Background: The ACTH receptor regulates adrenocortical function.
Results: Substitution of Phe7 in ACTH with d-Phe or d-Nal(2′) and mutation of MC2R TM3 resulted in decreased ACTH binding and signaling.
Conclusion: Phe7 in the ligand ACTH and TM3 of the ACTH receptor are crucial for ligand binding and signaling.
Significance: Elucidating the ACTH receptor is crucial for development of MC2R drugs.
Keywords: 7-Helix Receptor, Cyclic AMP (cAMP), G Protein-coupled Receptor (GPCR), Membrane Protein, Peptide Hormone
Abstract
The ACTH receptor, known as the melanocortin-2 receptor (MC2R), plays an important role in regulating and maintaining adrenocortical function. MC2R is a subtype of the melanocortin receptor (MCR) family and has unique characteristics among MCRs. Endogenous ACTH is the only endogenous agonist for MC2R, whereas the melanocortin peptides α-, β-, and γ-melanocyte-stimulating hormone and ACTH are full agonists for all other MCRs. In this study, we examined the molecular basis of MC2R responsible for ligand selectivity using ACTH analogs and MC2R mutagenesis. Our results indicate that substitution of Phe7 with d-Phe or d-naphthylalanine (d-Nal(2′)) in ACTH(1–24) caused a significant decrease in ligand binding affinity and potency. Substitution of Phe7 with d-Nal(2′) in ACTH(1–24) did not switch the ligand from agonist to antagonist at MC2R, which was observed in MC3R and MC4R. Substitution of Phe7 with d-Phe7 in ACTH(1–17) resulted in the loss of ligand binding and activity. Molecular analysis of MC2R indicated that only mutation of the third transmembrane domain of MC2R resulted in a decrease in d-Phe ACTH binding affinity and potency. Our results suggest that Phe7 in ACTH plays an important role in ligand selectivity and that the third transmembrane domain of MC2R is crucial for ACTH selectivity and potency.
Introduction
ACTH is a polypeptide hormone produced and secreted by the anterior pituitary gland. ACTH stimulates secretion of glucocorticoid steroid hormones from adrenal cortex cells, especially in the zona fasciculata of the adrenal gland in response to biological stress. The ACTH receptor, also known as the melanocortin-2 receptor (MC2R),2 is critical for ACTH-mediated adrenal glucocorticoid release. ACTH binds to its receptor, mainly expressed on adrenocortical cells of the adrenal cortex, and stimulates the production of cortisol. Deficiency of the human MC2R (hMC2R) have been identified to result in a potentially fatal disease called familial glucocorticoid deficiency (1, 2) which is due to the mutations of this receptor. Patients with MC2R mutation have high levels of serum ACTH and low levels of cortisol. Individuals with familial glucocorticoid deficiency are deficient in cortisol, subsequently resulting in childhood failure to thrive, weakness, and fatigue. These individuals are likely to die in adrenal crisis, such as hypoglycemia or overwhelming infection, in infancy or childhood if they are not treated (1, 3–14). Deficiency of MC2R caused by MC2R mutation is also associated with a number of pathologic conditions, such as Addison disease and Cushing syndrome. MC2R is therefore a potential therapeutic target for adrenal disorders. ACTH(1–39) was first synthesized as a replacement for Acthar gel. It is used to treat relapsing multiple sclerosis, infantile spasms, and nephrotic syndrome (a collection of symptoms that indicate kidney damage). Acthar gel is a Food and Drug Administration-approved option for dermatomyositis (a chronic inflammatory disease of skin and muscle associated with patches of raised reddish or scaly rash) and polymyositis (an autoimmune inflammatory disease of muscle). Another active synthetic form of ACTH consists of the first 24 amino acids of native ACTH. ACTH is available as a synthetic derivative in the forms of cosyntropin (trade name Cortrosyn) and Synacthen (synthetic ACTH). Synacthen is not Food and Drug Administration-approved, but it is used in the United Kingdom and Australia to conduct the ACTH stimulation test. Elucidating the molecular basis of MC2R responsible for ligand binding and signaling is therefore a prerequisite to the development of selective MC2R agonists and antagonists for the treatment of adrenal disorders.
MC2R is one of five known melanocortin receptors (MCRs) belonging to the seven-transmembrane G protein-coupled receptor (GPCR) family (15). It shares a nearly 40% homology with other melanocortin receptor subtypes, but it is unique among MCRs for its ligand selectivity. The melanocortin peptides α-, β-, and γ-melanocyte-stimulating hormone (MSH) and ACTH are a group of neuropeptides that are derived from the proopiomelanocortin prohormone and that contain a common amino acid sequence, His-Phe-Arg-Trp. ACTH is the only endogenous ligand for MC2R. A large number of MSH analogs have been characterized as MCR subtypes (16–18). One of the most important findings was the discovery of [Nle4,d-Phe7]α-MSH, an analog of endogenous α-MSH. Substituting Phe7 with d-Phe in MSH significantly increased ligand binding affinity and potency at MC1R, MC3R, MC4R, and MC5R (19, 20). Moreover, substitution of Phe7 in MSH with d-naphthylalanine (d-Nal(2′)) switches MSH from agonist to antagonist at MC3R and MC4R (21). All of these studies indicate that Phe7 in melanocortin peptides plays an important role in ligand binding and potency at MCRs. However, ACTH is an endogenous agonist only for MC2R, and ACTH shares the first 13 amino acid residues with α-MSH. Whether Phe7 in ACTH also plays an important role in ligand binding affinity and potency at MC2R remains unknown. In this study, we examined the role of Phe7 in ACTH responsible for ligand selectivity and potency at hMC2R using ACTH analogs and determined the molecular basis of MC2R for ligand selectivity and potency using receptor mutagenesis and receptor modeling. Our results suggest that the role of Phe7 in ACTH is different at MC2R compared with other MCRs in ligand selectivity and that the third transmembrane domain (TM3) of MC2R is crucial for this differentiation.
EXPERIMENTAL PROCEDURES
Peptides
ACTH(1–24) and the ACTH analogs were custom-prepared by GenScript USA Inc. (Belmont, CA) (Fig. 1).
FIGURE 1.

ACTH analog sequences. The core amino acid residue substituted is shown in boldface. AA, amino acids.
Construction of Chimeric MCRs
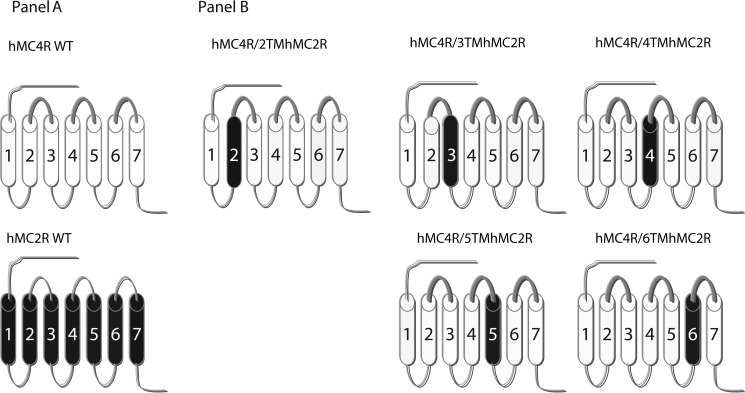
The amino acid sequences of hMC2R and hMC4R were manually examined by comparing their sequences with a previously published alignment of seven-transmembrane GPCR α-helices (22). The chimeras utilized in this study are schematically diagramed in Fig. 2. The chimeric receptors were constructed by PCR using Pfu polymerase (Stratagene, La Jolla, CA) (23). hMC4R served as a template. During an initial round of PCR, partial-length receptor fragments were generated. The sequence of one of the PCR primer oligonucleotides consisted of the transmembrane domain of interest, which was then coupled to a portion of the extracellular domain required to form the chimeric receptor. The second oligonucleotide primer consisted of the 5′- or 3′-end of MC4R. Receptor fragments were separated by agarose gel electrophoresis and used in a second round of PCR in which full-length chimeric receptor constructs were assembled by cycling the appropriate fragments together for 10 cycles prior to adding both 5′- and 3′-receptor primers. The chimeric receptors were subcloned into the eukaryotic expression vector pcDNA3.1 (Invitrogen).
FIGURE 2.
Schematic representation of the chimeric hMCRs used in these studies. A schematically depicts the seven-transmembrane structures of WT MC4R (thin lines) and MC2R (thick lines). B depicts the structures of chimeric MC4R.
Site-directed Mutagenesis
A single mutation was constructed using a QuikChange site-directed mutagenesis kit (Stratagene). The entire coding region of the MC2R mutants was sequenced at the University of Alabama at Birmingham Sequence Core to confirm that the desired mutation sequences were present and that no sequence errors had been introduced.
Cell Culture and Transfection
The OS3 and HEK cell lines, lacking endogenous MC2R, were used for hMC4R, hMC2R, and chimeric receptor transfection. The cells transfected with the receptor were cultured in DMEM containing 10% bovine fetal serum and HEPES. Cells at 80% confluence were washed twice, and the receptor constructs were transfected into cells using Lipofectamine (Life Technologies). The permanently transfected clonal cell lines were selected by resistance to the neomycin analog G418 (24).
Binding Assays
Binding experiments were performed using the conditions described previously (25). Briefly, after removal of the medium, cells were incubated with non-radioligand ACTH from 10−10 to 10−6 m in 0.5 ml of minimum Eagle's medium containing 0.2% BSA and 2 × 105 cpm of 125I-ACTH(1–24) for 1 h. The binding reactions were terminated by removing the medium and washing the cells twice with minimum Eagle's medium containing 0.2% BSA. The cells were then lysed with 0.2 n NaOH, and the radioactivity in the lysate was quantified in an analytical γ-counter (PerkinElmer Life Sciences). Nonspecific binding was determined by measuring the amount of 125I label bound on the cells in the presence of excess 10−6 m unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity. Binding data are reported as Bmax and IC50.
cAMP Assay
Cellular cAMP generation was measured using a cAMP assay kit (BioTek). Cells were plated on 96-well plates overnight. The culture medium was removed briefly, and the cells were incubated with 50 μl of Earle's balanced salt solution containing the melanocortin agonist ACTH (10−10 to 10−6 m) for 1 h at 37 °C in the presence of 10−3 m isobutylmethylxanthine. The reaction was stopped by the addition of lysis buffer (50 μl/well) and incubation for 20 min. cAMP content was measured according to the instructions accompanying the assay kit.
Receptor Expression Using Flow Cytometry (26)
The transfected cells were harvested in 0.2% EDTA and washed twice with PBS. Aliquots of 3 × 106 cells were centrifuged and fixed with 3% paraformaldehyde in PBS (pH 7.4). The cells were incubated with 50 μl of 10 μg/ml murine anti-FLAG monoclonal antibody M1 (Sigma catalog no. 316) in incubation buffer for 45 min. Under these conditions, the primary antibody binds only to receptors located at the cell surface. The cells were collected by centrifugation and washed three times with incubation buffer. The cell pellets were suspended in 100 μl of incubation buffer containing Cy3-conjugated Affinity Pure donkey anti-mouse IgG (ImmunoResearch Laboratories, West Grove, PA) and incubated at room temperature for 30 min. Flow cytometry was performed on a BD FACStarPLUS 6-parameter cytometer/sorter with a dual-argon ion laser (BD Biosciences). The results were analyzed using CellQuest software (BD Biosciences).
Statistical Analysis
Each experiment was performed in duplicate three separate times. The mean value of the dose-response data of binding and cAMP production was fit to a sigmoid curve with a variable slope factor using nonlinear square regression analysis (GraphPad Prism, GraphPad Software, San Diego, CA). Data are expressed as mean ± S.E. Significant differences were assessed by Student's t test, with p < 0.05 considered to be statistically significant.
RESULTS
Effect of Substitution of Phe7 in ACTH(1–24) with d-Phe or d-Nal(2′) on Ligand Binding Affinity and Potency
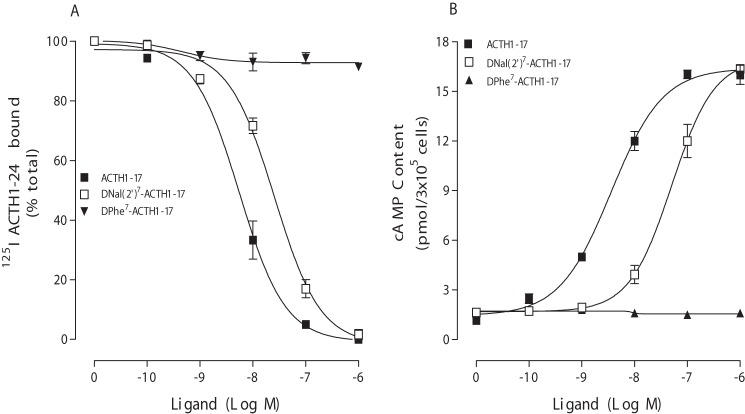
Extensive studies have identified that Phe7 in MSH plays an important role in ligand binding and receptor signaling at MC1R, MC3R, MC4R, and MC5R. To test whether Phe7 in ACTH also plays an important role in ligand binding affinity and signaling at MC2R, we examined several ACTH analogs in which Phe7 was modified. First, hMC2R was transfected into OS3 cells, which lack endogenous MC2R, and the receptor function was examined. Cells expressing hMC2R were incubated with 125I-ACTH(1–24) and different concentrations of unlabeled ACTH analogs. Ligand binding affinity was determined. Our results indicate that ACTH(1–24) dose-dependently displaced 125I-ACTH binding at WT hMC2R. Consistent with the binding results, ACTH(1–24) was able to stimulate cAMP production at WT hMC2R in a dose-dependent manner (Fig. 3). The IC50 and EC50 are shown in Table 1. Second, we examined several ACTH analogs at hMC2R. Our results indicate that substitution of Phe7 with d-Phe in ACTH(1–24) ([d-Phe7]ACTH(1–24)) dose-dependently displaced 125I-ACTH binding at hMC2R, but the binding affinity was significantly decreased compared with that of Phe7-ACTH(1–24). Consistent with the binding results, [d-Phe7]ACTH(1–24) was able to stimulate cAMP production at hMC2R in a dose-dependent manner, but its potency was significantly reduced (Fig. 3). The IC50 and EC50 values are shown in Table 1. Previous results indicate that substitution of Phe7 with d-Nal(2′) in [Nle4,d-Phe7]α-MSH switches agonist to antagonist at MC3R and MC4R (21). However, in this study, [d-Nal(2′)7]ACTH(1–24) (substitution of Phe7 with d-Nal(2′) in ACTH) dose-dependently displaced 125I-ACTH binding at hMC2R, but its binding affinity was significantly reduced. Surprisingly, [d-Nal(2′)7]ACTH(1–24) still dose dependently induced cAMP production at hMC2R, unlike [d-Nal(2′)7]MSH at MC3R and MC4R, for which SHU9119 is an antagonist (Fig. 3). SHU9119 is a synthetic peptide with a β-(2-naphthyl)-d-alanine (d-Nal) substituted at position 7 of MTII and is a potent but nonselective antagonist for MC3R and MC4R (19, 20).
FIGURE 3.
Binding affinities and potencies of ACTH(1–24) analogs at WT hMC2R. Cells transfected with hMC2R were incubated with 125I-ACTH(1–24) at 37 °C for 1 h in the presence of the indicated amounts of unlabeled ligands. Total 125I-ACTH(1–24) binding was then determined on duplicate wells as described under “Experimental Procedures.” Data points represent the mean ± S.E. of at least three independent experiments. A depicts the binding affinities of ACTH(1–24) analogs at WT hMC2R. B demonstrates the abilities of ACTH(1–24) analogs to stimulate the production of intracellular cAMP at WT hMC2R.
TABLE 1.
ACTH analogs in ligand binding and signaling
| ACTH analog peptides | hMC2R |
|
|---|---|---|
| IC50 | EC50 | |
| nm | ||
| ACTH(1–17) | 5.5 ± 1.0 | 5.5 ± 1.0 |
| ACTH(1–24) | 3.9 ± 1.2 | 1.3 ± 0.2 |
| ACTH(1–39) | 4.6 ± 1.2 | 3.2 ± 0.5 |
| [d-Phe7]ACTH(1–17) | —a | — |
| [d-Phe7]ACTH(1–24) | 28 ± 3.4 | 39 ± 11 |
| [d-Nal(2′)7]ACTH(1–17) | 26 ± 2.5 | 58 ± 4.5 |
| [d-Nal(2′)7]ACTH(1–24) | 23 ± 3.3 | 38 ± 5.5 |
a —, no binding and signaling.
Effect of Truncated [d-Nal(2′)7]ACTH Fragments on MC2R Binding and Activation
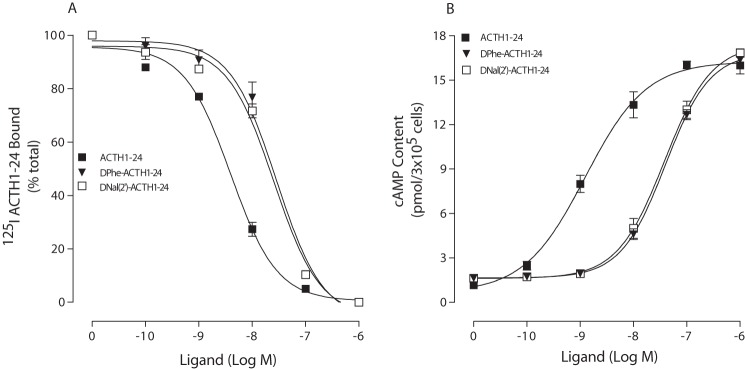
Our previous results indicate that ACTH(1–17) is the minimal ACTH peptide for hMC2R binding and receptor activation. To further examine the role of ACTH Phe7 in receptor binding affinity and potency, we examined different ACTH(1–17) analogous at MC2R. We examined the effects of Phe7-ACTH(1–17), [d-Phe7]ACTH(1–17), and [d-Nal(2′)7]ACTH(1–17) on MC2R binding and signaling. Our results indicate that Phe7-ACTH(1–17) and [d-Nal(2′)7]ACTH(1–17) were able to bind to the receptor and stimulate cAMP production in a dose-dependent manner. However, [d-Phe7]ACTH(1–17) lost its ability to bind and activate the receptor (Fig. 4). The binding affinity IC50 and potency EC50 values are shown in Table 1. To determine whether [d-Phe7]ACTH(1–17) is functional, we examined this peptide's activity at hMC1R. Our results indicate that [d-Phe7]ACTH(1–17) dose-dependently displaced 125I-ACTH(1–24) binding and induced cAMP production in a dose-dependent manner at hMC1R, suggesting that this peptide is functional (data not shown).
FIGURE 4.
Binding affinities and potencies of ACTH(1–17) analogs at WT hMC2R. Cells transfected with hMC2R were incubated with 125I-ACTH(1–24) in the presence of the indicated amounts of unlabeled ligands. Total 125I-ACTH(1–24) binding was then determined on duplicate wells as described under “Experimental Procedures.” Data points represent the mean ± S.E. of at least three independent experiments. A depicts the binding affinities of ACTH(1–17) analogs at WT hMC2R. B demonstrates the abilities of ACTH(1–17) analogs to stimulate the production of intracellular cAMP at WT hMC2R.
Regions of MC2R Responsible for ACTH Binding Affinity and Potency
To determine which region of hMC2R is responsible for the difference in ACTH analog selectivity and potency, a domain-exchange strategy was used to localize regions of hMC2R responsible for ligand selectivity and potency. We first used MC2R as a template, and TM2–TM6 of MC2R were substituted with the homologous regions of hMC4R. To determine whether the chimeric receptors are expressed at the cell surface and to quantify receptor expression levels, we utilized the antigenic epitope FLAG to examine receptor expression. Our results indicate that these chimeric receptors were expressed on the cell surface at a very low level, and we were unable to assess ligand binding affinity and potency. We then utilized MC4R as a template, and TM2–TM6 of hMC4R were substituted with the homologous regions of hMC2R. To assess the binding affinity of peptide [d-Phe7]ACTH(1–24) at these chimeric receptors, cells expressing the chimeric receptors were treated with 125I-Phe7-ACTH(1–24) and different doses of unlabeled [d-Phe7]ACTH analogs (Figs. 5 and 6). Our results indicate that these chimeric receptors did not significantly alter [d-Phe7]ACTH(1–24) binding affinity, except the chimeric receptor hMC4R/TM3 hMC2R. Ligand [d-Phe7]ACTH(1–24) binding affinity at this chimeric receptor was significantly decreased. The IC50 values are summarized in Table 2. To examine the ability of [d-Phe7]ACTH(1–24) to activate chimeric hMC4R/hMC2R, cells expressing the chimeric receptors were treated with different doses of [d-Phe7]ACTH analogs, and cAMP production was examined. Our results indicate that [d-Phe7]ACTH(1–24) increased cAMP generation in a dose-dependent manner at these hMC4R/hMC2R chimeras, which are similar to WT MC4R, except for chimeric hMC4R/TM3 hMC2R. Substitution of hMC4R TM3 with hMC2R TM3 (hMC4R/TM3 hMC2R) significantly decreased [d-Phe7]ACTH(1–24) potency (Figs. 5 and 6). The EC50 values are shown in Table 2.
FIGURE 5.
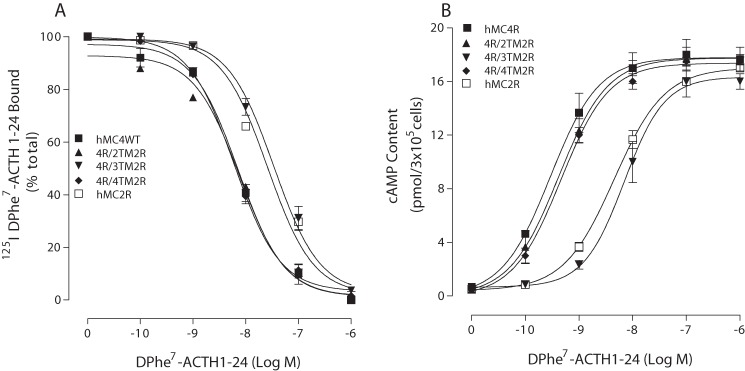
Binding affinity and potency of ACTH(1–24) at the chimeric receptors. HEK cells transfected with hMC4R chimeras were incubated with 125I-ACTH at 37 °C for 1 h in the presence of the indicated amounts of unlabeled ligands, and total 125I-ACTH(1–24) binding was determined. For the cAMP assay, the cells transfected with hMC4R chimeras were incubated with the indicated amounts of [Nle4,d-Phe7-ACTH(1–24) for 30 min, and total cAMP accumulation was determined on duplicate wells. Data points represent the mean ± S.E. of at least three independent experiments. A depicts the binding affinity of ACTH at the chimeric receptors. B demonstrates the ability of ACTH to stimulate the production of intracellular cAMP at the chimeric receptors.
FIGURE 6.
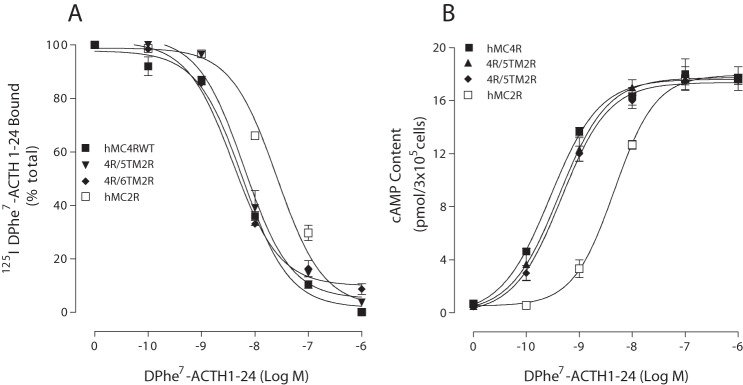
Binding affinity and potency of ACTH at the chimeric receptors. HEK cells transfected hMC4R chimeras were incubated with 125I-ACTH at 37 °C for 1 h in the presence of the indicated amounts of unlabeled ligands, and total 125I-ACTH binding was determined on duplicate wells as described under “Experimental Procedures.” For the cAMP assay, the cells transfected with hMC4R chimeras were incubated with the indicated amounts of [d-Phe7]ACTH(1–24) for 30 min, and total cAMP accumulation was determined on duplicate wells. Data points represent the mean ± S.E. of at least three independent experiments. A depicts the binding affinity of [d-Phe7]ACTH(1–24) at the chimeric receptors. B demonstrates the ability of [d-Phe7]ACTH(1–24) to stimulate the production of intracellular cAMP at the chimeric receptors.
TABLE 2.
Effect of receptor domain exchanges in hMC4R on ligand binding and signaling
| [d-Phe7] ACTH(1–24) Ki | [d-Phe7]ACTH (1–24)-mediated cAMP production (EC50) | |
|---|---|---|
| nm | nm | |
| hMC4RWT | 3.7 ± 0.2 | 0.9 ± 0.1 |
| hMC4R/TM2 hMC2R | 8.1 ± 0.3 | 1.7 ± 0.1 |
| hMC4R/TM3 hMC2R | 35.6 ± 11.2a | 32 ± 7.2 |
| hMC4R/TM4 hMC2R | 5.8 ± 0.2 | 1.5 ± 0.2 |
| hMC4R/TM5 hMC2R | 4.8 ± 1.6 | 1.8 ± 0.1 |
| hMC4R/TM6 hMC2R | 5.3 ± 0.3 | 1.5 ± 0.3 |
| hMC2RWT | 29 ± 11 | 38 ± 7.4 |
a p < 0.05 compared to hMC4RWT.
Role of Amino Acid Residues of MC2R TM3 in [d-Phe7]ACTH(1–24) or [d-Nal(2′)7]ACTH(1–24) Binding Affinity and Potency
TM3 of hMC2R has been identified to play an important role in [d-Phe7]ACTH(1–24) binding affinity and potency. Amino acid sequence alignment between TM3 of hMC2R and TM3 of hMC4R was performed. Three amino acids were identified not to be conserved between TM3 of hMC4R and TM3 of hMC2R. We then performed a site-directed mutagenesis study to determine whether these amino acid residues are crucial for ligand binding affinity and potency. Three MC2R mutations were made (D104N, I105V, and L109V), and effect of [d-Phe7]ACTH(1–24) on these mutations was examined. Cells expressing these mutations were treated with [d-Phe7]ACTH(1–24), and ligand binding affinity and potency were evaluated. Our results indicate that D104N, I105V, and L109V did not dramatically increase [Nle4,d-Phe7]ACTH(1–24) agonist potency (EC50 for [d-Phe7]ACTH(1–24)): WT MC2R, 39 ± 11 nm; D104N, 35 ± 8 nm; I105V, 37 ± 9 nm; and L109V, 41 ± 12 nm, suggesting that the conformation of TM3 is crucial for [d-Phe7]ACTH(1–24) agonist binding and potency.
We also examined the effects of ligands ACTH(1–39), ACTH(1–24), and ACTH(1–17), as well as α-MSH, at chimeric MC4R/TM3 MC2R (Fig. 7). Our results further support that Phe7 in ACTH plays an important role in ligand selectivity and potency in the MCR subtype. For chimeric MC4R/TM3 MC2R, the ACTH ligand with d-Phe had high selectivity and potency, whereas the ACTH ligand with Phe had less potency, suggesting that the conformation of Phe in ACTH plays different roles in MC2R and MC4R.
FIGURE 7.
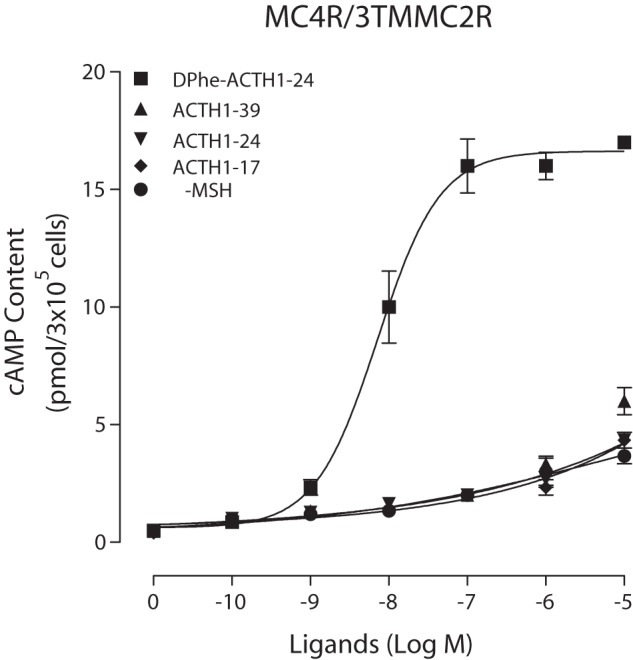
Effects of different ligands on cAMP production at the chimeric receptor. HEK cells transfected with hMC4R/TM3 MC2R were incubated with different ligands for 30 min, and total cAMP accumulation was determined on duplicate wells. Data points represent the mean ± S.E. of at least three independent experiments.
DISCUSSION
We have shown that substitution of Phe7 in ACTH with d-Phe or d-Nal(2′) resulted in decreased ligand binding affinity and potency. Substitution of Phe7 with d-Nal(2′) in ACTH did not switch the ligand from agonist to antagonist at MC2R, suggesting that MC2R, unlike other MCRs, has different binding sites for d-Phe and d-Nal(2′) in ACTH.
Ligand Modification Approach for Elucidating the Role of Key ACTH Residues in Ligand Potency
The melanocortin peptides α-MSH and ACTH are derived from the proopiomelanocortin prohormone. α-MSH shares the first 13 amino acids with ACTH, and they share the core sequence His6-Phe7-Arg8-Trp9. This region was identified as important for ligand binding and activities at hMC1R, hMC2R, hMC3R, hMC4R, and hMC5R (27–34). Our previous results indicate that [Nle4]ACTH(1–39) (with norleucine substituted for methionine at position 4) is equipotent to ACTH(1–39) in ligand binding and signaling (35). Our previous results also indicate that first 17 amino acid residues in ACTH are crucial for ligand binding and signaling at MC2R. ACTH analogs of fewer than 17 residues resulted in loss of ligand binding affinity and potency at MC2R. A d-Phe7 substitution in α-MSH ([Nle4,d-Phe7]α-MSH) increased ligand binding affinity and potency at MC1R, MC3R, MC4R, and MC5R. However, the results from our present study indicate that the d-Phe7 substitution in ACTH not only deceased ligand binding affinity but also decreased ligand potency at MC2R, suggesting that the role of Phe in ACTH at MC2R is different from that at other MCRs. Our previous results indicate that d-Nal(2′)7 replacement in α-MSH (SHU9119) switches the ligand from agonist to antagonist at MC3R and MC4R (21). However, in our present study, d-Nal(2′) substitution in ACTH did not switch the ligand from agonist to antagonist at hMC2R, suggesting that an aromatic side chain in position 7 of α-MSH is crucial for receptor activation at MC3R and MC4R, but not at MC2R. The role of Phe7 in ACTH at MC2R is different from that in MSH at other MCRs.
Receptor Modification Approach for Elucidating MC2R Structure and Function
GPCRs play fundamental roles in regulating the activity of human body. Upon binding of extracellular ligands, GPCRs interact with a specific subset of heterotrimeric G proteins that can then, in their activated forms, inhibit or activate various effector enzymes and/or ion channels. Ligand binding to GPCRs causes defined conformational changes in the receptor protein that promote its interaction with distinct classes of G protein heterotrimers (consisting of α-, β-, and γ-subunits) that are attached to the cytoplasmic surface of the plasma membrane. This ligand-GPCR interaction eventually leads to the desired physiological response. The MCR family has five receptor subtypes. These MCRs share common receptor architecture, but these subtype receptors display different pharmacological profiles as evidenced by their ability to recognize a diverse array of endogenous or synthetic agonists. MC2R is unique of all MCRs, and ACTH is the only endogenous agonist at MC2R. Sequence analysis of MC2R indicated that MC2R is different from the other MCRs. In this study, we utilized chimeric receptors to determine which region of MC2R is crucial for ligand selectivity. Chimeric receptors have been widely used for delineating the domains involved in the ligand binding specificity of GPCRs. The chimeric receptors constructed between MC2R and MC4R transmembrane segments have provided specific insights into the regions in MC2R that confer ACTH selectivity. Due to the fact that the chimeric receptors that use MC2R as a template have very low receptor protein expression on the cell surface and make receptor pharmacological analysis not feasible, chimeric receptors that use MC4R as a template provide us with a good alternative. Our results indicate that the chimeric receptors that use MC4R as a template were highly expressed at the cell surface, and functional analysis of these chimeric receptors provided us with very important information on the MC2R transmembrane region in ligand selectivity. Our results indicate that replacement of TM3 in MC4R with the corresponding region in MC2R significantly decreased [d-Phe7]ACTH(1–24) binding affinity and potency, suggesting that TM3 of MC2R is important for Phe7 selectivity and potency at MC2R. Furthermore, substitution of MC4R TM3 with MC2R TM3 significantly reduced ligand potency with Phe7, suggesting that the conformation of Phe in the ligand plays different roles in MC2R and MC4R.
Based on our MCR work and that of others, electrostatic and hydrophobic forces have been proposed to be involved in ACTH binding and receptor activation at MC2R. An MC2R computer model has been developed (Peptide Synthesis and Molecular Recognition Lab, University of Michigan). In this model, Asp103 and Asp107 of hMC2R have been hypothesized to form an ionic interaction with Arg8 of ACTH(1–24). In the model, the positively charged Arg8 of peptides is located close to the extracellular surface of the receptor, and it participates in the H-bond network with acidic residues from TM3 (Asp103 and Asp107). Phe7 is most essential for ligand docking into the MC2R-binding pocket, although this residue has no direct interaction with receptor residues. Its aromatic ring is situated on the bottom of the receptor pocket. The structure and conformation of Phe, d-Phe, and d-Nal(2′) are different, and therefore, their positions in the binding pocket are different. The conformation of these residues may play an important role in different ACTH analog selectivities. d-Phe or d-Nal(2′) in the ligand may prevent Arg8 interacting with Asp103 and Asp107.
GPCRs are the major targets of today's medicines. To determine the mechanism of activation of GPCRs and the interaction of these receptors with their ligands, mutagenesis studies have proven to be a powerful tool and have provided insight into the structure and function of GPCRs. However, GPCR mutagenesis studies have limitations. They have identified that the peptide and non-peptide ligands have different binding sites at the receptor, but they could not provide further detailed information on whether different agonists will result in different GPCR conformations. A new approach is therefore required to determine the conformational change mediated by different ligands. Biophysical NMR may provide a new approach to ligand binding (either kinetic or conformational details). In future studies, we will combine mutagenesis studies with structural analyses (with crystals and NMR), including iterative modeling and detailed spectroscopy (fluorescence resonance energy transfer and bioluminescence resonance energy transfer). We will use solution- and solid-state NMR approaches to examine receptor-ligand interaction and receptor activation. This approach will provide structural information on receptor-ligand interactions and the dynamic aspects of GPCR structure.
In conclusion, we have demonstrated that Phe7 in ACTH plays an important role in ligand binding affinity and potency at MC2R, and its role is different from that of other MCRs. TM3 of MC2R plays an important role in ligand selectivity and potency. This study may be valuable for future design of selective MCR ligands.
Note Added in Proof
A different version of Table 2 appears in the version of this article that was published on January 20, 2015 as a Paper in Press. The revised Table 2 reflects the inclusion of new data from two additional trials (n = 5 instead of n = 3). This revision does not change the interpretation of the results or the conclusions.
Footnotes
- MC2R
- melanocortin-2 receptor
- hMC2R
- human MC2R
- GPCR
- G protein-coupled receptor
- MCR
- melanocortin receptor
- MSH
- melanocyte-stimulating hormone
- d-Nal
- d-naphthylalanine
- TM3
- transmembrane domain 3.
REFERENCES
- 1. Clark A. J., Weber A. (1998) Adrenocorticotropin insensitivity syndromes. Endocr. Rev. 19, 828–843 [DOI] [PubMed] [Google Scholar]
- 2. Clark A. J., Metherell L. A., Cheetham M. E., Huebner A. (2005) Inherited ACTH insensitivity illuminates the mechanisms of ACTH action. Trends Endocrinol. Metab. 16, 451–457 [DOI] [PubMed] [Google Scholar]
- 3. Shepard T. H., Landing B. H., Mason D. G. (1959) Familial Addison's disease; case reports of two sisters with corticoid deficiency unassociated with hypoaldosteronism. AMA J. Dis. Child 97, 154–162 [PubMed] [Google Scholar]
- 4. Migeon C. J., Kenny E. M., Kowarski A., Snipes C. A., Spaulding J. S., Finkelstein J. W., Blizzard R. M. (1968) The syndrome of congenital adrenocortical unresponsiveness to ACTH. Report of six cases. Pediatr. Res. 2, 501–513 [DOI] [PubMed] [Google Scholar]
- 5. Clark A. J., McLoughlin L., Grossman A. (1993) Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 341, 461–462 [DOI] [PubMed] [Google Scholar]
- 6. Tsigos C., Arai K., Hung W., Chrousos G. P. (1993) Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J. Clin. Invest. 92, 2458–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber A., Toppari J., Harvey R. D., Klann R. C., Shaw N. J., Ricker A. T., Näntö-Salonen K., Bevan J. S., Clark A. J. (1995) Adrenocorticotropin receptor gene mutations in familial glucocorticoid deficiency: relationships with clinical features in four families. J. Clin. Endocrinol. Metab. 80, 65–71 [DOI] [PubMed] [Google Scholar]
- 8. Naville D., Barjhoux L., Jaillard C., Faury D., Despert F., Esteva B., Durand P., Saez J. M., Begeot M. (1996) Demonstration by transfection studies that mutations in the adrenocorticotropin receptor gene are one cause of the hereditary syndrome of glucocorticoid deficiency. J. Clin. Endocrinol. Metab. 81, 1442–1448 [DOI] [PubMed] [Google Scholar]
- 9. Weber A., Clark A. J. (1994) Mutations of the ACTH receptor gene are only one cause of familial glucocorticoid deficiency. Hum. Mol. Genet. 3, 585–588 [DOI] [PubMed] [Google Scholar]
- 10. Slavotinek A. M., Hurst J. A., Dunger D., Wilkie A. O. (1998) ACTH receptor mutation in a girl with familial glucocorticoid deficiency. Clin. Genet. 53, 57–62 [DOI] [PubMed] [Google Scholar]
- 11. Flück C. E., Martens J. W., Conte F. A., Miller W. L. (2002) Clinical, genetic, and functional characterization of adrenocorticotropin receptor mutations using a novel receptor assay. J. Clin. Endocrinol. Metab. 87, 4318–4323 [DOI] [PubMed] [Google Scholar]
- 12. Selva K. A., LaFranchi S. H., Boston B. (2004) A novel presentation of familial glucocorticoid deficiency (FGD) and current literature review. J. Pediatr. Endocrinol. Metab. 17, 85–92 [DOI] [PubMed] [Google Scholar]
- 13. Imamine H., Mizuno H., Sugiyama Y., Ohro Y., Sugiura T., Togari H. (2005) Possible relationship between elevated plasma ACTH and tall stature in familial glucocorticoid deficiency. Tohoku J. Exp. Med. 205, 123–131 [DOI] [PubMed] [Google Scholar]
- 14. Matsuura H., Shiohara M., Yamano M., Kurata K., Arai F., Koike K. (2006) Novel compound heterozygous mutation of the MC2R gene in a patient with familial glucocorticoid deficiency. J. Pediatr. Endocrinol. Metab. 19, 1167–1170 [DOI] [PubMed] [Google Scholar]
- 15. Mountjoy K. G., Robbins L. S., Mortrud M. T., Cone R. D. (1992) The cloning of a family of genes that encode the melanocortin receptors. Science 257, 1248–1251 [DOI] [PubMed] [Google Scholar]
- 16. Sawyer T. K., Sanfilippo P. J., Hruby V. J., Engel M. H., Heward C. B., Burnett J. B., Hadley M. E. (1980) 4-Norleucine, 7-d-phenylalanine-α-melanocyte-stimulating hormone: a highly potent α-melanotropin with ultralong biological activity. Proc. Natl. Acad. Sci. U.S.A. 77, 5754–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawyer T. K., Hruby V. J., Wilkes B. C., Draelos M. T., Hadley M. E., Bergsneider M. (1982) Comparative biological activities of highly potent active-site analogues of α-melanotropin. J. Med. Chem. 25, 1022–1027 [DOI] [PubMed] [Google Scholar]
- 18. Sawyer T. K., Hruby V. J., Darman P. S., Hadley M. E. (1982) [half-Cys4,half-Cys10]-α-Melanocyte-stimulating hormone: a cyclic α-melanotropin exhibiting superagonist biological activity. Proc. Natl. Acad. Sci. U.S.A. 79, 1751–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hruby V. J., Lu D., Sharma S. D., Castrucci A. L., Kesterson R. A., al-Obeidi F. A., Hadley M. E., Cone R. D. (1995) Cyclic lactam α-melanotropin analogues of Ac-Nle4-cyclo[Asp5,d-Phe7,Lys10]α-melanocyte-stimulating hormone-(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J. Med. Chem. 38, 3454–3461 [DOI] [PubMed] [Google Scholar]
- 20. Schiöth H. B., Muceniece R., Mutulis F., Bouifrouri A. A., Mutule I., Wikberg J. E. (1999) Further pharmacological characterization of the selective melanocortin 4 receptor antagonist HS014: comparison with SHU9119. Neuropeptides 33, 191–196 [DOI] [PubMed] [Google Scholar]
- 21. Yang Y., Chen M., Lai Y., Gantz I., Georgeson K. E., Harmon C. M. (2002) Molecular determinants of human melanocortin-4 receptor responsible for antagonist SHU9119 selective activity. J. Biol. Chem. 277, 20328–20335 [DOI] [PubMed] [Google Scholar]
- 22. Baldwin J. M. (1993) The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 12, 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Y., Dickinson C. J., Zeng Q., Li J. Y., Thompson D. A., Gantz I. (1999) Contribution of melanocortin receptor exoloops to Agouti-related protein binding. J. Biol. Chem. 274, 14100–14106 [DOI] [PubMed] [Google Scholar]
- 24. Yang Y., Dickinson C., Haskell-Luevano C., Gantz I. (1997) Molecular basis for the interaction of [Nle4,d-Phe7]melanocyte stimulating hormone with the human melanocortin-1 receptor (melanocyte α-MSH receptor). J. Biol. Chem. 272, 23000–23010 [DOI] [PubMed] [Google Scholar]
- 25. Yang Y., Fong T. M., Dickinson C. J., Mao C., Li J. Y., Tota M. R., Mosley R., Van Der Ploeg L. H., Gantz I. (2000) Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry 39, 14900–14911 [DOI] [PubMed] [Google Scholar]
- 26. Conrad D. M., Hanniman E. A., Watson C. L., Mader J. S., Hoskin D. W. (2004) Ryanodine receptor signaling is required for anti-CD3-induced T cell proliferation, interleukin-2 synthesis, and interleukin-2 receptor signaling. J. Cell. Biochem. 92, 387–399 [DOI] [PubMed] [Google Scholar]
- 27. Holder J. R., Bauzo R. M., Xiang Z., Haskell-Luevano C. (2002) Structure-activity relationships of the melanocortin tetrapeptide Ac-His-dPhe-Arg-Trp-NH2 at the mouse melanocortin receptors. Part 2: modifications at the Phe position. J. Med. Chem. 45, 3073–3081 [DOI] [PubMed] [Google Scholar]
- 28. Holder J. R., Xiang Z., Bauzo R. M., Haskell-Luevano C. (2003) Structure-activity relationships of the melanocortin tetrapeptide Ac-His-dPhe-Arg-Trp-NH2 at the mouse melanocortin receptors. Part 3: modifications at the Arg position. Peptides 24, 73–82 [DOI] [PubMed] [Google Scholar]
- 29. Holder J. R., Marques F. F., Xiang Z., Bauzo R. M., Haskell-Luevano C. (2003) Characterization of aliphatic, cyclic, and aromatic N-terminally “capped” His-d-Phe-Arg-Trp-NH2 tetrapeptides at the melanocortin receptors. Eur. J. Pharmacol. 462, 41–52 [DOI] [PubMed] [Google Scholar]
- 30. Todorovic A., Holder J. R., Bauzo R. M., Scott J. W., Kavanagh R., Abdel-Malek Z., Haskell-Luevano C. (2005) N-terminal fatty acylated His-dPhe-Arg-Trp-NH2 tetrapeptides: influence of fatty acid chain length on potency and selectivity at the mouse melanocortin receptors and human melanocytes. J. Med. Chem. 48, 3328–3336 [DOI] [PubMed] [Google Scholar]
- 31. Holder J. R., Bauzo R. M., Xiang Z., Haskell-Luevano C. (2002) Structure-activity relationships of the melanocortin tetrapeptide Ac-His-dPhe-Arg-Trp-NH2 at the mouse melanocortin receptors. 1. Modifications at the His position. J. Med. Chem. 45, 2801–2810 [DOI] [PubMed] [Google Scholar]
- 32. Haskell-Luevano C., Hendrata S., North C., Sawyer T. K., Hadley M. E., Hruby V. J., Dickinson C., Gantz I. (1997) Discovery of prototype peptidomimetic agonists at the human melanocortin receptors MC1R and MC4R. J. Med. Chem. 40, 2133–2139 [DOI] [PubMed] [Google Scholar]
- 33. Shizume K., Lerner A. B., Fitzpatrick T. B. (1954) In vitro bioassay for the melanocyte stimulating hormone. Endocrinology 54, 553–560 [DOI] [PubMed] [Google Scholar]
- 34. Goldman J. M., Hadley M. E. (1970) Evidence for separate receptors for melanophore stimulating hormone and catecholamine regulation of cyclic AMP in the control of melanophore responses. Br. J. Pharmacol. 39, 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen M., Aprahamian C. J., Kesterson R. A., Harmon C. M., Yang Y. (2007) Molecular identification of the human melanocortin-2 receptor responsible for ligand binding and signaling. Biochemistry 46, 11389–11397 [DOI] [PMC free article] [PubMed] [Google Scholar]