Background: GX sPLA2 is expressed as an inactive pro-enzyme; the protease(s) responsible for pro-GX sPLA2 processing are unknown.
Results: Epitope-tagged pro-GX sPLA2 is proteolytically activated by furin and PCSK6 in adrenal cells.
Conclusion: Furin-like proprotein convertases regulate GX sPLA2 activity.
Significance: Identifying the factors involved in activating GX sPLA2 provides novel insight into the mechanisms surrounding its regulation.
Keywords: Adrenal, Glucocorticoid, Nuclear Receptor, Phospholipase, Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9)
Abstract
Group X secretory phospholipase A2 (GX sPLA2) hydrolyzes mammalian cell membranes, liberating free fatty acids and lysophospholipids. GX sPLA2 is produced as a pro-enzyme (pro-GX sPLA2) that contains an N-terminal 11-amino acid propeptide ending in a dibasic motif, suggesting cleavage by a furin-like proprotein convertase (PC). Although propeptide cleavage is clearly required for enzymatic activity, the protease(s) responsible for pro-GX sPLA2 activation have not been identified. We previously reported that GX sPLA2 negatively regulates adrenal glucocorticoid production, likely by suppressing liver X receptor-mediated activation of steroidogenic acute regulatory protein expression. In this study, using a FLAG epitope-tagged pro-GX sPLA2 expression construct (FLAG-pro-GX sPLA2), we determined that adrenocorticotropic hormone (ACTH) enhanced FLAG-pro-GX sPLA2 processing and phospholipase activity secreted by Y1 adrenal cells. ACTH increased the expression of furin and PCSK6, but not other members of the PC family, in Y1 cells. Overexpression of furin and PCSK6 in HEK 293 cells significantly enhanced FLAG-pro-GX sPLA2 processing, whereas siRNA-mediated knockdown of both PCs almost completely abolished FLAG-pro-GX sPLA2 processing in Y1 cells. Expression of either furin or PCSK6 enhanced the ability of GX sPLA2 to suppress liver X receptor reporter activity. The PC inhibitor decanoyl-Arg-Val-Lys-Arg-chloromethyl ketone significantly suppressed FLAG-pro-GX sPLA2 processing and sPLA2 activity in Y1 cells, and it significantly attenuated GX sPLA2-dependent inhibition of steroidogenic acute regulatory protein expression and progesterone production. These findings provide strong evidence that pro-GX sPLA2 is a substrate for furin and PCSK6 proteolytic processing and define a novel mechanism for regulating corticosteroid production in adrenal cells.
Introduction
The secreted phospholipase A2 (sPLA2)2 enzymes hydrolyze membrane phospholipids at the sn-2 position to liberate free fatty acids and lysophospholipids. The sPLA2 family is characterized by their low molecular mass (∼14–18 kDa), requirement for millimolar concentrations of Ca2+, and utilization of a highly conserved catalytic histidine within their active sites. Eleven sPLA2 members have been identified (group IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA, and XIIB PLA2-like protein that lacks catalytic activity) and placed into different groups based on the number and position of conserved cysteine residues that form disulfide bridges, generating a semi-rigid three-dimensional structure (1). Members of the sPLA2 family exhibit unique tissue distributions and disparate substrate specificities, reflecting their distinct roles in a range of physiological processes.
Among the sPLA2s, group X (GX) sPLA2 has the most potent hydrolytic activity toward zwitterionic phospholipids, including phosphatidylcholine (2), the most abundant phospholipid in mammalian plasma membranes and lipoprotein particles. GX sPLA2 has a wide tissue distribution, including the small intestine, testes, brain, pancreas, lung, thymus, spleen, peripheral blood leukocytes, among others (3, 4). Notably, GX sPLA2 appears to preferentially hydrolyze arachidonate and linoleate at the sn-2 position of phosphatidylcholine-containing lipoproteins (3, 5). The predilection of GX sPLA2 for arachidonate has important consequences with respect to the generation of bioactive lipids. For example, in vitro studies suggest that hydrolysis of phosphatidylcholine by GX sPLA2 results in cyclooxygenase-2 (COX-2)-dependent prostaglandin E2 (PGE2) production (6). In lipopolysaccharide (LPS)-treated mouse peritoneal macrophages, the addition of recombinant GX sPLA2, but not GIB or GIIA, results in a robust increase in the production of PGE2 and thromboxane A4 (7). In a Th2 cytokine-driven mouse model of asthma, GX sPLA2 has been implicated in the production of eicosanoids, including PGE2, PGD2, leukotriene B4, and cysteinyl leukotrienes (8).
The generation of C57BL/6 mice with targeted deletion of GX sPLA2 (GX KO mice) has led to new insights into novel mechanisms by which GX sPLA2 modulates physiological processes. Our laboratory reported that GX KO mice fed a normal rodent diet gain more weight and exhibit increased adiposity compared with wild-type mice (9). We also determined that stromal vascular cells isolated from adipose tissue of GX KO mice accumulate significantly more triglyceride when induced to differentiate into adipocytes compared with cells from wild-type mice. Conversely, overexpression of GX sPLA2 in OP9 pre-adipocytes results in a significant 50% reduction in triglyceride accumulation during differentiation into mature adipocytes, an effect that was associated with significantly reduced induction of adipogenic genes, including Pparγ, Srebf1, Scd1, and Fasn. Activation of the liver X receptor (LXR), a nuclear receptor known to up-regulate adipogenic gene expression, was suppressed in OP9 cells when GX sPLA2 was overexpressed, leading us to conclude that GX sPLA2 negatively regulates adipogenesis, possibly by suppressing LXR activation. We also determined that GX sPLA2 suppresses LXR activation in macrophages, resulting in reduced expression of ATP-binding cassette transporters A1 (ABCA1) and G1 (ABCG1) (10). Consequently, macrophages from GX KO mice exhibit increased cellular cholesterol efflux and decreased cellular cholesterol content (10). GX sPLA2 is expressed in adrenal cells, where it suppresses corticosteroid production through a mechanism that also appears to involve LXR. Compared with wild-type mice, GX KO mice have significantly increased plasma corticosterone levels under both basal and adrenocorticotropic hormone (ACTH)-induced conditions. The expression of steroidogenic acute regulatory protein (StAR), the rate-limiting protein in corticosteroid production, is significantly increased in adrenal glands from GX KO mice compared with wild-type adrenal glands. Conversely, in the mouse adrenal Y1 cell line, overexpression of GX sPLA2 suppresses StAR expression. Results from luciferase reporter assays indicated that GX sPLA2 antagonizes StAR promoter activity and LXR-mediated StAR promoter activation in adrenal cells. In summary, results from gain-of-function and loss-of-function studies in multiple tissues indicate that GX sPLA2 modulates cellular metabolism by negatively regulating LXR target gene expression through a mechanism that is dependent on GX sPLA2 hydrolytic activity.
Given the potent ability of GX sPLA2 to hydrolyze cell membranes and generate bioactive lipid mediators, its hydrolytic activity is likely under tight regulation. GX sPLA2 is one of only three sPLA2s produced as an inactive pro-enzyme, such that cleavage of an N-terminal pro-segment is necessary for its enzymatic activity (3). Studies in transgenic mice with constitutive GX sPLA2 expression indicate that the inactive precursor is the predominant form expressed in most tissues under normal conditions (11). However, enzymatically active GX sPLA2 is detected in the transgenic mice in tissues with inflammatory granulation, suggesting that proteolytic activation may occur during inflammation. The N-terminal pro-segment of GX sPLA2 includes 11 amino acids ending in a dibasic motif, suggesting cleavage by member(s) of the furin-like proprotein convertase (PC) family. Recently, Jemel et al. (12) showed that in transfected HEK 293 cells, the second residue within the dibasic doublet is necessary and sufficient for GX sPLA2 processing and hydrolytic activity. Furthermore, using a panel of nonspecific protease inhibitors, the involvement of PCs in GX sPLA2 maturation and activation in transfected 293 cells was confirmed. However, the identity of the individual PCs involved in GX sPLA2 processing in physiologically relevant tissues remains to be investigated.
During the course of studying GX sPLA2 in adrenal cells, we noted significantly increased phospholipase activity secreted by Y1 cells stably transfected with a GX sPLA2 expression construct and, to a lesser extent, control-transfected Y1 cells, in response to ACTH treatment (13). We reasoned that this increase in secretion reflected post-transcriptional regulation of GX sPLA2, because the promoter driving recombinant GX sPLA2 expression in our cell system would not be expected to be regulated by ACTH. Thus, mouse Y1 cells provide us a physiologically relevant model for understanding GX sPLA2 regulation. In this study, we establish that an epitope-tagged form of pro-GX sPLA2 is proteolytically activated in Y1 adrenal cells by furin and PCSK6, two members of the PC family. We also provide evidence that PC-dependent proteolytic activation of pro-GX sPLA2 is enhanced under ACTH-stimulated conditions, suggesting a novel mechanism for regulating adrenal steroidogenesis.
EXPERIMENTAL PROCEDURES
Biochemical Reagents and Assays
Adrenocorticotropic hormone (ACTH) was purchased from Sigma. The quantification of progesterone levels in cell media was achieved using a progesterone EIA kit (Cayman Chemical); sPLA2 activity was measured using our previously published method with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphorylglycerol (Matreya) as substrate (14) or a commercially available sPLA2 assay kit (Cayman Chemical).
GX sPLA2 Expression in Y1 Adrenal Cells
Murine Y1 adrenal cells were purchased from American Type Culture Collection (ATCC) and maintained in F-12K media (ATCC) supplemented with 2.5% nonheat-inactivated fetal bovine serum (Invitrogen), 15% nonheat-inactivated horse serum (Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin. A C-terminal 3× FLAG-tagged mouse GX sPLA2 cDNA was developed by PCR using forward (5′-CTGAAGCTTATGCTGCTGCTACTG-3′) and reverse (5′-ATGAATTCTCACTTGTCATCGTCGTCCTTGTAGTCGATATCGTGGTCCTTGTAGTCTCCATCGTGGTCCTTGTAGTAGTCATT-3′) primers containing HindIII and EcoRI restriction sites, respectively, and a previously generated pcDNA3.0 (Invitrogen) expression construct encoding GX sPLA2 fused to a single C-terminal FLAG tag as template (13). Thus, the amplified fragment containing the GX sPLA2 cDNA did not include any 5′- or 3′-noncoding sequences derived from the GX sPLA2 gene. Y1 cells were cultured on 100-mm dishes and transfected with either 3× FLAG-tagged GX sPLA2 or the corresponding pcDNA3.0 using Lipofectamine 2000 according to the manufacturer's instructions. G418 was then used to select for stable transfectants.
Gene Silencing with Small Interfering RNA (siRNA)
A set of ON-TARGET plus SMART pool siRNA synthetic oligonucleotides directed toward the mouse PCSK6 target sequences (5′-UAAACAAGCUUUCGAGUAU-3′, 5′-GGUCAGAGAUGAACGUCCA-3′, 5′-CGAGAUGCCUGGCGUCACA-3′, and 5′-GAUGAGACCUUCUGCGCGA-3′) and toward the mouse furin target sequences (5′-CGACAUCGGCAAACGGCUA-3′, 5′-GAAGAAUCAUCCCGACCUA-3′, 5′-GAAAGUGAGCCAUUCGUAU-3′, and 5′-GCGCCACACAGUUCGGCAA-3′) were purchased from Thermo Scientific. The ON-TARGET plus nontargeting pool was used as a control. Cells were transfected with siRNAs using Dharmafect 1 transfection reagent (Thermo Scientific) according to the manufacturer's instructions.
Quantitative RT-PCR
Total RNA was isolated from mouse Y1 adrenal cells using the RNeasy mini kit (Qiagen). RNA (1 μg) was reverse-transcribed using the High Capacity cDNA reverse transcription kit (Applied Biosystems). cDNA was diluted 4-fold at which point quantitative PCR was performed with Power SYBR Green PCR master mix kit (Applied Biosystems) and the iQ5 multicolor RT-PCR Detection System (Bio-Rad) through 40 cycles of amplification. Primer sequences used in qRT-PCR are available upon request.
Immunoblotting
Proteins were resolved using SDS-PAGE. The 3× FLAG-tagged GX sPLA2 chimeric protein was detected by Western blot analysis using anti-FLAG M2 primary antibody (Stratagene). StAR was detected using anti-StAR primary antibody (Santa Cruz Biotechnology). The secondary HRP-conjugated antibody was from Abcam.
Reporter Assays
HEK 293 cells were grown to ∼75% confluence and then transfected with mouse 3× FLAG-tagged GX sPLA2 expression vector or a control vector encoding green fluorescent protein (GFP) (0.4 μg), along with pTK-3-LXRE-Luc reporter construct (0.4 μg), mouse liver X receptor (mLXRα) (0.1 μg), mouse retinoid X receptor (mRXR) (0.1 μg), Renilla luciferase (Promega, 0.02 μg), and either furin, PCSK6 (Origene), or GFP expression constructs (0.4 μg) using Lipofectamine 2000 according to the manufacturer's protocol. The mLXR and mRXR expression constructs and the 3× LXR element reporter plasmid were gifts from Dr. Peter Tontonoz, UCLA. After 18 h, cells were incubated in fresh media (high glucose DMEM (HyClone), supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin), and either 0 or 1 μm T0901317 for 24 h. Cells were washed with DPBS and harvested in 1× PLB buffer (Promega). Luminescence was measured using the dual-luciferase reporter assay system (Promega).
Statistics
Statistical significance is reported in the figures and/or figure legends. Statistical analysis of data sets was performed using GraphPad Prism. For experiments in which only two groups were compared, a two-tailed Student's t test was performed. For comparison of means between more than two groups, a one- or two-way analysis of variance was used where appropriate. Post hoc analysis was done using Bonferroni's test. All data sets conformed to the constraints of the parametric analysis.
RESULTS
ACTH Increases Ectopically Expressed GX sPLA2 Proteolytic Activation in Y1 Adrenal Cells
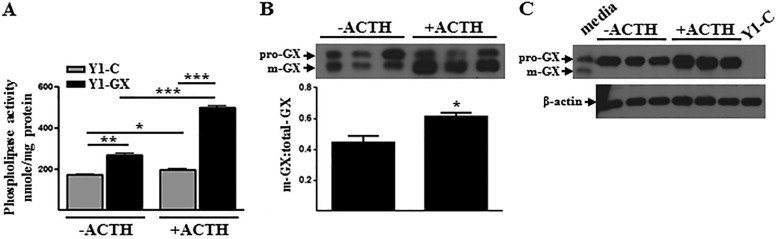
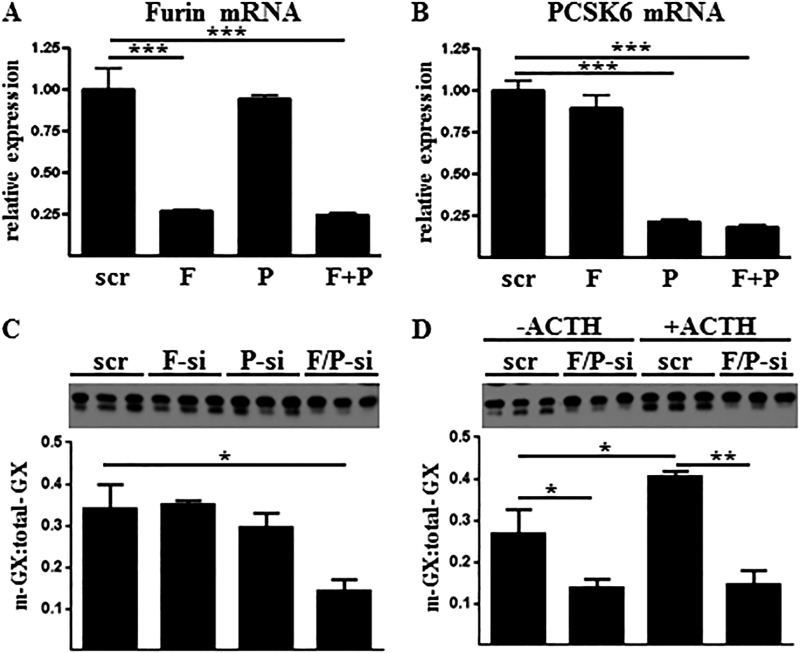
To investigate post-translational mechanisms involved in regulating GX sPLA2 activity in adrenal cells, we generated mouse Y1 cells stably expressing GX sPLA2 fused to a C-terminal 3× FLAG sequence (Y1-GX) under the control of a CMV-driven promoter. This chimeric construct provided us with a model for studying the post-translational processing of GX sPLA2 based on differences in the molecular mass of the inactive precursor, pro-GX (∼15.2 kDa) and the mature form of the enzyme, m-GX (∼13.9 kDa). Phospholipase activity secreted by Y1-GX cells was increased ∼1.5-fold compared with Y1-C cells (Fig. 1A) indicating that GX sPLA2 expression was only modestly increased over endogenous levels in Y1-GX cells. In accordance with our previous findings (13), ACTH treatment resulted in a significant increase in the phospholipase activity secreted by control-transfected Y1 cells (Y1-C) (Fig. 1A). The increase in phospholipase activity in response to ACTH was even more robust in Y1-GX cells (Fig. 1A). Associated with the increase in phospholipase activity in ACTH-treated Y1-GX cells was an increase in FLAG-pro-GX sPLA2 processing, expressed as the ratio of m-GX/total-GX sPLA2, in response to ACTH stimulation (Fig. 1B). In the case of untreated Y1-GX cells, ∼44% of total FLAG-GX sPLA2 present in the media was processed to the mature form. This contrasts to ACTH-treated cells, where ∼61% of secreted FLAG-GX sPLA2 had undergone proteolytic processing. m-GX sPLA2 was not detected in cell lysates of Y1-GX cells in either the absence or presence of ACTH (Fig. 1C). Taken together, these results suggest that ACTH enhances the phospholipase activity secreted by Y1-GX cells, at least partly by increasing the proteolytic processing of pro-GX sPLA2, and that pro-GX sPLA2 is the major, if not exclusive, form detected intracellularly.
FIGURE 1.
ACTH increases FLAG-pro-GX sPLA2 processing and phospholipase activity secreted by Y1 adrenal cells. Y1 adrenal cells were stably transfected with either a control expression vector (Y1-C) or a vector expressing 3× FLAG-tagged GX sPLA2 (Y1-GX) and then incubated with 0 or 100 nm ACTH for 20 h. A, phospholipase activity in conditioned media was measured (n = 6). B, conditioned media (20 μl) was immunoblotted using an anti-FLAG antibody (top); results from densitometric analyses are shown below (n = 3). Data are expressed as the ratio of m-GX sPLA2/total-GX sPLA2 in the media. C, whole-cell lysates (10 μg of protein) were immunoblotted using anti-FLAG antibody (top) or β-actin (bottom). For comparison, conditioned media from vehicle-treated Y1-GX cells (media) and lysates from Y1-C cells (Y1-C) were also analyzed. Data are means ± S.E. and are representative of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FLAG-Pro-GX sPLA2 Processing and Activity Are Blocked by the Furin-like Proprotein Convertase Inhibitor RVKR
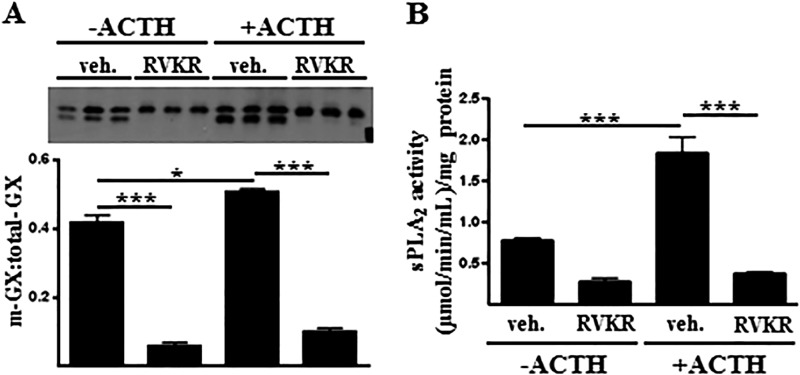
Based on results from studies using a panel of protease inhibitors, the family of furin-like PCs has recently been implicated in the proteolytic activation of GX sPLA2 in transfected HEK 293 cells (12). To investigate the role of PCs in regulating GX sPLA2 proteolytic activation in mouse Y1 adrenal cells, sPLA2 activity and FLAG-pro-GX sPLA2 processing was assessed in Y1-GX cells treated with the PC inhibitor, decanoyl-Arg-Val-Lys-Arg-chloromethyl ketone. After overnight incubation, both the precursor and mature forms of FLAG-GX sPLA2 were detected in the media of vehicle-treated Y1-GX cells, and ACTH treatment significantly increased the ratio of m-GX sPLA2/total-GX sPLA2 (Fig. 2A). However, the processing of FLAG-pro-GX-sPLA2 was almost completely abolished in RVKR-treated cells, both in the absence and presence of ACTH. This decrease in processing was accompanied by a significant reduction in sPLA2 activity secreted by the cells (Fig. 2B). These results demonstrate a role for furin-like PCs in the proteolytic activation of GX sPLA2 in Y1 adrenal cells under both basal and ACTH-stimulated conditions.
FIGURE 2.
FLAG-pro-GX sPLA2 processing and activity are blocked by the furin-like proprotein convertase inhibitor RVKR. A, Y1-GX cells were incubated with 0 or 100 nm ACTH in the presence or absence of 25 μm RVKR for 20 h, at which time media were collected for immunoblotting with anti-FLAG (top); results from densitometric analyses are shown below. Data are expressed as the ratio of m-GX sPLA2/total-GX sPLA2 in the media. B, sPLA2 activity in the media was quantified. Data are means ± S.E. and are representative of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; veh, vehicle.
Furin and PCSK6 Expression Is Increased in Y1 Cells Treated with ACTH
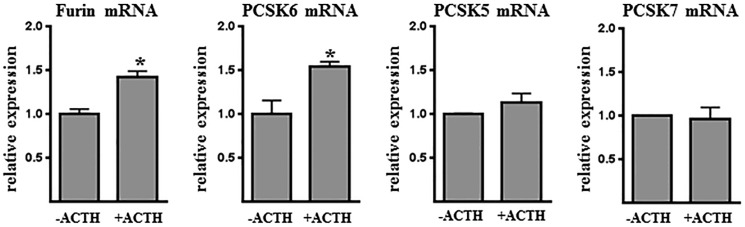
To identify candidate PCs that may be responsible for regulating GX sPLA2 activity in adrenal cells, we quantified mRNA abundance for each member of the PC family in Y1 cells under basal and ACTH-stimulated conditions. Among the six candidate convertases, only four were expressed at appreciable levels (Ct value <35) as follows: furin, PCSK5, PCSK6, and PCSK7 (Fig. 3). Interestingly, both furin and PCSK6 mRNA abundance were significantly increased in response to overnight incubation with ACTH, consistent with the enhanced pro-GX sPLA2 processing. Therefore, we further investigated the role of furin and PCSK6 in regulating GX sPLA2 processing in Y1 cells.
FIGURE 3.
ACTH increases furin and PCSK6 gene expression in adrenal cells. Y1 cells were incubated for 16 h with either 0 or 100 nm ACTH, and furin-like proprotein convertase gene expression was quantified by qRT-PCR (n = 3). Data are means ± S.E. and are representative of two independent experiments. *, p < 0.05.
FLAG-Pro-GX sPLA2 Is Proteolytically Cleaved by Furin and PCSK6
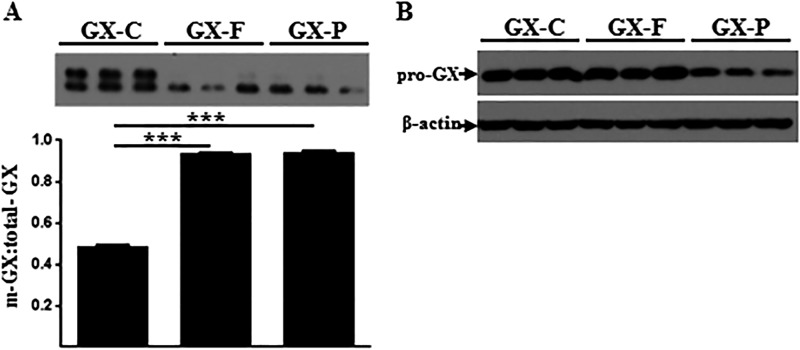
Previous reports characterizing the substrate specificity of PCs suggest some redundancy in functionality for some, but not all, targets (15). To assess whether pro-GX sPLA2 is a substrate for furin and/or PCSK6 proteolytic activation, FLAG-pro-GX sPLA2 was co-expressed with either furin or PCSK6 in HEK 293 cells. The ratio of m-GX sPLA2/total-GX sPLA2 in media from HEK 293 cells expressing GX sPLA2 alone was ∼1:1 (Fig. 4A). Expression of either furin or PCSK6 resulted in an almost complete conversion of FLAG-pro-GX sPLA2 to the mature form, indicating that pro-GX sPLA2 is a substrate for both PCs. m-GX sPLA2 was not detected in whole-cell lysates, indicating that the active form of the enzyme does not accumulate intracellularly after pro-GX sPLA2 is processed by furin or PCSK6 (Fig. 4B).
FIGURE 4.
FLAG-pro-GX sPLA2 is proteolytically cleaved by furin and PCSK6. HEK 293 cells were transiently co-transfected with 3× FLAG-tagged GX sPLA2 and either a control vector (GX) or a vector encoding furin (GX+F) or PCSK6 (GX+P). Cells were then incubated in fresh media for 24 h. A, conditioned media were immunoblotted using anti-FLAG antibody (top); results from densitometric analyses are shown below. Data are expressed as the ratio of m-GX sPLA2/total-GX sPLA2 in the media. B, whole-cell lysates (10 μg of protein) were immunoblotted using anti-FLAG antibody (top) and anti-β-actin (bottom). Data are means ± S.E. and are representative of two independent experiments. ***, p < 0.001.
Both Furin and PCSK6 Contribute to FLAG-Pro-GX sPLA2 Processing in Y1 Cells
To determine whether furin and/or PCSK6 mediate GX sPLA2 proteolytic activation in adrenal cells, we employed small interfering RNAs (siRNAs) to suppress the expression of furin mRNA, PCSK6 mRNA, or both mRNAs. Both furin and PCSK6 mRNA expression was effectively reduced ∼75% using this approach (Fig. 5, A and B). Neither knockdown of furin nor PCSK6 alone was able to significantly inhibit processing of FLAG-pro-GX sPLA2 secreted by Y1 cells (Fig. 5C). However, knockdown of both furin and PCSK6 together resulted in a significant reduction in FLAG-pro-GX sPLA2 cleavage. As expected, ACTH treatment significantly increased the ratio of m-GX sPLA2/total-GX sPLA2 in the media compared with vehicle-treated cells (Fig. 5D). However, enhanced FLAG-pro-GX sPLA2 processing in response to ACTH was significantly decreased when furin and PCSK6 expression was suppressed. These findings strongly suggest that both furin and PCSK6 mediate GX sPLA2 processing in Y1 adrenal cells, and ACTH-induced increases in GX sPLA2 activity is at least partly due to up-regulation of these convertases.
FIGURE 5.
FLAG-pro-GX sPLA2 processing in mouse Y1 adrenal cells is dependent on furin and PCSK6 gene expression. Y1-GX cells were transiently transfected with either control siRNA (scr) or siRNA targeting furin (F-si), PCSK6 (P-si), or both siRNAs (F/+P-si). A and B, Furin (A) and PCSK6 (B) mRNAs were quantified by qRT-PCR. C, conditioned media were immunoblotted using anti-FLAG antibody (top); results from densitometric analyses are shown below. D, Y1-GX cells transiently transfected with either control siRNA (scr) or siRNA targeting both furin and PCSK6 (F/P-si) were then incubated in fresh media containing either 0 or 100 nm ACTH for 20 h. Conditioned media were immunoblotted using anti-FLAG antibody (top); and densitometric analysis is shown below. Data (means ± S.E.) are expressed as the ratio of m-GX sPLA2/total-GX sPLA2 in the media and are representative of two independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Suppression of LXR Activation by GX sPLA2 Is Enhanced When Furin or PCSK6 Are Overexpressed
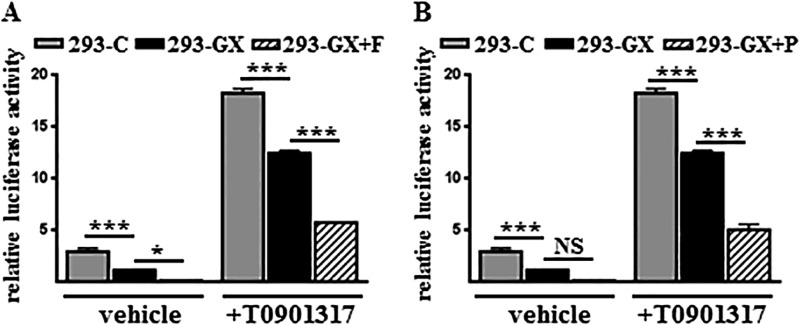
We previously reported that GX sPLA2 inhibits LXR-mediated target gene activation through a mechanism that is dependent on its enzymatic activity (10). Therefore, we investigated whether proteolytic activation of GX sPLA2 by furin or PCSK6 enhances GX sPLA2-dependent inhibition of LXR activation. As expected, GX sPLA2 overexpression in HEK 293 cells suppressed the transcriptional activation of an LXR reporter construct under both basal and T0901317-treated conditions (Fig. 6, A and B). The inhibitory effect of GX sPLA2 on LXR activation was augmented when either furin or PCSK6 was co-expressed, indicating that GX sPLA2 processing by furin-like proprotein convertases may represent an important mechanism for regulating GX sPLA2-mediated inhibition of LXR transcriptional activation.
FIGURE 6.
GX sPLA2 processing by furin or PCSK6 enhances GX sPLA2-dependent inhibition of LXR-mediated gene activation. HEK 293 cells were transiently co-transfected with ptk-3× LXRE-luc reporter construct and vectors encoding mLXRα, mRXR, Renilla luciferase, and either a control vector encoding GFP (293-C) or a vector encoding 3× FLAG-tagged GX sPLA2 in the absence (293-GX) or presence of furin (293-GX+F) (A) or PCSK6 (293-GX+P) (B). Cells were then incubated in media containing either 0 or 1 μm T0901317 for 24 h prior to measurements of luciferase activity. Data are means ± S.E. (n = 4) and are representative of two independent experiments. *, p < 0.05; ***, p < 0.001; NS, not significant.
GX sPLA2-mediated Suppression of Steroidogenesis Requires Furin-like Proprotein Convertase Activity
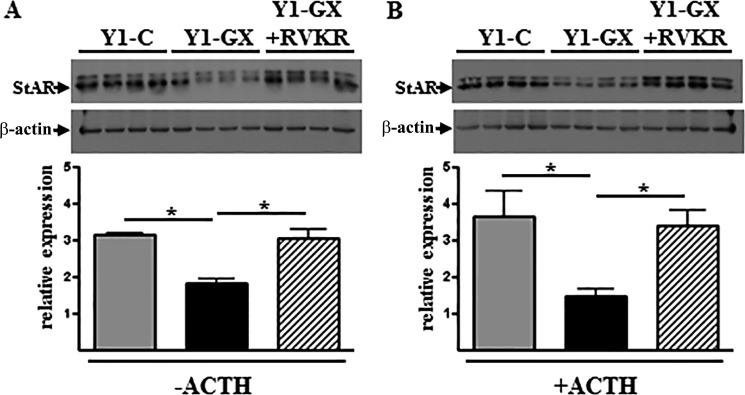
StAR plays a critical function in adrenal corticoid synthesis by delivering cholesterol to steroidogenic enzymes located in the inner mitochondrial membrane. Given its key role in corticosteroid production, StAR mRNA expression is under both positive and negative control by a variety of transcription factors, including LXR (16). Given our previous finding that GX sPLA2 suppresses StAR expression in an LXR-dependent manner (13), it was of interest to investigate whether inhibiting PC activity impacted the ability of GX sPLA2 to regulate StAR, and hence steroid production in adrenal cells. As we reported previously, GX sPLA2 overexpression inhibited StAR protein expression in Y1 cells under both basal (Fig. 7A) and ACTH-stimulated conditions (Fig. 7B). This inhibitory effect was abolished when cells were treated with RVKR. In a subsequent study, we determined that RVKR increased StAR protein abundance in control Y1 cells, but this effect was not significant (p = 0.08). The trend for increased StAR in the absence of GX sPLA2 overexpression could be due to the effect of the PC inhibitor on endogenous GX sPLA2 processing. Collectively, our results indicate that furin-like proprotein convertases are required for GX sPLA2-mediated StAR regulation.
FIGURE 7.
Inhibition of StAR protein expression by GX-sPLA2 is abolished by RVKR. Y1 cells were transiently transfected with either a control vector (Y1-C) or a vector encoding 3×-FLAG tagged GX sPLA2 (Y1-GX). Cells were then incubated in media containing 0 or 100 nm ACTH and either DMSO vehicle or 25 μm RVKR for 18 h. Immunoblot analysis of total cell lysates was performed using antibodies specific for StAR and β-actin. A, StAR and β-actin expression in Y1-C cells (1st to 4th lanes), Y1-GX cells (5th to 8th lanes), and Y1-GX cells treated with RVKR (9th to 12th lanes) in the absence of ACTH (top). Results from densitometric analyses (bottom) are expressed as the ratio of StAR/β-actin. B, StAR and β-actin expression in Y1-C cells (1st to 4th lanes), Y1-GX cells (5th to 8th lanes), and Y1-GX cells treated with RVKR (9th to 12th lanes) in the presence of 100 nm ACTH (top). Results from densitometric analyses (bottom) are expressed as the ratio of StAR/β-actin. Data are means ± S.E. (n = 3). *, p < 0.05.
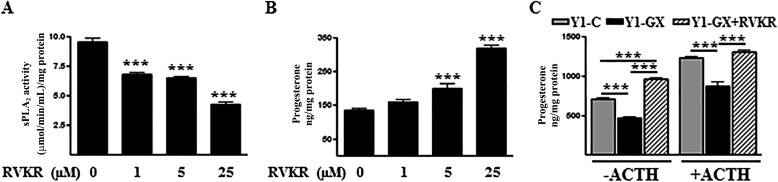
We next assessed the effect of inhibiting PCs on steroid production in Y1-GX cells. Because Y1 cells do not express 21-hydroxylase, the enzyme required for conversion of progesterone to corticosterone (17), progesterone levels in conditioned media were measured as an indicator of steroid production by these cells. As expected, treatment with increasing concentrations of RVKR resulted in a dose-dependent decrease in phospholipase activity secreted by Y1-GX cells (Fig. 8A). Notably, progesterone production was reciprocally increased in response to increasing concentrations of RVKR (Fig. 8B). In accordance with previous findings, GX sPLA2 overexpression significantly decreased progesterone production by Y1 cells under both basal and ACTH-stimulated conditions (Fig. 8C). The inhibition was ablated when cells were treated with RVKR at a dose that significantly reduced sPLA2 activity secreted by these cells. This result is consistent with the conclusion that pro-GX sPLA2 processing by PCs is necessary for GX sPLA2-mediated suppression of adrenal steroidogenesis.
FIGURE 8.
GX sPLA2-mediated inhibition of progesterone production by adrenal cells requires furin-like proprotein convertase activity. A and B, Y1 cells were transiently transfected with 3× FLAG-tagged GX sPLA2. After 16 h, cells were incubated with the indicated concentrations of RVKR for 20 h. sPLA2 activity (A) and progesterone concentrations (B) were assayed in the media and expressed relative to total cellular protein (n = 6). Data are means ± S.E. ***, p < 0.001 compared with control-treated cells. C, Y1 cells were transiently transfected with either a control vector (Y1-C) or a vector encoding 3× FLAG-tagged GX sPLA2 (Y1-GX). Cells were then incubated in media containing 0 or 100 nm ACTH and either DMSO vehicle or 25 μm RVKR for 20 h. The concentration of progesterone in the media was measured and expressed relative to total cellular protein (n = 6). Data are means ± S.E. ***, p < 0.001.
DISCUSSION
Furin-like PCs are calcium-dependent serine proteases that mediate the post-translational processing and activation of numerous molecules important for tissue and whole-body homeostasis, such as cell surface receptors, pro-hormones, growth factors, matrix metalloproteinases, and adhesion molecules (15). Perturbations in PC activity have been implicated in multiple pathological conditions, including various endocrinopathies, infectious diseases, cancer, and Alzheimer disease, reflecting their fundamental role in diverse physiological processes. Here, we provide strong evidence that pro-GX sPLA2 is a previously unrecognized substrate for two members of the PC family, furin and PCSK6, and we identify proteolytic activation of pro-GX sPLA2 by PCs as a novel mechanism for regulating glucocorticoid production in adrenal cells.
The initial observation that prompted our study was the finding that phospholipase activity secreted by Y1-GX cells, and to a lesser extent Y1-C cells, was significantly increased when cells were stimulated by ACTH (13). We speculated that the increase in sPLA2 activity was due to enhanced conversion of both endogenous and ectopically expressed pro-GX sPLA2 to m-GX sPLA2 in ACTH-treated cells. A recent study by Jemel et al. (12) suggested that pro-GX sPLA2 is proteolytically activated by a furin-like PC, although the identity of the specific PC(s) was not determined. In an initial screening by qRT-PCR, we determined that four members of the PC family are expressed in Y1 adrenal cells, furin, PCSK5, PCSK6, and PCSK7. We conclude that both furin and PCSK6 play a major role in pro-GX sPLA2 processing in adrenal cells based on the following finding. 1) Furin and PCSK6 mRNA abundance were both significantly induced by ACTH in Y1 adrenal cells. 2) FLAG-pro-GX sPLA2 processing and activation were blocked in ACTH-treated Y1 cells by the PC inhibitor RVKR. 3) Co-transfection of FLAG-pro-GX sPLA2 with either furin or PCSK6 significantly enhanced FLAG-pro-GX sPLA2 processing in HEK 293 cells. 4) FLAG-pro-GX sPLA2 processing in adrenal cells was effectively blocked when the expression of both furin and PCSK6 was suppressed. Analysis of PC cleavage preferences and substrate specificities reveals considerable overlap among several members of the PC family (18). However, caution should be taken when attempting to draw conclusions from studies using short unfolded peptides as substrates. Redundancy in furin substrate cleavage specificity has been described in the liver using an interferon-inducible Mx-Cre/loxP furin-deficient mouse model (19). Both soluble furin and PCSK6 are able to cleave pro-Nodal while bound to its co-receptor at the cell surface (20, 21). However, unique furin substrates, including the iron regulatory protein, pro-hepcidin, and pro-bone morphogenic protein 10 (pro-BMP10) in the developing heart, have also been described (22, 23). Our data indicate that suppression of both furin and PCSK6 by siRNA-mediated gene silencing is required to effectively block FLAG-pro-GX sPLA2 processing and provide strong evidence for redundancy, at least in transfected Y1-GX cells.
In this study, using a FLAG-tagged pro-GX sPLA2, we could detect m-GX sPLA2 only in the media and not in the cell lysates, although both pro-GX and m-GX sPLA2 were found in conditioned media. Because Western blotting would detect steady state levels of the precursor and processed forms of GX sPLA2, we carried out radioimmunoprecipitation experiments to determine whether the mature form could be detected in cells metabolically labeled for a relatively brief period (4 h). However, only pro-GX sPLA2 was detected in cell lysates (data not shown), indicating that if intracellular processing does occur, m-GX sPLA2 must be either rapidly degraded or secreted. Furin is thought to process substrates in the trans-Golgi network, at the cell surface, and in endosomes, although PCSK6-dependent processing is believed to take place predominantly in the extracellular matrix (15). Notably, there appear to be distinct mechanisms underlying the compartment-specific regulation of substrate processing by furin. Pro-ADAMTS4 co-localizes with furin in the trans-Golgi network, where its pro-segment is proteolytically removed (24). However, pro-ADAMTS9 processing by furin takes place at the cell surface before being secreted into the media such that no evidence for mature- ADAMTS9 is detected in the cell lysates (25). Our conclusion that m-GX sPLA2 does not accumulate intracellularly is in contrast to a previous report that utilized cell-permeable and cell-impermeable inhibitors to define the cellular location of pro-GX sPLA2 processing. In this study, the authors concluded that pro-GX sPLA2 processing takes place both before and after secretion (12). This conclusion was supported by the finding that exogenously added pro-GX sPLA2 does not result in significant cellular membrane hydrolysis. The reason for the discrepancy between these two findings is unclear. One possibility is that PC-dependent processing of pro-GX sPLA2 occurs coincident with secretion in a cellular compartment near the cell surface that is inaccessible to cell-impermeable PC inhibitors.
This study confirms previous findings from our laboratory documenting the role of GX sPLA2 in modulating adrenal steroidogenesis (13). GX KO mice have increased plasma corticosterone levels under both basal and ACTH-induced stress conditions (13). This phenotype is due at least partly to a direct effect of GX sPLA2 in the adrenal gland, because primary adrenal cells isolated from GX KO mice produce higher levels of glucocorticoids in response to ACTH compared with cells from wild-type mice (13). We determined that GX sPLA2-mediated suppression of progesterone production in Y1 adrenal cells is dependent on its hydrolytic activity, as evidenced by the fact that GX sPLA2, but not a catalytically inactive mutant lacking the active-site histidine residue, suppressed basal and ACTH-induced progesterone production in Y1 cells. Our finding that treatment with the PC inhibitor, RVKR, abolished GX sPLA2-dependent suppression of progesterone production under both basal and ACTH-stimulated conditions is consistent with previous observations that removal of the N-terminal pro-segment is necessary for GX sPLA2 hydrolytic activity (4). Importantly, the derepression of progesterone production in Y1-GX cells treated with RVKR was associated with both decreased FLAG-pro-GX sPLA2 processing and sPLA2 activity in conditioned media from these cells. Furthermore, RVKR resulted in significantly reduced sPLA2 activity secreted by Y1-C cells (data not shown), suggesting that upon processing endogenous GX sPLA2 contributes significantly to the phospholipase activity secreted by Y1 adrenal cells. Taken together, these data indicate that PCs proteolytically activate pro-GX sPLA2, which in turn acts to suppress glucocorticoid production in adrenal cells. Although the mechanism has not been completely delineated, GX sPLA2 appears to negatively regulate adrenal glucocorticoid production through transcriptional suppression of StAR, most likely by reducing the activation of LXR (13). StAR represents the rate-limiting protein in steroid hormone production, and many of the factors known to regulate steroidogenesis, including LXR, have their effect by targeting StAR (16, 25, 26). Results from this study demonstrate that suppression of LXR reporter activation by GX sPLA2 is enhanced in the presence of either furin or PCSK6 and that inhibition of pro-GX sPLA2 processing by RVKR restores StAR protein expression in Y1-GX cells to levels comparable with Y1-C cells. Together, these findings suggest that processing of pro-GX sPLA2 by PCs is necessary for GX sPLA2-dependent suppression of LXR target gene activation.
Mammals have evolved a complex regulatory network for fine-tuning adrenal steroid production. Pituitary-derived ACTH stimulates the adrenals to produce glucocorticoids, which feedback on both the anterior pituitary and the hypothalamus to inhibit ACTH and corticotropin-releasing hormone, respectively. Here, we provide evidence for a negative feedback loop in adrenal cells whereby ACTH increases PC expression, resulting in conversion of pro-GX sPLA2 to m-GX sPLA2, which in turn acts to suppress glucocorticoid production. What remains to be determined are the mechanism(s) responsible for turning off the activity of GX sPLA2. One potential pathway is the M-type sPLA2 receptor (sPLA2-R), which has been implicated in the lysosomal degradation of GX sPLA2. Chinese hamster ovary (CHO) cells overexpressing the sPLA2-R rapidly degrade GX sPLA2, resulting in a marked reduction in PGE2 production compared with non-sPLA2 receptor-expressing cells (27). Mice deficient in sPLA2-R have higher levels of GX sPLA2 present in the bronchoalveolar lavage fluid in response to ovalbumin-induced airway inflammation, and this is associated with the increased production of eicosanoids, Th2 cytokines, and infiltration of inflammatory neutrophils and eosinophils (28). A soluble form of the sPLA2-R has been identified in mouse plasma that binds and inactivates GX sPLA2. The in vitro incubation of GX sPLA2 with plasma from wild-type mice but not sPLA2-R-deficient mice decreases phospholipase activity (29). We determined that the sPLA2-R is expressed in mouse adrenal glands and Y1 cells (13). Interestingly, silencing sPLA2-R expression in YI-GX cells results in significantly reduced progesterone production, consistent with the possibility that the sPLA2-R internalizes and/or inactivates GX sPLA2, thereby reducing the magnitude of GX sPLA2's suppressive effect (13).
Understanding the tissue-specific regulation of pro-GX sPLA2 processing will provide novel insights into the regulatory mechanisms governing both the physiological and pathophysiological processes in which GX sPLA2 is thought to play a role, including asthma, ischemia-reperfusion injury, and atherosclerosis to name a few (30–32). In addition to adrenal cells, studies in our laboratory document that GX sPLA2 negatively regulates LXR target gene expression in macrophages and adipocytes, with significant consequences with respect to macrophage cholesterol efflux capacity (10), macrophage-mediated inflammatory responses (33), and adipocyte lipogenesis (9). Given that both furin and PCSK6 are ubiquitously expressed (15), it is tempting to speculate that these PCs are the predominant proteases involved in the conversion of pro-GX sPLA2 to m-GX sPLA2 in multiple cell types. Interestingly, furin mRNA expression and sPLA2 activity are significantly increased in J774 macrophages stably expressing GX sPLA2 when treated with lipopolysaccharide.3 Clearly, further studies are needed to solidify the roles of individual PCs in modulating GX sPLA2 activity in different tissues.
Acknowledgments
We thank Kathy Forrest and Lubna Zahoor for their excellent technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK082419 (to N. R. W.) and T32 Training Grant 2T32DK007778-11 (to J. L.).
J. D. Layne, P. Shridas, and N. R. Webb, unpublished data.
- sPLA2
- secreted phospholipase A2
- GX
- group X
- pro-GX sPLA2
- GX sPLA2 produced as pro-enzyme
- PC
- proprotein convertase
- StAR
- steroidogenic acute regulatory protein
- LXR
- liver X receptor
- PG
- prostaglandin
- qRT
- quantitative RT
- sPLA2-R
- sPLA2 receptor
- mLXR
- mouse liver X receptor
- mRXR
- mouse retinoid X receptor.
REFERENCES
- 1. Boyanovsky B. B., Webb N. R. (2009) Biology of secretory phospholipase A2. Cardiovasc. Drugs Ther. 23, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singer A. G., Ghomashchi F., Le Calvez C., Bollinger J., Bezzine S., Rouault M., Sadilek M., Nguyen E., Lazdunski M., Lambeau G., Gelb M. H. (2002) Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 277, 48535–48549 [DOI] [PubMed] [Google Scholar]
- 3. Morioka Y., Saiga A., Yokota Y., Suzuki N., Ikeda M., Ono T., Nakano K., Fujii N., Ishizaki J., Arita H., Hanasaki K. (2000) Mouse group X secretory phospholipase A2 induces a potent release of arachidonic acid from spleen cells and acts as a ligand for the phospholipase A2 receptor. Arch. Biochem. Biophys. 381, 31–42 [DOI] [PubMed] [Google Scholar]
- 4. Cupillard L., Koumanov K., Mattéi M. G., Lazdunski M., Lambeau G. (1997) Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J. Biol. Chem. 272, 15745–15752 [DOI] [PubMed] [Google Scholar]
- 5. Pruzanski W., Lambeau L., Lazdunsky M., Cho W., Kopilov J., Kuksis A. (2005) Differential hydrolysis of molecular species of lipoprotein phosphatidylcholine by groups IIA, V and X secretory phospholipases A2. Biochim. Biophys. Acta 1736, 38–50 [DOI] [PubMed] [Google Scholar]
- 6. Morioka Y., Ikeda M., Saiga A., Fujii N., Ishimoto Y., Arita H., Hanasaki K. (2000) Potential role of group X secretory phospholipase A2 in cyclooxygenase-2-dependent PGE2 formation during colon tumorigenesis. FEBS Lett. 487, 262–266 [DOI] [PubMed] [Google Scholar]
- 7. Saiga A., Morioka Y., Ono T., Nakano K., Ishimoto Y., Arita H., Hanasaki K. (2001) Group X secretory phospholipase A2 induces potent productions of various lipid mediators in mouse peritoneal macrophages. Biochim. Biophys. Acta 1530, 67–76 [DOI] [PubMed] [Google Scholar]
- 8. Henderson W. R., Jr., Chi E. Y., Bollinger J. G., Tien Y. T., Ye X., Castelli L., Rubtsov Y. P., Singer A. G., Chiang G. K., Nevalainen T., Rudensky A. Y., Gelb M. H. (2007) Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 204, 865–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X., Shridas P., Forrest K., Bailey W., Webb N. R. (2010) Group X secretory phospholipase A2 negatively regulates adipogenesis in murine models. FASEB J. 24, 4313–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shridas P., Bailey W. M., Gizard F., Oslund R. C., Gelb M. H., Bruemmer D., Webb N. R. (2010) Group X secretory phospholipase A2 negatively regulates ABCA1 and ABCG1 expression and cholesterol efflux in macrophages. Arterioscler. Thromb. Vasc. Biol. 30, 2014–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohtsuki M., Taketomi Y., Arata S., Masuda S., Ishikawa Y., Ishii T., Takanezawa Y., Aoki J., Arai H., Yamamoto K., Kudo I., Murakami M. (2006) Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. J. Biol. Chem. 281, 36420–36433 [DOI] [PubMed] [Google Scholar]
- 12. Jemel I., Ii H., Oslund R. C., Payré C., Dabert-Gay A. S., Douguet D., Chargui K., Scarzello S., Gelb M. H., Lambeau G. (2011) Group X secreted phospholipase A2 pro-enzyme is matured by a furin-like proprotein convertase and releases arachidonic acid inside of human HEK 293 cells. J. Biol. Chem. 286, 36509–36521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shridas P., Bailey W. M., Boyanovsky B. B., Oslund R. C., Gelb M. H., Webb N. R. (2010) Group X secretory phospholipase A2 regulates the expression of steroidogenic acute regulatory protein (StAR) in mouse adrenal glands. J. Biol. Chem. 285, 20031–20039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wooton-Kee C. R., Boyanovsky B. B., Nasser M. S., de Villiers W. J., Webb N. R. (2004) Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation. Arterioscler. Thromb. Vasc. Biol. 24, 762–767 [DOI] [PubMed] [Google Scholar]
- 15. Artenstein A. W., Opal S. M. (2011) Proprotein convertases in health and disease. N. Engl. J. Med. 365, 2507–2518 [DOI] [PubMed] [Google Scholar]
- 16. Cummins C. L., Volle D. H., Zhang Y., McDonald J. G., Sion B., Lefrançois-Martinez A. M., Caira F., Veyssière G., Mangelsdorf D. J., Lobaccaro J. M. (2006) Liver X receptors regulate adrenal cholesterol balance. J. Clin. Invest. 116, 1902–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parker K. L., Chaplin D. D., Wong M., Seidman J. G., Smith J. A., Schimmer B. P. (1985) Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells. Proc. Natl. Acad. Sci. U.S.A. 82, 7860–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Remacle A. G., Shiryaev S. A., Oh E. S., Cieplak P., Srinivasan A., Wei G., Liddington R. C., Ratnikov B. I., Parent A., Desjardins R., Day R., Smith J. W., Lebl M., Strongin A. Y. (2008) Substrate cleavage analysis of furin and related proprotein convertases. A comparative study. J. Biol. Chem. 283, 20897–20906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roebroek A. J., Taylor N. A., Louagie E., Pauli I., Smeijers L., Snellinx A., Lauwers A., Van de Ven W. J., Hartmann D., Creemers J. W. (2004) Limited redundancy of the proprotein convertase furin in mouse liver. J. Biol. Chem. 279, 53442–53450 [DOI] [PubMed] [Google Scholar]
- 20. Mesnard D., Donnison M., Fuerer C., Pfeffer P. L., Constam D. B. (2011) The microenvironment patterns the pluripotent mouse epiblast through paracrine Furin and Pace4 proteolytic activities. Genes Dev. 25, 1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blanchet M. H., Le Good J. A., Mesnard D., Oorschot V., Baflast S., Minchiotti G., Klumperman J., Constam D. B. (2008) Cripto recruits Furin and PACE4 and controls Nodal trafficking during proteolytic maturation. EMBO J. 27, 2580–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guillemot J., Canuel M., Essalmani R., Prat A., Seidah N. G. (2013) Implication of the proprotein convertases in iron homeostasis: proprotein convertase 7 sheds human transferrin receptor 1 and furin activates hepcidin. Hepatology 57, 2514–2524 [DOI] [PubMed] [Google Scholar]
- 23. Susan-Resiga D., Essalmani R., Hamelin J., Asselin M. C., Benjannet S., Chamberland A., Day R., Szumska D., Constam D., Bhattacharya S., Prat A., Seidah N. G. (2011) Furin is the major processing enzyme of the cardiac-specific growth factor bone morphogenetic protein 10. J. Biol. Chem. 286, 22785–22794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang P., Tortorella M., England K., Malfait A. M., Thomas G., Arner E. C., Pei D. (2004) Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (Aggrecanase-1) in the trans-Golgi network. J. Biol. Chem. 279, 15434–15440 [DOI] [PubMed] [Google Scholar]
- 25. Cherradi N., Capponi A. M., Gaillard R. C., Pralong F. P. (2001) Decreased expression of steroidogenic acute regulatory protein: a novel mechanism participating in the leptin-induced inhibition of glucocorticoid biosynthesis. Endocrinology 142, 3302–3308 [DOI] [PubMed] [Google Scholar]
- 26. Fon W. P., Li P. H. (2007) Dexamethasone-induced suppression of steroidogenic acute regulatory protein gene expression in mouse Y-1 adrenocortical cells is associated with reduced histone H3 acetylation. Endocrine 32, 155–165 [DOI] [PubMed] [Google Scholar]
- 27. Yokota Y., Notoya M., Higashino K., Ishimoto Y., Nakano K., Arita H., Hanasaki K. (2001) Clearance of group X secretory phospholipase A(2) via mouse phospholipase A(2) receptor. FEBS Lett. 509, 250–254 [DOI] [PubMed] [Google Scholar]
- 28. Tamaru S., Mishina H., Watanabe Y., Watanabe K., Fujioka D., Takahashi S., Suzuki K., Nakamura T., Obata J. E., Kawabata K., Yokota Y., Murakami M., Hanasaki K., Kugiyama K. (2013) Deficiency of phospholipase A2 receptor exacerbates ovalbumin-induced lung inflammation. J. Immunol. 191, 1021–1028 [DOI] [PubMed] [Google Scholar]
- 29. Higashino Ki K., Yokota Y., Ono T., Kamitani S., Arita H., Hanasaki K. (2002) Identification of a soluble form phospholipase A2 receptor as a circulating endogenous inhibitor for secretory phospholipase A2. J. Biol. Chem. 277, 13583–13588 [DOI] [PubMed] [Google Scholar]
- 30. Hallstrand T. S., Lai Y., Altemeier W. A., Appel C. L., Johnson B., Frevert C. W., Hudkins K. L., Bollinger J. G., Woodruff P. G., Hyde D. M., Henderson W. R., Jr., Gelb M. H. (2013) Regulation and function of epithelial secreted phospholipase A2 group X in asthma. Am. J. Respir. Crit. Care Med. 188, 42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujioka D., Saito Y., Kobayashi T., Yano T., Tezuka H., Ishimoto Y., Suzuki N., Yokota Y., Nakamura T., Obata J. E., Kanazawa M., Kawabata K., Hanasaki K., Kugiyama K. (2008) Reduction in myocardial ischemia/reperfusion injury in group X secretory phospholipase A2-deficient mice. Circulation 117, 2977–2985 [DOI] [PubMed] [Google Scholar]
- 32. Karabina S. A., Brochériou I., Le Naour G., Agrapart M., Durand H., Gelb M., Lambeau G., Ninio E. (2006) Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB J. 20, 2547–2549 [DOI] [PubMed] [Google Scholar]
- 33. Shridas P., Bailey W. M., Talbott K. R., Oslund R. C., Gelb M. H., Webb N. R. (2011) Group X secretory phospholipase A2 enhances TLR4 signaling in macrophages. J. Immunol. 187, 482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]