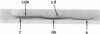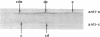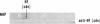Abstract
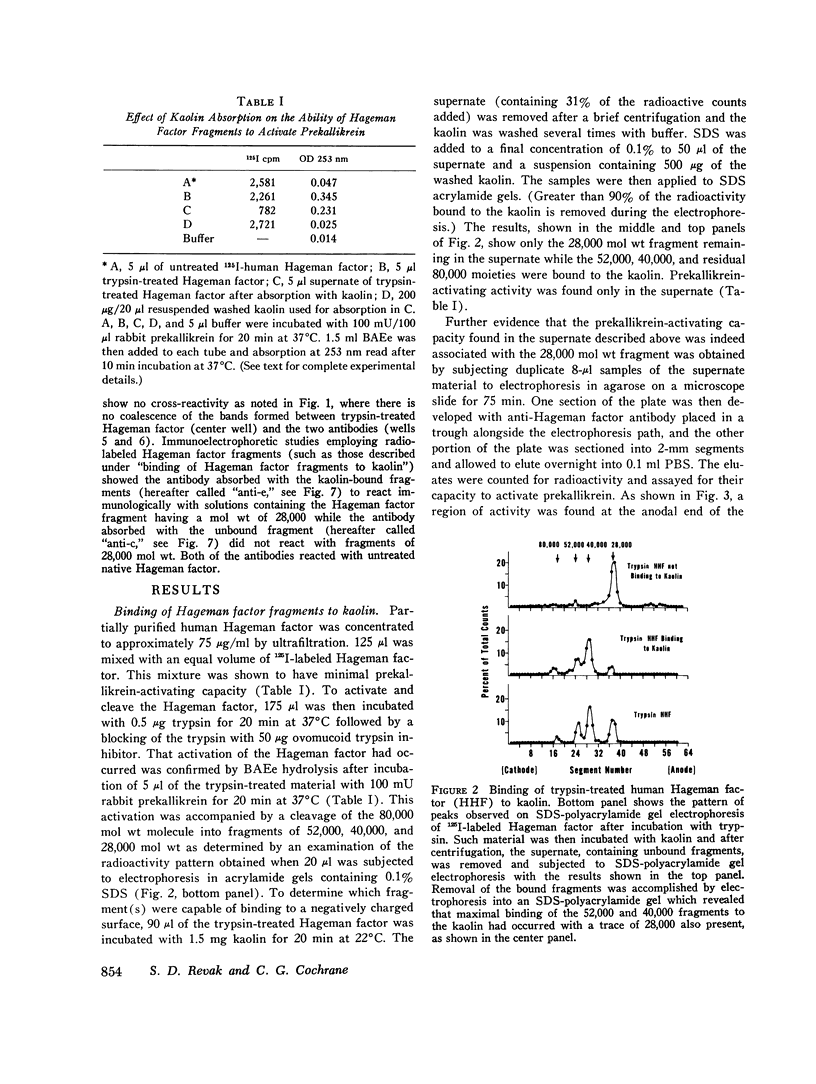
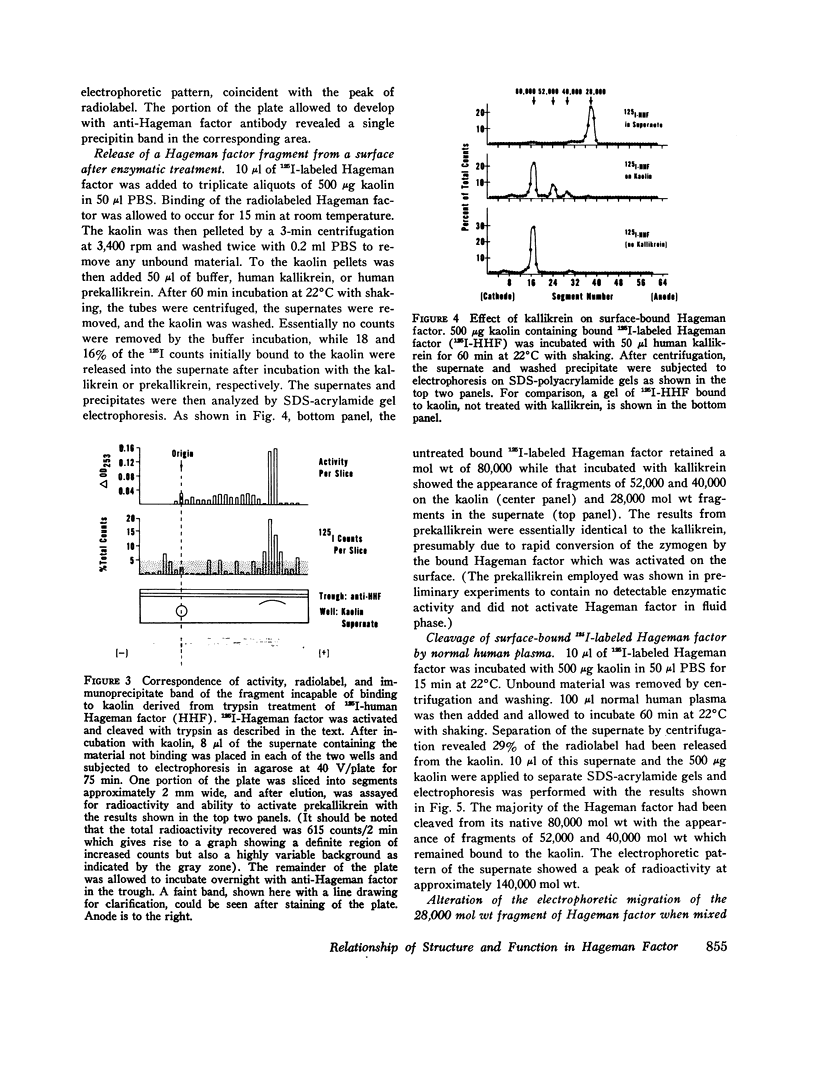
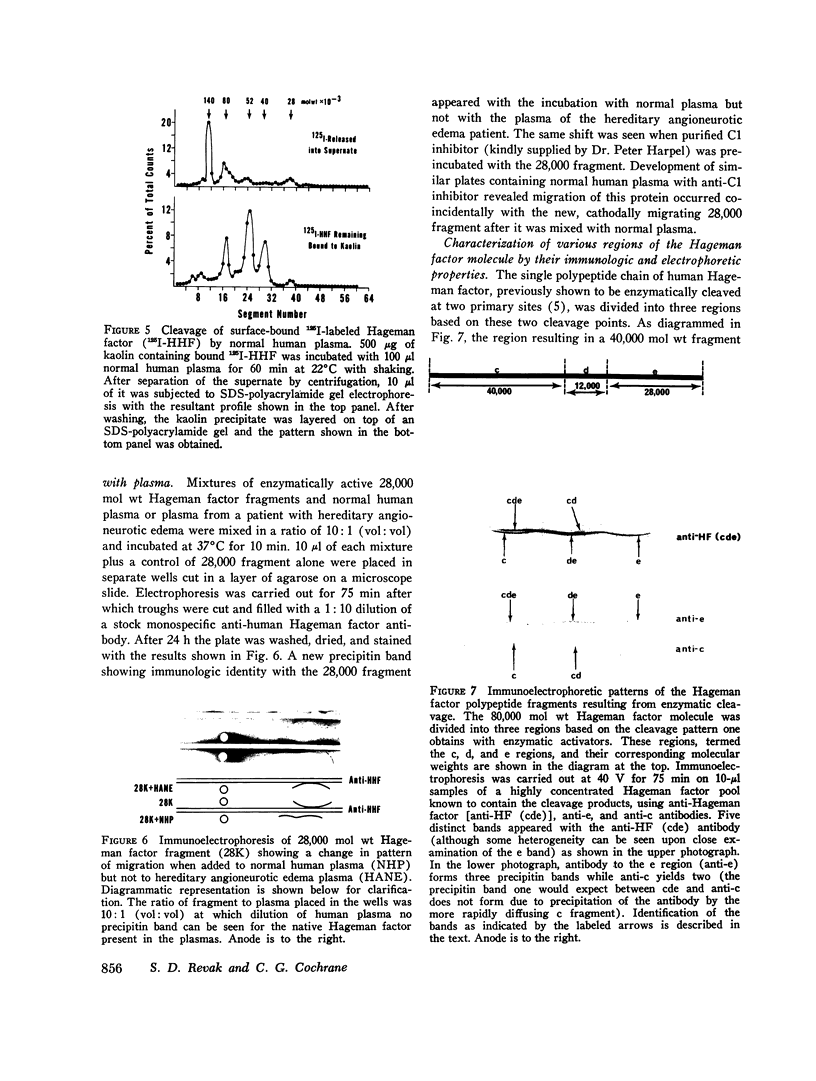
Three regions of the human Hageman factor molecule termed the c, d, and e regions have been defined. Division of the molecule into these three regions is based on the analysis of fragments obtained by enzymatic cleavage during fluid-phase activation. The three regions have the following properties: (a) the c region has a mol wt of 40,000, has the capacity to bind to negatively charged surfaces, and does not have detectable enzymatic activity; (b) the e region possess a mol wt of 28,000 has enzymatic activity, and does not bind to negatively charged surfaces; (c) the d region has a mol wt of 12,000, is located between the c and e fragments but has not been detected as a freely existing polypeptide, and can bind firmly to negatively charged surfaces. The preparation of antibodies specific for the c and e regions is described as well as their use in defining the electrophoretic characteristics of the cde, cd, de, c, and e polypeptide fragments of Hageman factor. Evidence is given showing that the e region, but not the c or d, is released from a negatively charged surface when bound Hageman factor is exposed to proteolytic enzymes or whole plasma and that when this occurs in the presence of normal plasma, the e fragment becomes bound to C1 esterase inhibitor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian A., Talamo R. C., Colman R. W. Isolation of high molecular weight activators of human plasma prekallikrein. J Biol Chem. 1973 May 25;248(10):3456–3463. [PubMed] [Google Scholar]
- Cochrane C. G., Revak S. D., Wuepper K. D. Activation of Hageman factor in solid and fluid phases. A critical role of kallikrein. J Exp Med. 1973 Dec 1;138(6):1564–1583. doi: 10.1084/jem.138.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Wuepper K. D. The first component of the kinin-forming system in human and rabbit plasma. Its relationship to clotting factor XII (Hageman Factor). J Exp Med. 1971 Oct 1;134(4):986–1004. doi: 10.1084/jem.134.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes C. D., Pensky J., Ratnoff O. D. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J Lab Clin Med. 1970 Nov;76(5):809–815. [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A pre-albumin activator of prekallikrein. J Immunol. 1970 Oct;105(4):802–811. [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med. 1971 Apr 1;133(4):696–712. doi: 10.1084/jem.133.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIS J. Activation of plasma by contact with glass: evidence for a common reaction which releases plasma kinin and initiates coagulation. J Physiol. 1958 Nov 10;144(1):1–22. doi: 10.1113/jphysiol.1958.sp006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin C. R., Saito H., Ratnoff O. D., Walton A. G. The secondary structure of human Hageman factor (factor XII) and its alteration by activating agents. J Clin Invest. 1974 Dec;54(6):1312–1322. doi: 10.1172/JCI107877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revak S. D., Cochrane C. G., Johnston A. R., Hugli T. E. Structural changes accompanying enzymatic activation of human Hageman factor. J Clin Invest. 1974 Sep;54(3):619–627. doi: 10.1172/JCI107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Schreiber A. D., Kaplan A. P., Austen K. F. Inhibition by C1INH of Hagemann factor fragment activation of coagulation, fibrinolysis, and kinin generation. J Clin Invest. 1973 Jun;52(6):1402–1409. doi: 10.1172/JCI107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer R. J., Ridgway H., Hill J. M. Activated human Hageman factor (XII). Thromb Diath Haemorrh. 1965 Sep 1;14(1-2):1–11. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Webster M. E. Human plasma kallikrein, its activation and pathological role. Fed Proc. 1968 Jan-Feb;27(1):84–89. [PubMed] [Google Scholar]
- Wuepper K. D., Cochrane C. G. Plasma prekallikrein: isolation, characterization, and mechanism of activation. J Exp Med. 1972 Jan;135(1):1–20. doi: 10.1084/jem.135.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]