontogiannea M., Thorarinsdottir T., Fallon L., Williams A. J., Djarmati A., Fantaneanu T., Paulson H. L., Fon E. A. (2011) The Machado-Joseph disease-associated mutant form of ataxin-3 regulates parkin ubiquitination and stability. Hum Mol. Genet. 20, 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsai Y. C., Fishman P. S., Thakor N. V., Oyler G. A. (2003) Parkin facilitates the elimination of expanded polyglutamine proteins and leads to preservation of proteasome function. J. Biol. Chem. 278, 22044–22055 [DOI] [PubMed] [Google Scholar]
- 32. Sakata E., Yamaguchi Y., Kurimoto E., Kikuchi J., Yokoyama S., Yamada S., Kawahara H., Yokosawa H., Hattori N., Mizuno Y., Tanaka K., Kato K. (2003) Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep. 4, 301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dächsel J. C., Lücking C. B., Deeg S., Schultz E., Lalowski M., Casademunt E., Corti O., Hampe C., Patenge N., Vaupel K., Yamamoto A., Dichgans M., Brice A., Wanker E. E., Kahle P. J., Gasser T. (2005) Parkin interacts with the proteasome subunit α4. FEBS Lett. 579, 3913–3919 [DOI] [PubMed] [Google Scholar]
- 34. Deveraux Q., Ustrell V., Pickart C., Rechsteiner M. (1994) A 26 S protease subunit that binds ubiquitin conjugates. J. Biol. Chem. 269, 7059–7061 [PubMed] [Google Scholar]
- 35. Husnjak K., Elsasser S., Zhang N., Chen X., Randles L., Shi Y., Hofmann K., Walters K. J., Finley D., Dikic I. (2008) Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 453, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schreiner P., Chen X., Husnjak K., Randles L., Zhang N., Elsasser S., Finley D., Dikic I., Walters K. J., Groll M. (2008) Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature 453, 548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hiyama H., Yokoi M., Masutani C., Sugasawa K., Maekawa T., Tanaka K., Hoeijmakers J. H., Hanaoka F. (1999) Interaction of hHR23 with S5a. The ubiquitin-like domain of hHR23 mediates interaction with S5a subunit of 26 S proteasome. J. Biol. Chem. 274, 28019–28025 [DOI] [PubMed] [Google Scholar]
- 38. Elsasser S., Finley D. (2005) Delivery of ubiquitinated substrates to protein-unfolding machines. Nat. Cell Biol. 7, 742–749 [DOI] [PubMed] [Google Scholar]
- 39. Kleijnen M. F., Shih A. H., Zhou P., Kumar S., Soccio R. E., Kedersha N. L., Gill G., Howley P. M. (2000) The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol. Cell 6, 409–419 [DOI] [PubMed] [Google Scholar]
- 40. Funakoshi M., Sasaki T., Nishimoto T., Kobayashi H. (2002) Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc. Natl. Acad. Sci. U.S.A. 99, 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crosas B., Hanna J., Kirkpatrick D. S., Zhang D. P., Tone Y., Hathaway N. A., Buecker C., Leggett D. S., Schmidt M., King R. W., Gygi S. P., Finley D. (2006) Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell 127, 1401–1413 [DOI] [PubMed] [Google Scholar]
- 42. Xie Y., Varshavsky A. (2002) UFD4 lacking the proteasome-binding region catalyses ubiquitination but is impaired in proteolysis. Nat. Cell Biol. 4, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 43. Bienko M., Green C. M., Crosetto N., Rudolf F., Zapart G., Coull B., Kannouche P., Wider G., Peter M., Lehmann A. R., Hofmann K., Dikic I. (2005) Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310, 1821–1824 [DOI] [PubMed] [Google Scholar]
- 44. Hecker C. M., Rabiller M., Haglund K., Bayer P., Dikic I. (2006) Specification of SUMO1- and SUMO2-interacting motifs. J. Biol. Chem. 281, 16117–16127 [DOI] [PubMed] [Google Scholar]
- 45. Safadi S. S., Barber K. R., Shaw G. S. (2011) Impact of autosomal recessive juvenile Parkinson's disease mutations on the structure and interactions of the parkin ubiquitin-like domain. Biochemistry 50, 2603–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Safadi S. S., Shaw G. S. (2007) A disease state mutation unfolds the parkin ubiquitin-like domain. Biochemistry 46, 14162–14169 [DOI] [PubMed] [Google Scholar]
- 47. Saeki Y., Sone T., Toh-e A., Yokosawa H. (2002) Identification of ubiquitin-like protein-binding subunits of the 26S proteasome. Biochem. Biophys. Res. Commun. 296, 813–819 [DOI] [PubMed] [Google Scholar]
- 48. Elsasser S., Gali R. R., Schwickart M., Larsen C. N., Leggett D. S., Müller B., Feng M. T., Tübing F., Dittmar G. A., Finley D. (2002) Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat. Cell Biol. 4, 725–730 [DOI] [PubMed] [Google Scholar]
- 49. Safadi S. S., Shaw G. S. (2010) Differential interaction of the E3 ligase parkin with the proteasomal subunit S5a and the endocytic protein Eps15. J. Biol. Chem. 285, 1424–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chaugule V. K., Burchell L., Barber K. R., Sidhu A., Leslie S. J., Shaw G. S., Walden H. (2011) Autoregulation of Parkin activity through its ubiquitin-like domain. EMBO J. 30, 2853–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen X., Lee B. H., Finley D., Walters K. J. (2010) Structure of proteasome ubiquitin receptor hRpn13 and its activation by the scaffolding protein hRpn2. Mol. Cell 38, 404–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Finley D. (2009) Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78, 477–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leggett D. S., Hanna J., Borodovsky A., Crosas B., Schmidt M., Baker R. T., Walz T., Ploegh H., Finley D. (2002) Multiple associated proteins regulate proteasome structure and function. Mol. Cell 10, 495–507 [DOI] [PubMed] [Google Scholar]
- 54. Hamazaki J., Iemura S., Natsume T., Yashiroda H., Tanaka K., Murata S. (2006) A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J. 25, 4524–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yao T., Song L., Xu W., DeMartino G. N., Florens L., Swanson S. K., Washburn M. P., Conaway R. C., Conaway J. W., Cohen R. E. (2006) Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat. Cell Biol. 8, 994–1002 [DOI] [PubMed] [Google Scholar]
- 56. Sarraf S. A., Raman M., Guarani-Pereira V., Sowa M. E., Huttlin E. L., Gygi S. P., Harper J. W. (2013) Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496, 372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Donzeau M., Káldi K., Adam A., Paschen S., Wanner G., Guiard B., Bauer M. F., Neupert W., Brunner M. (2000) Tim23 links the inner and outer mitochondrial membranes. Cell 101, 401–412 [DOI] [PubMed] [Google Scholar]
- 58. Finney N., Walther F., Mantel P. Y., Stauffer D., Rovelli G., Dev K. K. (2003) The cellular protein level of parkin is regulated by its ubiquitin-like domain. J. Biol. Chem. 278, 16054–16058 [DOI] [PubMed] [Google Scholar]
- 59. Um J. W., Im E., Lee H. J., Min B., Yoo L., Yoo J., Lübbert H., Stichel-Gunkel C., Cho H. S., Yoon J. B., Chung K. C. (2010) Parkin directly modulates 26S proteasome activity. J. Neurosci. 30, 11805–11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tomoo K., Mukai Y., In Y., Miyagawa H., Kitamura K., Yamano A., Shindo H., Ishida T. (2008) Crystal structure and molecular dynamics simulation of ubiquitin-like domain of murine parkin. Biochim. Biophys. Acta 1784, 1059–1067 [DOI] [PubMed] [Google Scholar]
- 61. Elsasser S., Chandler-Militello D., Müller B., Hanna J., Finley D. (2004) Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J. Biol. Chem. 279, 26817–26822 [DOI] [PubMed] [Google Scholar]