Background: Macrophages become resistant to apoptosis in response to pathogenic cues.
Results: Tissue-type plasminogen activator (tPA) protects the classically activated macrophages from apoptosis.
Conclusion: tPA is an endogenous factor that promotes macrophage survival.
Significance: tPA modulates inflammation by promoting the survival of polarized macrophages through a new signaling cascade.
Keywords: Apoptosis, Cell Signaling, Macrophage, Serine Protease, Tissue Plasminogen Activator (tPA)
Abstract
Macrophage accumulation is one of the hallmarks of progressive kidney disease. Resting macrophages have a finite lifespan, but become resistant to apoptosis in response to pathogenic cues, whereas the underlying mechanism remains unknown. Tissue-type plasminogen activator (tPA), a protease up-regulated in the kidneys with chronic injury, has been shown to promote macrophage accumulation and renal inflammation. We hypothesized that tPA may be the endogenous factor that promotes macrophage survival and extends their lifespan that leads to their accumulation in the injured kidneys. We examined the role of tPA in macrophage survival, and found that tPA protected macrophages from both staurosporine and H2O2-induced apoptosis. tPA promoted the survival of both resting and lipopolysaccharide- or interferon-γ-induced M1 macrophages, but failed to do so in the interleukin 4 (IL4)-induced M2 macrophages. In the kidneys with unilateral ureteral obstruction, there were significantly more apoptotic M1 macrophages in tPA-deficient mice than their wild-type counterparts, and obstruction-induced M1 macrophages accumulation and M1 chemokine expression were markedly reduced in these knock-out mice. The cytoprotective effect of tPA required its receptor, LDL receptor-related protein-1 (LRP-1). tPA induced the phosphorylation of Erk1/2, p90 ribosomal S6 kinase (RSK), and p38 in a temporal order. The tPA-mediated macrophage survival was eliminated by PD98059, BI-D1870, or sc68376, the specific inhibitors for Erk1/2, p90RSK, or p38, respectively. Thus, it is clear that tPA promoted M1 macrophage survival through its receptor LRP-1-mediated novel signaling cascade involving Erk1/2, p90RSK, and p38, which leads to the accumulation of these cells in the injured kidneys.
Introduction
Macrophage accumulation is one of the histological hallmarks of most interstitial and glomerular kidney diseases (1, 2). Sustained macrophage accumulation in the damaged kidneys eventually becomes pathological, resulting in irreversible fibrosis, tissue destruction, and progressive chronic kidney disease (CKD)2 (1). The number of renal macrophages is finely regulated by the balance among the expansion through proliferation, clearance by apoptotic death, and the recruitment of circulating monocytes (1, 3, 4). Resting macrophages have a finite lifespan and presumably undergo apoptotic death locally (1). Intriguingly, differentiated macrophages in response to pathogenic cues display an extended lifespan and are resistant to apoptosis (5, 6), leading to the accumulation of these cells at the sites of injury. However, the underlying mechanisms remain largely unknown.
In disease conditions, macrophages are differentiated into two broad but distinct subsets that are categorized as either classically activated (M1) or alternatively activated (M2) (1). During classical activation, M1 macrophages express a panoply of proinflammatory genes to promote inflammation and damage through a combination of transcription factors, including NF-κB, and mitogen-activated protein kinases (MAPKs) (7). In contrast, M2 macrophages, differentiated from alternative activation, help to resolve inflammation and promote tissue remodeling.
tPA, a member of the serine protease family, has been shown to act as a profibrotic cytokine to promote the progression of CKD by triggering profound receptor-mediated intracellular signaling events including MAPKs (8–15). Increasing evidence indicates that tPA modulates inflammation in various disease models (14–20). Our recent work demonstrated that tPA promotes macrophage accumulation and renal inflammation, and induces NF-κB-dependent chemokine expression in a CKD model (14, 19). Notably, increased macrophage accumulation in the obstructed kidneys is accompanied by the concomitant up-regulation of tPA and its receptor LRP-1 (10, 11), suggesting that tPA signaling may play an important role in the modulation of the fate and polarity of the differentiated macrophages during chronic kidney disease.
In the present study, we investigated the role of tPA in macrophage survival and elucidated the underlying signaling mechanisms. Our data demonstrated that tPA differentially promotes M1 macrophage accumulation in the obstructed kidneys by inducing their survival through a novel LRP-1-mediated signaling cascade of Erk1/2, p90 ribosomal S6 kinase (RSK), and p38.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
The anti-phospho- and total-Erk, p90RSK, p38 antibodies, as well as the cleaved caspase 3 antibody, were purchased from Cell Signaling Technology (Beverly, MA). Mouse α-tubulin antibody, staurosporine, and lipopolysaccharide (LPS) were obtained from Sigma. Anti-CD 11b and anti-F4/80 antibodies, MIP-1α and IP-10 ELISA kits, and mouse recombinant IL-4 were purchased from eBioscience (San Diego, CA). Anti-Ym1 antibody was from Stemcell Technologies (Vancouver, BC, Canada). Mouse recombinant IFN-γ was supplied by Peprotech (Rocky Hill, NJ). Mouse monoclonal anti-LRP-1 (11H4) antibody was prepared as previously described (11, 12). The secondary HRP-conjugated antibodies, fetal bovine serum (FBS), and supplements were obtained from Fisher Scientific. The non-enzymatic tPA was supplied by Molecular Innovations Inc. (Southfield, MI). The recombinant human single-chain wild-type tPA was purchased from American Diagnostica Inc. (Stamford, CT). Dulbecco's modified Eagle's medium (DMEM) was obtained from American Type Culture Collection (ATCC, Manassas, VA). All other chemicals of analytic grade were obtained from Sigma or Fisher Scientific unless otherwise indicated.
Cell Culture
Mouse macrophages J774.A1 were purchased from ATCC and maintained as previously described (14). Primary bone marrow-derived macrophages were prepared and maintained as previously reported (19). After a 24-h serum-free starvation, the macrophages were treated with vehicle or non-enzymatic or wild-type tPA for various periods of time as indicated and then collected for different assays.
Animal Model
Homozygous tPA knock-out (KO) and wild-type (WT) mice on C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained as previously described (8, 10, 12, 14). Macrophage-specific LRP-1 KO mice (LysMCre+LRPflox/flox) were generated by cross-breeding LRP-1 floxed mice on C57BL/6 background (provided by Drs. Wendy M. Mars and Joachim Herz) with macrophage-specific LysMCre C57BL/6 mice (Jackson Laboratory) (21–24). The animal protocol was approved by the Institutional Animal Care and Use Committee at the Penn State University College of Medicine. Unilateral ureteral obstruction (UUO) was performed in 20–22 g male mice (3–5 mice per group) using established procedures (8, 10, 12, 14).
Flow Cytometry
Single-cell suspensions from the whole kidneys were prepared as preciously described (14, 19). The cells were stained with antibodies against CD45, CD11b, F4/80, CD206, TNFα, arginase 1, IL-1β, and cleaved caspase-3, followed by flow cytometry analysis using LSR II SORP machine (BD Biosciences, San Diego, CA) and FlowJo 7.6.1 software (Tree Star Inc., Ashland, OR).
Western Blot Analysis
Samples were prepared and separated on 10% SDS-polyacrylamide gels as previously described (8, 10–12, 14), followed by protein transfer to a PVDF membrane and incubation with various primary antibodies and HRP-conjugated secondary antibodies. Signals were visualized by a Chemiluminescent Substrate kit (Thermo Fisher Scientific).
Quantitative RT-PCR
Total RNA was extracted and reverse transcribed into cDNA and amplified using the SYBR Green PCR kit (Qiagen, Valencia, CA) as previously described (10, 14). The sequence of the primers was reported elsewhere (25, 26). Relative level of mRNAs was quantified and normalized to β-actin.
Statistical Analysis
All experimental data were presented as mean ± S.E. Statistical analysis of the data were performed using SigmaStat software (Jandel Scientific Software). Comparison between multiple groups was performed using one-way analysis of variance followed by Student-Newman-Keuls test or Student's t test between two groups. A p value of less than 0.05 was considered statistically significant.
RESULTS
tPA Promotes M1 Macrophages Accumulation in the Obstructed Kidneys
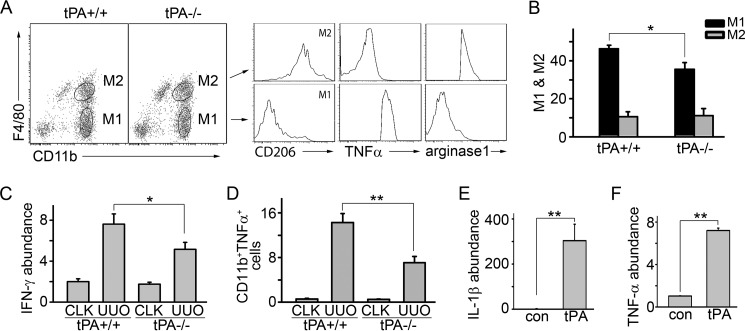
Our previous work showed that macrophage accumulation in obstruction-induced fibrotic kidneys was alleviated in tPA-deficient mice (14, 19). We further examined the macrophage polarity in the obstructed kidneys from WT and tPA KO mice. UUO was performed for 7 days in these mice. Flow cytometry analyses showed that WT mice had a significantly higher number of CD11b+F4/80loCD206− M1 macrophages (27, 28), which were further validated as TNFα+arginase-1− cells, than tPA KO mice (Fig. 1, A and B). However, there was no statistical significance in the numbers of M2 (CD11b+F4/80hiCD206+) macrophages (27, 28), which were also arginase-1+TNFα−, between tPA KO mice and WT mice. We found that tPA also induces the in vivo mRNA expression of IFN-γ (Fig. 1C), as well as the in vitro expression of IL-1β (Fig. 1E) and TNF-α (Fig. 1F) in macrophages. Flow cytometry data further confirmed that there were more TNFα-expressing CD11b-positive macrophages in the obstructed kidneys from WT mice than from tPA KO mice (Fig. 1D), suggesting that tPA promotes M1 chemokine expression both in vivo and in vitro. This result is also consistent with our previous work that tPA promotes NF-κB-dependent M1 chemokine expression, such as IP-10 and MIP-1α (14). Thus, tPA preferably promotes M1 macrophage accumulation at UUO 7 days.
FIGURE 1.
tPA promotes M1 macrophage accumulation and induces M1 chemokine expression. A, representative figure showing flow cytometry analyses. Cells in the ovals were M1 or M2 macrophages as indicated. UUO was performed in tPA WT and KO mice (n = 5 mice per group) for 7 days. Single cell suspensions were prepared from whole kidneys and subjected to flow cytometry analyses to identify M1 or M2 macrophages. B, quantitative illustration of the ratio of M1 or M2 macrophages in the gated cells. *, p < 0.05, n = 5 mice per group. Quantitative IFN-γ mRNA expression (C) and flow cytometry analysis of TNFα-expressing CD11b+ macrophages (D) in WT and tPA KO mice with UUO 7 days. *, p < 0.05; **, p < 0.01, n = 5 mice per group. CLK, contralateral control kidneys. J774 macrophages were treated with 10 nm non-enzymatic tPA for 6 h, followed by quantitative RT-PCR for IL-1β (E) and TNF-α (F) abundance. **, p < 0.01, n = 3.
tPA Promotes the Survival of M1 Macrophages but Not M2 Macrophages
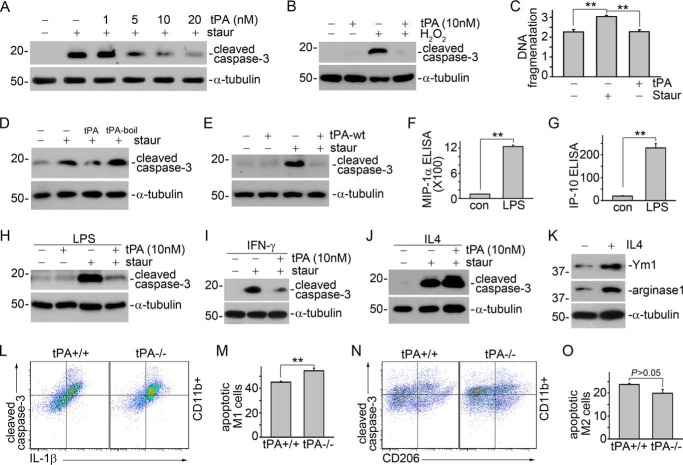
We examined the role of tPA in cell survival in the resting macrophages. J774 macrophages were treated with 100 nm staurosporine with or without tPA for 4 h, followed by Western blot for cleaved caspase-3 or DNA fragmentation assay, the two classical markers of apoptosis (8). We found that tPA dose-dependently protected J774 cells from staurosporine-induced apoptosis (Fig. 2A). tPA also protected J774 macrophages against apoptosis triggered by H2O2 (Fig. 2B), another typical apoptotic inducer (8). A DNA fragmentation assay further validated the anti-apoptotic effect of tPA in macrophages (Fig. 2C). Both non-enzymatic (Fig. 2, A–C) and wild-type tPA (Fig. 2E) had similar effects on macrophage survival suggesting that the anti-apoptotic effect of tPA is independent of its protease activities. The cytoprotective effect of tPA was also operative in the LPS, a well known M1 stimulator (29–31), converted M1 or M(LPS) macrophages (32), as indicated by the reduction of staurosporine-induced cleaved caspase-3 after tPA treatment (Fig. 2H). M(LPS) phenotype was confirmed by the induction of M1 chemokine expression in J774 macrophages (Fig. 2, F and G). The interference of possible endotoxin contamination in tPA solution was excluded because the heat-inactivated tPA had little effect on macrophage apoptosis (Fig. 2D). We further found that tPA also protected IFN-γ-induced M1 or M(IFN-γ) macrophages from staurosporine-induced apoptosis (Fig. 2I). However, tPA had little effect on the survival of IL4-induced M2 or M(IL4) macrophages (29–31) (Fig. 2J). The M(IL4) phenotype was confirmed by induction of Ym1 and arginase-1 expression in these cells (Fig. 2K). To examine the in vivo relevance of tPA effects, CD11b-positive macrophages in the whole obstructed kidneys from tPA WT and KO mice were sorted by flow cytometry, and the apoptotic M1 (cleaved caspase-3+IL1β+) and M2 (cleaved caspase-3+CD206+) macrophages were further quantified. We found that there were more apoptotic M1 macrophages in the obstructed kidneys from tPA KO mice than from WT mice (Fig. 2, L and M). In contrast, tPA WT mice appeared to have more apoptotic M2 macrophages than KO mice, although the difference did not reach statistical significance (Fig. 2, N and O). Thus, it is clear that tPA differentially promotes the survival of M1, which contributes to the accumulation of these cells in the fibrotic kidneys.
FIGURE 2.
tPA protects M1 macrophage against apoptosis. A, J774 macrophages were treated with 100 nm staurosporine with or without tPA (1, 5, 10, and 20 nm) for 4 h, followed by Western blot for cleaved caspase-3 and α-tubulin. B, J774 cells were treated with 500 μm H2O2 plus 10 nm tPA or vehicle for 16 h, followed by Western blot for cleaved caspase-3 and α-tubulin. C, J774 cells were treated with 100 nm staurosporine with or without 10 nm tPA for 4 h, followed by DNA fragmentation assay. **, p < 0.01, n = 3. D, J774 cells were treated with 100 nm staurosporine with or without 10 nm heat-inactivated tPA (tPA-boil) for 4 h, followed by Western blot for cleaved caspase-3 and α-tubulin. E, J774 cells were treated with 100 nm staurosporine with or without 10 nm wild-type tPA (tPA-wt) for 4 h, followed by Western blot for cleaved caspase-3 and α-tubulin. The supernatants of J774 treated with 1 μg/ml of LPS overnight were subjected to ELISA for MIP-1α (F) and IP-10 (G). **, p < 0.01, n = 5–6. J774 cells were treated with 1 μg/ml of LPS (H), 10 ng/ml of IFN-γ (I), or 100 ng/ml of IL-4 (J) overnight, followed by incubation with 100 nm staurosporine with or without 10 nm tPA for 4 h. Apoptosis was assessed by Western blot for cleaved caspase-3. K, Western blot for Ym1, arginase-1, and α-tubulin in J774 cells treated with 100 ng/ml of IL-4 overnight. Single cell suspensions prepared from whole obstructed kidneys from tPA WT and KO mice were subjected to flow cytometry analyses to quantify cleaved caspse-3-positive IL-1β-expressing (L and M) or CD206-expressing (N and O) CD11b+ macrophages. L and N, representative pictures of flow cytometry analyses. M and O, quantitative illustrations. **, p < 0.01, n = 4 mice per group.
tPA Activates a Sequential Survival Signaling of Erk1/2, p90RSK, and p38
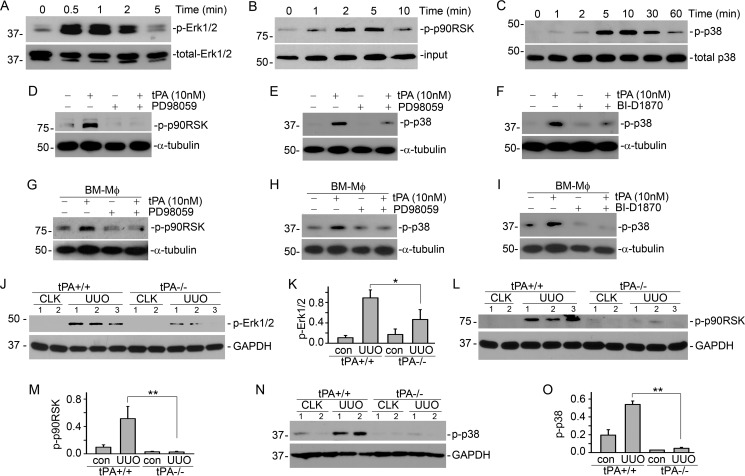
J774 macrophages were treated with 10 nm non-enzymatic tPA for various time periods, followed by Western blot for phospho-Erk1/2, p90RSK, and p38. It was found that tPA induced the phosphorylation of Erk1/2 as early as 0.5 min (Fig. 3A), followed by phosphorylation of p90RSK from 1 to 10 min (Fig. 3B). Blockade of Erk1/2 signaling by its specific inhibitor PD98059 abrogated tPA-induced p90RSK phosphorylation (Fig. 3D). Thus, p90RSK acted as the downstream effector of Erk1/2 in the tPA-mediated survival signaling cascade, which is consistent with our previous finding in fibroblasts (8). Notably, tPA also activated p38 MAPK from 5 to 30 min (Fig. 3C). The temporal order of tPA-induced phosphorylation of Erk1/2, p90RSK, and p38 MAPK suggested that p38 is the downstream mediator of the Erk1/2/p90RSK pathway. This was confirmed by the inhibition experiments that both PD98059 (Fig. 3E) and BI-D1870 (Fig. 3F), a specific p90RSK inhibitor (33–35), suppressed tPA-induced p38 phosphorylation in J774 macrophages. Of note, tPA-activated sequential order of the survival signal events were also reproducible in the primary bone marrow macrophages extracted from WT mice (Fig. 3, G–I).
FIGURE 3.
tPA activates sequential survival signaling of Erk1/2, p90RSK, and p38. J774 macrophages were treated with 10 nm tPA for the indicated time periods, followed by Western blot for phospho- and total Erk1/2 (A), phospho-p90RSK (B), and phospho- and total p38 (C). J774 macrophages (D–F) or primary bone marrow macrophages from WT mice (G–I) were treated with 20 μm PD98059 (D, E, G, and H) or 125 nm BI-D1870 (F and I), followed by incubation with 10 nm tPA for 5 or 10 min. D and G, Western blots for phospho-p90RSK and α-tubulin. E, F, H, and I, Western blots for phospho-p38 and α-tubulin. Kidney homogenates from WT and tPA KO mice with UUO for 7 days were subjected to Western blot for phospho-specific Erk1/2 (J and K), p90RSK (L and M), p38 (N and O), and GAPDH. J, L, and N, representative Western blots. K, M, and O, quantitative illustrations. *, p < 0.05; **, p < 0.01, n = 5 mice per group. CLK, control kidneys. The number indicates the individual mouse.
To determine whether tPA promotes survival signaling of Erk1/2, p90RSK, and p38 pathway in vivo, UUO was performed in WT and tPA KO mice for 7 days, followed by Western blot for phospho-Erk1/2, p90RSK, and p38. As shown in Fig. 3, J–O, phosphorylation of Erk1/2, p90RSK, and p38 were induced in the WT mice after UUO; but tPA KO mice displayed dramatically decreased Erk1/2, p90RSK, or p38 activation. Thus, tPA also activates the above survival signaling cascade involving Erk1/2, p90RSK, and p38 in vivo.
LRP-1 Mediates the Anti-apoptosis Effect of tPA in Macrophages
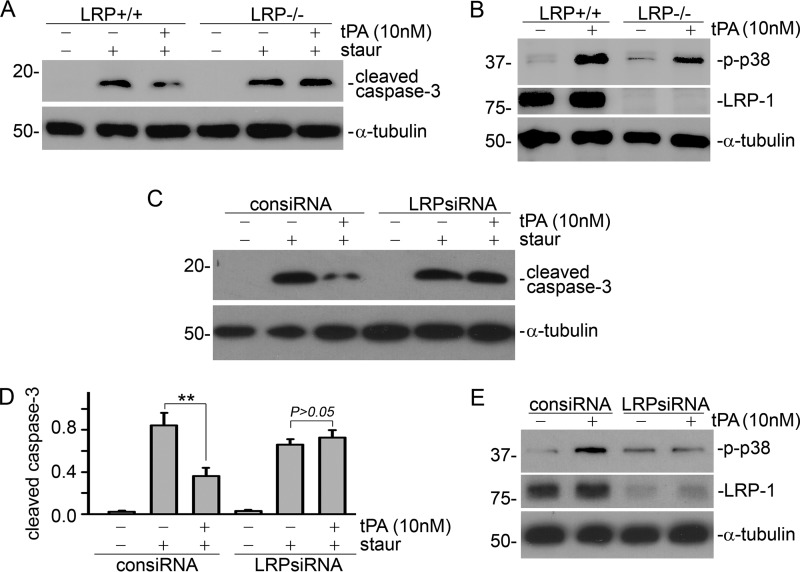
Our previous work demonstrated that tPA receptor LRP-1 transduces tPA-induced Erk1/2/p90RSK signaling and mediates its cytoprotective effect in fibroblasts (8, 20). We further examined the role of LRP-1 in macrophage survival. Bone marrow-derived macrophages were extracted from macrophage-specific LRP-1 KO mice (LysMCre+LRPflox/flox) (21–24) and their littermate controls (LysMCre−LRPflox/flox), followed by treatment with staurosporine (100 nm) with or without 10 nm tPA for 4 h. As shown in Fig. 4, A and B, tPA activated the survival of p38 signaling (Fig. 4B) and protected against staurosporine-induced apoptosis (Fig. 4A) in the LRP-1+/+ macrophages, but failed to do so in the LRP-1−/− macrophages. We also knocked down the expression of LRP-1 in J774 macrophages using siRNA interference, and found that transfection of LRP-1 siRNA abrogated tPA-mediated suppression of caspase-3 cleavage (Fig. 4, C and D) and p38 phosphorylation (Fig. 4E). Thus, LRP-1 mediated the cytoprotective effects of tPA in macrophages.
FIGURE 4.
LRP-1 mediates tPA-induced macrophage survival. Bone marrow-derived macrophages were extracted from the macrophage-specific LRP-1 KO mice (LysMCre+LRPflox/flox) and their littermate controls (LysMCre−LRPflox/flox). A, LRP-1+/+ and LRP-1−/− macrophages were treated with 100 nm staurosporine with or without 10 nm tPA for 4 h, followed by Western blot for cleaved caspase-3 and α-tubulin. B, LRP-1+/+ and LRP-1−/− macrophages were treated with 10 nm tPA for 10 min, followed by Western blot for phospho-p38 and α-tubulin. LRP-1 deficiency was validated by Western blot for LRP-1 (B). J774 macrophages were transfected with control siRNA or LRP-1 siRNA, followed by treatments with 100 nm staurosporine in the presence of 10 nm tPA or vehicle for 4 h (C and D) or 10 nm tPA for 10 min (E). Cell lysates were subjected to Western blot for cleaved caspase-3 (C) or phospho-p38 (E). D, quantitative analysis of the relative cleaved caspase-3 abundance. **, p < 0.01, n = 3.
Erk1/2, p90RSK, and p38 Signaling Is Indispensable to tPA-induced Macrophage Survival
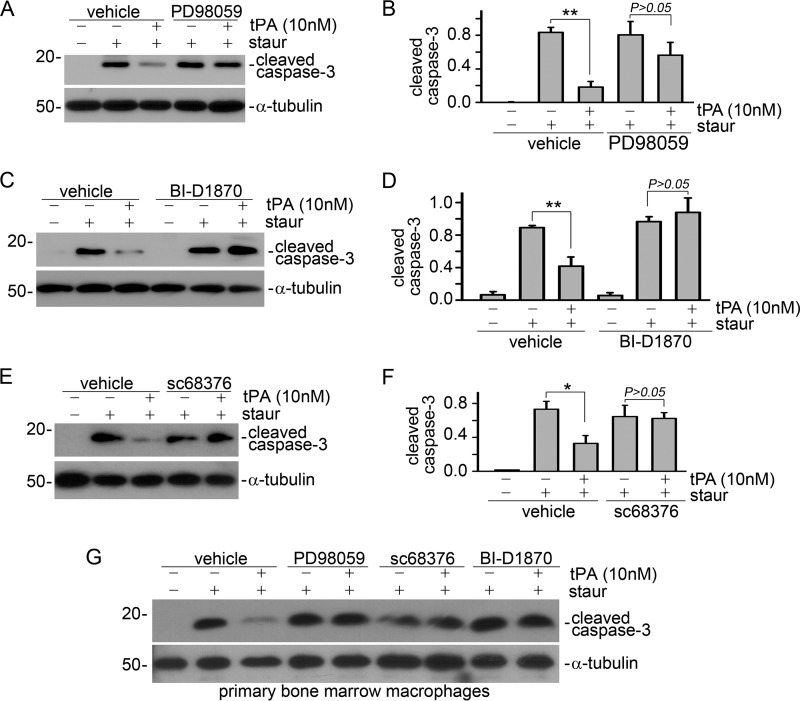
Next, we examined the role of Erk1/2, p90RSK, and p38 in tPA-induced macrophage survival. J774 macrophages were treated with various inhibitors including PD98059 (20 μm), BI-D1870 (125 nm), and sc68376 (20 μm) for 1 h, followed by incubation of 100 nm staurosporine with or without 10 nm tPA for an additional 4 h. Apoptosis was evaluated by Western blot for cleaved caspase-3. It was found that these specific kinase inhibitors restored the tPA-suppressed protein level of cleaved caspase-3 (Fig. 5, A–F). Of note, the roles of Erk1/2, p90RSK, and p38 in tPA-mediated macrophage survival were also validated in primary bone marrow macrophages derived from WT mice (Fig. 5G). Thus, it is clear that tPA promotes macrophage survival through the LRP-1-mediated Erk1/2, p90RSK, and p38 pathways.
FIGURE 5.
tPA promotes macrophage survival through the Erk1/2, p90RSK, and p38 pathways. J774 macrophages (A–F) or primary bone marrow macrophages (G) were treated with 20 μm PD98059 (A, B, and G), 125 nm BI-D1870 (C, D, and G), or 20 μm sc68376 (E, F, and G) for 1 h, followed by incubation with 100 nm staurosporine plus vehicle or 10 nm tPA for an additional 4 h. Cell apoptosis was evaluated by Western blot for cleaved caspase-3. A, C, E, and G, representative Western blots. B, D, and F, quantitative illustrations. *, p < 0.05; **, p < 0.01, n = 3.
DISCUSSION
Interstitial macrophage accumulation is one of the histological characteristics of both acute kidney injury and CKD. In response to persistent inflammatory injury, macrophages start to accumulate within tissue and organs through enhanced infiltration, augmented proliferation, or decreased apoptosis (1, 3, 4). tPA has been shown to modulate macrophage accumulation in various organs (14–19). In an acute brain injury model, tPA was shown to mediate F4/80 macrophage accumulation and activation in the ischemic brain (16). It was also found that tPA promotes the accumulation of macrophages or other leukocytes in both models of acute (18) and chronic kidney injury (14, 19). Intriguingly, the increased macrophage accumulation in the above models is also accompanied by the concomitant induction of tPA (10, 11, 36, 37), suggesting that tPA may be a common endogenous factor that modulates macrophage functions and inflammatory response in multiple organ systems.
In response to injury, macrophages differentiate into two distinct subsets through classical activation (M1) or alternative activation (M2) (1). Generally, M1 macrophages, stimulated by LPS and IFN-γ, promote inflammation and exaggerate damage, whereas M2 macrophages, stimulated by IL-4/IL-13, help to resolve inflammation and promote tissue remodeling (1, 2). Although we have shown that accumulation of CD11b macrophages, as well as fibroblast activation and matrix deposition, are attenuated in the obstruction-induced fibrotic kidneys from tPA-deficient mice (12, 14, 19), it is not clear whether tPA differentially promotes the infiltration and accumulation of one subset of macrophages over the other. Based on our previous observation that tPA activates NF-κB and induces the expression of M1-like chemokines in J774 macrophages (14), we hypothesized that tPA might preferably induce M1 macrophage accumulation and promote renal inflammation. As shown in Fig. 1, A–D, obstruction-induced accumulation of M1 macrophages was attenuated in fibrotic kidneys from tPA-deficient mice, accompanied by the according alteration of M1 chemokines such as IFN-γ and TNFα. tPA also induced M1 chemokine IL-1β and TNF-α expression in vitro (Fig. 1, E and F), suggesting that tPA promotes the M1 phenotype of macrophages. In contrast, tPA had little effect on the numbers of renal M2 macrophages in either WT or tPA KO mice (Fig. 1B). Therefore, our data clearly supported our hypothesis.
Although the fate of differentiated macrophages has not been fully examined, recruited macrophages within individual organs usually stay local and are cleared through apoptosis (1). In response to pathogenic cues, differentiated macrophages at the sites of injury display a prolonged lifespan and are resistant to apoptotic death (5, 6). It is possible that tPA, concomitantly induced in the injured organs (10, 11, 36, 37), is an endogenous factor regulating this process. We examined the role of tPA in the survival of both resting and differentiated macrophages. We found that tPA not only protected resting macrophages from both staurosporine- and H2O2-induced apoptosis (Fig. 2, A–C), but also promoted the survival of LPS and IFN-γ-stimulated M1 macrophages (Fig. 2, F–I). However, tPA failed to protect the IL4-induced M2 macrophage against apoptosis (Fig. 2, J and K). To further clarify the effects of tPA on the fate of M1 and M2 macrophages in vivo, we analyzed and quantified the apoptotic M1 and M2 macrophages in the kidneys from WT and tPA KO mice using flow cytometry, and found that tPA KO mice had significantly more apoptotic M1 macrophages than WT mice (Fig. 2, L and M). In contrast, both WT and tPA KO mice had similar numbers of apoptotic M2 macrophages (Fig. 2, N and O). Thus, it is clear that tPA preferably promotes the survival of M1 macrophages, which together with our recent finding that tPA promotes macrophage migration into diseased kidneys (19) eventually leads to the accumulation of these cells during chronic renal injury.
The cytoprotective effect of tPA was mediated through its receptor LRP-1 (Fig. 4). tPA activated a series of intracellular signaling events that promotes macrophage survival. As shown in Fig. 3, tPA induced the phosphorylation of Erk1/2, p90RSK, and p38 in a temporal order. We further confirmed that p90RSK acted as the downstream mediator of Erk1/2, but functioned as the upstream kinase of the p38 pathway by employing various specific kinase inhibitors (Fig. 3, D–I). The 90-kDa ribosomal S6 kinases (RSKs) are a group of serine/threonine kinases that regulate diverse cellular processes including cell survival (35, 38). There are 4 RSK isoforms (RSK1–4), of which RSK1, the predominant isoform in the kidney, is also designated as p90RSK. RSKs play an important role in the Ras-MAPK signaling cascade and are the direct downstream effectors of the Ras-Erk1/2 signaling. Erk1/2 activation directly phosphorylates and activates RSKs (35, 38, 39), which, in turn, activate various signaling events through selection of different phosphorylation substrates including GSK3β, Bad, and IκB (35, 38). In contrast to our previous finding that Bad acts as a downstream substrate of p90RSK in mediating tPA-induced fibroblast survival (8), our present study indicates that p90RSK-mediated tPA-induced macrophage survival through the p38 pathway (Figs. 3 and 5). Thus far, there are three known MAPK members: Erk1/2, the c-Jun N-terminal kinases (JNKs), and the p38 (40). It is known that p38 functions in parallel to Erk1/2 MAPK, and usually promotes apoptosis (41). However, our current results indicate that p38 acts as a downstream effector of Erk1/2 and p90RSK signaling to promote the survival of macrophages. Although the exact mechanism remains unknown, it is possible that p38 or its upstream MAPK kinases are among one of the expanding list of p90RSK substrates. Our current finding is in line with a previous report that p38 can be activated by Erk1/2 and acts as its downstream effector to modulate cell senescence (42). Because p90RSK can activate numerous signaling cascades through phosphorylating a wide range of substrates, our present study indicates that p90RSK may play an important role in the cross-talk between different MAPKs, as well as MAPKs and other signaling pathways.
As summarized in Fig. 6, the present study has established that tPA promotes M1 macrophage accumulation in the diseased kidneys through a novel receptor LRP-1-mediated signaling cascade involving Erk1/2, p90RSK, and p38 MAPK. Blockade of each step within this survival signaling eliminates tPA-induced cytoprotection of macrophages.
FIGURE 6.

Schematic illustration of tPA-induced survival signaling. tPA binds to receptor LRP-1 and induces sequential phosphorylation of Erk1/2, p90RSK, and p38, which in turn promotes the survival of M1 macrophages. Knock-out or siRNA knockdown of LRP-1 or pretreatment with the respective inhibitor of Erk1/2, p90RSK, or p38 blocks the cytoprotective effects of tPA.
Acknowledgments
We thank Drs. Wendy M. Mars and Joachim Herz for providing the necessary LRP-1 floxed mice.
This work was supported, in whole or in part, by National Institutes of Health Grant DK102624, American Heart Association Grants 14GRNT20380289 and 10SDG3900029, Barsumian Trust Grant 157904, and the Kidney Foundation of Central Pennsylvania (to K. H.).
- CKD
- chronic kidney disease
- LRP-1
- LDL receptor-related protein-1
- NF-κB
- nuclear factor-κB
- p90RSK
- p90 ribosomal S6 kinase
- tPA
- tissue-type plasminogen activator
- UUO
- unilateral ureteral obstruction.
REFERENCES
- 1. Ricardo S. D., van Goor H., Eddy A. A. (2008) Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y., Harris D. C. (2011) Macrophages in renal disease. J. Am. Soc. Nephrol. 22, 21–27 [DOI] [PubMed] [Google Scholar]
- 3. Zhang M. Z., Yao B., Yang S., Jiang L., Wang S., Fan X., Yin H., Wong K., Miyazawa T., Chen J., Chang I., Singh A., Harris R. C. (2012) CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Invest. 122, 4519–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 5. Steinberg B. E., Grinstein S. (2008) Pathogen destruction versus intracellular survival: the role of lipids as phagosomal fate determinants. J. Clin. Invest. 118, 2002–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marriott H. M., Bingle C. D., Read R. C., Braley K. E., Kroemer G., Hellewell P. G., Craig R. W., Whyte M. K., Dockrell D. H. (2005) Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J. Clin. Invest. 115, 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu K., Lin L., Tan X., Yang J., Bu G., Mars W. M., Liu Y. (2008) tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis. J. Am. Soc. Nephrol. 19, 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu K., Mars W. M., Liu Y. (2008) Novel actions of tissue-type plasminogen activator in chronic kidney disease. Front. Biosci. 13, 5174–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu K., Wu C., Mars W. M., Liu Y. (2007) Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J. Clin. Invest. 117, 3821–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu K., Yang J., Tanaka S., Gonias S. L., Mars W. M., Liu Y. (2006) Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J. Biol. Chem. 281, 2120–2127 [DOI] [PubMed] [Google Scholar]
- 12. Lin L., Bu G., Mars W. M., Reeves W. B., Tanaka S., Hu K. (2010) tPA activates LDL receptor-related protein 1-mediated mitogenic signaling involving the p90RSK and GSK3β pathway. Am. J. Pathol. 177, 1687–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi Y., Mantuano E., Inoue G., Campana W. M., Gonias S. L. (2009) Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci. Signal. 2, ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin L., Wu C., Hu K. (2012) Tissue plasminogen activator activates NF-κB through a pathway involving annexin A2/CD11b and integrin-linked kinase. J. Am. Soc. Nephrol. 23, 1329–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin L., Hu K. (2014) Tissue plasminogen activator and inflammation: from phenotype to signaling mechanisms. Am. J. Clin. Exp. Immunol. 3, 30–36 [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang C., An J., Strickland D. K., Yepes M. (2009) The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am. J. Pathol. 174, 586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higazi A. A., El-Haj M., Melhem A., Horani A., Pappo O., Alvarez C. E., Muhanna N., Friedman S. L., Safadi R. (2008) Immunomodulatory effects of plasminogen activators on hepatic fibrogenesis. Clin. Exp. Immunol. 152, 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roelofs J. J., Rouschop K. M., Leemans J. C., Claessen N., de Boer A. M., Frederiks W. M., Lijnen H. R., Weening J. J., Florquin S. (2006) Tissue-type plasminogen activator modulates inflammatory responses and renal function in ischemia reperfusion injury. J. Am. Soc. Nephrol. 17, 131–140 [DOI] [PubMed] [Google Scholar]
- 19. Lin L., Jin Y., Mars W. M., Reeves W. B., Hu K. (2014) Myeloid-derived tissue-type plasminogen activator promotes macrophage motility through FAK, Rac1, and NF-κB pathways. Am. J. Pathol. 184, 2757–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin L., Hu K. (2014) LRP-1: functions, signaling and implications in kidney and other diseases. Int. J. Mol. Sci. 15, 22887–22901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rohlmann A., Gotthardt M., Willnow T. E., Hammer R. E., Herz J. (1996) Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat. Biotechnol. 14, 1562–1565 [DOI] [PubMed] [Google Scholar]
- 22. Cao C., Lawrence D. A., Li Y., Von Arnim C. A., Herz J., Su E. J., Makarova A., Hyman B. T., Strickland D. K., Zhang L. (2006) Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 25, 1860–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu L., Boesten L. S., May P., Herz J., Bovenschen N., Huisman M. V., Berbée J. F., Havekes L. M., van Vlijmen B. J., Tamsma J. T. (2006) Macrophage low-density lipoprotein receptor-related protein deficiency enhances atherosclerosis in ApoE/LDLR double knockout mice. Arterioscler. Thromb. Vasc. Biol. 26, 2710–2715 [DOI] [PubMed] [Google Scholar]
- 24. Overton C. D., Yancey P. G., Major A. S., Linton M. F., Fazio S. (2007) Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ. Res. 100, 670–677 [DOI] [PubMed] [Google Scholar]
- 25. Lange-Sperandio B., Trautmann A., Eickelberg O., Jayachandran A., Oberle S., Schmidutz F., Rodenbeck B., Hömme M., Horuk R., Schaefer F. (2007) Leukocytes induce epithelial to mesenchymal transition after unilateral ureteral obstruction in neonatal mice. Am. J. Pathol. 171, 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang B., Ramesh G., Uematsu S., Akira S., Reeves W. B. (2008) TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J. Am. Soc. Nephrol. 19, 923–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujiu K., Manabe I., Nagai R. (2011) Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J. Clin. Invest. 121, 3425–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwata Y., Boström E. A., Menke J., Rabacal W. A., Morel L., Wada T., Kelley V. R. (2012) Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J. Immunol. 188, 4568–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez F. O., Sica A., Mantovani A., Locati M. (2008) Macrophage activation and polarization. Front. Biosci. 13, 453–461 [DOI] [PubMed] [Google Scholar]
- 30. Sica A., Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Locati M., Mantovani A., Sica A. (2013) Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 120, 163–184 [DOI] [PubMed] [Google Scholar]
- 32. Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., Natoli G., Saeij J. P., Schultze J. L., Shirey K. A., Sica A., Suttles J., Udalova I., van Ginderachter J. A., Vogel S. N., Wynn T. A. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sapkota G. P., Cummings L., Newell F. S., Armstrong C., Bain J., Frodin M., Grauert M., Hoffmann M., Schnapp G., Steegmaier M., Cohen P., Alessi D. R. (2007) BI-D1870 is a specific inhibitor of the p90RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem. J. 401, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen T. L. (2008) Targeting RSK: an overview of small molecule inhibitors. Anticancer Agents Med. Chem. 8, 710–716 [DOI] [PubMed] [Google Scholar]
- 35. Romeo Y., Zhang X., Roux P. P. (2012) Regulation and function of the RSK family of protein kinases. Biochem. J. 441, 553–569 [DOI] [PubMed] [Google Scholar]
- 36. Yepes M., Sandkvist M., Moore E. G., Bugge T. H., Strickland D. K., Lawrence D. A. (2003) Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 112, 1533–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y. F., Tsirka S. E., Strickland S., Stieg P. E., Soriano S. G., Lipton S. A. (1998) Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat. Med. 4, 228–231 [DOI] [PubMed] [Google Scholar]
- 38. Anjum R., Blenis J. (2008) The RSK family of kinases: emerging roles in cellular signalling. Nat. Rev. Mol. Cell Biol. 9, 747–758 [DOI] [PubMed] [Google Scholar]
- 39. Carriere A., Ray H., Blenis J., Roux P. P. (2008) The RSK factors of activating the Ras/MAPK signaling cascade. Front. Biosci. 13, 4258–4275 [DOI] [PubMed] [Google Scholar]
- 40. Raman M., Chen W., Cobb M. H. (2007) Differential regulation and properties of MAPKs. Oncogene 26, 3100–3112 [DOI] [PubMed] [Google Scholar]
- 41. Thornton T. M., Rincon M. (2009) Non-classical p38 MAP kinase functions: cell cycle checkpoints and survival. Int. J. Biol. Sci. 5, 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang W., Chen J. X., Liao R., Deng Q., Zhou J. J., Huang S., Sun P. (2002) Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell. Biol. 22, 3389–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]