Background: Although nitric oxide inhibits β-cell function, the impact of superoxide on nitric oxide signaling remains unknown.
Results: Superoxide produced within β-cells inhibits nitric oxide-dependent responses, whereas extracellular generation does not.
Conclusion: The reaction of nitric oxide with superoxide regulates the β-cell response to nitric oxide.
Significance: The location of radical generation dictates the functional response of β-cells to reactive species.
Keywords: Beta Cell (B-cell), Cytokine, Diabetes, Nitric Oxide, Oxidative Stress, Peroxynitrite, Type 1 Diabetes
Abstract
Cytokines impair the function and decrease the viability of insulin-producing β-cells by a pathway that requires the expression of inducible nitric oxide synthase (iNOS) and generation of high levels of nitric oxide. In addition to nitric oxide, excessive formation of reactive oxygen species, such as superoxide and hydrogen peroxide, has been shown to cause β-cell damage. Although the reaction of nitric oxide with superoxide results in the formation of peroxynitrite, we have shown that β-cells do not have the capacity to produce this powerful oxidant in response to cytokines. When β-cells are forced to generate peroxynitrite using nitric oxide donors and superoxide-generating redox cycling agents, superoxide scavenges nitric oxide and prevents the inhibitory and destructive actions of nitric oxide on mitochondrial oxidative metabolism and β-cell viability. In this study, we show that the β-cell response to nitric oxide is regulated by the location of superoxide generation. Nitric oxide freely diffuses through cell membranes, and it reacts with superoxide produced within cells and in the extracellular space, generating peroxynitrite. However, only when it is produced within cells does superoxide attenuate nitric oxide-induced mitochondrial dysfunction, gene expression, and toxicity. These findings suggest that the location of radical generation and the site of radical reactions are key determinants in the functional response of β-cells to reactive oxygen species and reactive nitrogen species. Although nitric oxide is freely diffusible, its biological function can be controlled by the local generation of superoxide, such that when this reaction occurs within β-cells, superoxide protects β-cells by scavenging nitric oxide.
Introduction
Type 1 diabetes is characterized by the selective destruction of insulin-producing β-cells. Inflammatory cytokines, such as IL-1, released from infiltrating macrophages and leukocytes have been shown to induce β-cell damage and are believed to participate in the loss of β-cell mass during diabetes development (1). The mechanisms responsible for mediating cytokine toxicity involve the formation of micromolar levels of nitric oxide by the inducible nitric oxide synthase (iNOS3 or NOS2) using arginine as a substrate. Nitric oxide inhibits mitochondrial oxidative metabolism, causing a decrease in the synthesis of ATP, and this is responsible for the inhibitory actions of cytokines on insulin secretion (2–4). Inhibitory actions of cytokines on mitochondrial oxidation and insulin secretion can be prevented with NOS inhibitors (5). Also, islets isolated from iNOS-deficient animals are resistant to cytokine-induced toxicity (6). In addition to nitric oxide, reactive oxygen species (ROS) have been suggested to contribute to the destruction of pancreatic islets in type 1 diabetes (7–9). ROS can be formed enzymatically by NADPH oxidases (10), by uncoupled endothelial NOS (11), and by respiratory chain complexes due to electron leaks (12). When produced at physiological levels, ROS, such as superoxide anion and hydrogen peroxide, can function as signaling intermediates in response to growth factor stimulation as well as cytokine exposure and have been reported to regulate insulin secretion and insulin action (13). In contrast, excessive formation of ROS has been proposed to contribute to the destruction of pancreatic β-cells during the development of type 1 diabetes (14, 15). It is well known that ROS and RNS interact. One example is the diffusion-controlled reaction of superoxide with nitric oxide forming peroxynitrite (16), and studies have reported that this powerful oxidant may participate in the pathogenesis of diabetes (17–19).
Pancreatic β-cells are often described as vulnerable to oxidant-induced damage due to low levels of catalase and glutathione peroxidase, enzymes that detoxify hydrogen peroxide (20–22). However, these cells express detectable levels of superoxide dismutase and several other antioxidant enzymes including thioredoxin, thioredoxin reductase, glutathione reductase, glutaredoxins, and peroxiredoxins (23, 24). The differential expression of various antioxidant genes suggests that β-cells may be acutely sensitive to some oxidants but resistant to others depending on the chemical nature of the specific reactive species. Here, we examined the sensitivity of pancreatic β-cells to various forms of ROS and RNS and show, surprisingly, that β-cells are resistant to peroxynitrite-induced damage. ROS and RNS modify β-cell function and viability, and both forms of reactive species are capable of differentially activating signaling cascades; however, the fate of the β-cell is controlled by the location in which ROS or RNS are generated. We report here that the extracellular production of superoxide does not modify the response of β-cells to nitric oxide. In contrast, when produced within β-cells, superoxide functions to scavenge nitric oxide and thereby attenuates nitric oxide-dependent responses of β-cells.
EXPERIMENTAL PROCEDURES
Materials
RPMI 1640 tissue culture medium was purchased from Gibco, and FBS was from HyClone (Logan, UT). DPTA/NO and SIN-1 were obtained from Cayman Chemical (Ann Arbor, MI). All other chemicals were of analytical grade and were purchased from Sigma-Aldrich.
Cell Culture
INS832/13 cells were cultured in RPMI supplemented with 10% FBS, 2 mm glutamine, 1 mm sodium pyruvate, 10 mm HEPES, 50 μg/ml β-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin (complete medium). Cells were maintained at 37 °C under an atmosphere of 95% air and 5% CO2. For real-time monitoring of peroxynitrite, β-cells were washed to remove the tissue culture media and then cultured in Dulbecco's phosphate-buffered saline containing glucose (5.5 mm) and pyruvate (0.33 mm).
ROS and RNS Treatments
Insulinoma cells were exposed to reactive species in complete RPMI 1640 tissue culture medium. The nitric oxide donor DPTA/NO has a half-life of 3 h under cell culture conditions (37 °C, pH 7.4) and liberates two molecules of nitric oxide per molecule of parent compound. Administration of 500 μm DPTA/NO results in the flux of nitric oxide of ∼3.8 μm/min. SIN-1 generates equimolar amounts of superoxide and nitric oxide that rapidly react to form peroxynitrite. The calculated maximal flux of peroxynitrite is ∼1.8 μm/min for 250 μm SIN-1 under the cell culture conditions used in this study. Menadione is a quinone that produces superoxide by intracellular redox cycling using the cytosolic enzymes NAD(P)H quinone oxidoreductase and NADPH-cytochrome P450 reductase (25). We have demonstrated previously that the addition of menadione results in intracellular superoxide generation in pancreatic β-cells (26).
Viability
Cell viability was measured using the neutral red dye uptake assay as described previously (27, 28). Briefly, INS832/13 cells were cultured on 96-well plates in 100 μl of complete medium. After the treatment, the medium was replaced with complete culture medium containing 40 μg/ml neutral red, and the cells were incubated at 37 °C for 1 h. The medium was then removed, and cells were fixed with formaldehyde (1%, (v/v) in 1% CaCl2). Neutral red was extracted from the cells in 100 μl of 50% ethanol containing 1% acetic acid (v/v). The absorbance was read at 540 nm, and viability was calculated based on the uptake of neutral red by untreated cells.
Nucleotide Measurements
HPLC was used to quantify the cellular levels of ATP and NAD+ as reported previously (29, 30). Nucleotides were extracted by perchloric acid precipitation (31), and solvent A (75 μl; 0.1 m potassium phosphate and 4 mm tetrabutylammonium bisulfate (pH 6.0), diluted 64:36 in water (v/v)) was added to supernatants. Precipitated protein was isolated by centrifugation and solubilized in 0.5 n NaOH (200 μl), and protein concentrations were determined using the Bradford assay (32). HPLC analysis of nucleotides was performed on a Kinetex C18 column (2.6 μm, 100 × 4.6-mm internal diameter) following previously published methods (29). ATP and NAD+ peaks were measured for each sample and expressed in nanomoles per milligram of protein.
Quantitative Real-time PCR
RNA was isolated from whole cell lysates using the RNeasy kit (Qiagen, Valencia, CA). Subsequent first-strand cDNA synthesis was performed using oligo(dT)12–18 primers and the reverse transcriptase SuperScript preamplification system (Life Technologies) according to the manufacturer's instructions. Quantitative real-time PCR was performed using SsoFast EvaGreen supermix with the CFX96 real-time system (Bio-Rad). Values were normalized to GAPDH, and -fold changes were calculated by the ΔΔCt method. The following primer sequences were used: GADD45α, 5′-GTGTGCTGGTGACGAACCCACAT-3′ (forward), 5′-CCGTTCGGGGAATCACCGTCCG-3′ (reverse); PUMA, 5′-CAGGGGCAGGCAAGGGAAGC-3′ (forward), 5′-GAAGCCGCACTGGGGACACC-3′ (reverse); and GAPDH 5′-TCGGTGTGAACGGATTTGGCCG-3′ (forward), 5′TGAAGGGGTCGTTGATGGCAACA-3′ (reverse). Primers were purchased from Integrated DNA Technologies (Coralville, IA).
Aconitase Activity
Mitochondrial aconitase activity in INS832/13 cells was determined using a coupled assay with isocitrate dehydrogenase as described previously (33).
Western Blot Analysis
Equal amounts of protein from cell lysates were resolved by reducing SDS-PAGE and transferred to nitrocellulose membranes. Proteins were detected using primary antibodies at a 1:1000 dilution: phospho-AMPK (Thr-172), phospho-acetyl-CoA carboxylase (phospho-ACC), phospho-eIF2α, phospho-GSK3β (Cell Signaling), phospho-ERK (anti-active MAPK polyclonal antibody), phospho-p38 (Promega, Madison, WI), PAR (Trevigen), γH2AX (Millipore), and GAPDH (Ambion), and detection was performed by enhanced chemiluminescence (34) using species-specific HRP-conjugated goat anti-mouse (1:10,000 dilution) or goat-anti-rabbit (1:7000) secondary antibodies.
Peroxynitrite Measurements
The formation of peroxynitrite was monitored in real time using the selective fluorescent probe, coumarin-7-boronate (10 μm). Immediately after the addition of the probe, the formation of peroxynitrite was monitored by fluorescence (excitation, 332 nm; emission, 450 nm) for up to 4 h at 37 °C as described previously (35).
Statistical Analysis
Statistical comparisons were made between groups using one-way analysis of variance with Tukey post hoc test. The minimum level of significance was set at p < 0.05.
RESULTS
Differential Sensitivity of β-Cells to ROS and RNS
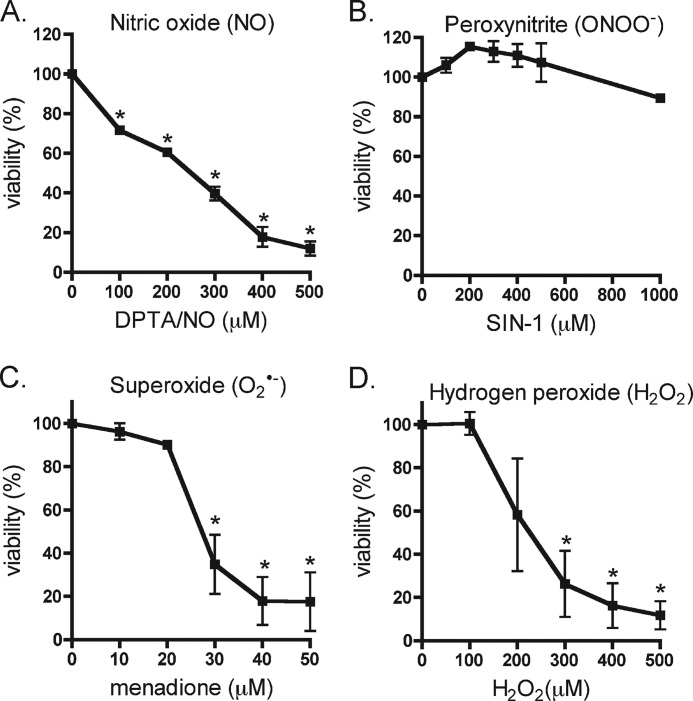
The effects of ROS (superoxide and hydrogen peroxide) and RNS (nitric oxide and peroxynitrite), on INS832/13 cell viability was examined following a 4-h incubation in the presence of the indicated concentrations of donors of each reactive species or redox cycling agent (Fig. 1). In a concentration-dependent fashion, the nitric oxide donor DPTA/NO decreases INS832/13 cell viability with half-maximal death observed at ∼200 μm (Fig. 1A). In contrast, the peroxynitrite donor SIN-1 does not impact β-cell viability when added at concentrations up to 1 mm (Fig. 1B). Superoxide formation in response to treatment with the redox cycling agent menadione does not modify cell viability at concentrations up to 20 μm; however, a sharp decrease in viability (70%) was induced in INS832/13 cells at 30 μm (Fig. 1C). Similar results were obtained with superoxide generated by a second redox cycling agent 2,3-dimethoxy-1,4-naphthoquinone (data not shown). As reported previously (26), hydrogen peroxide is highly toxic to β-cells at concentrations above 100 μm (Fig. 1D). These findings indicate that β-cells are acutely sensitive to nitric oxide, superoxide, and hydrogen peroxide, but are not sensitive to peroxynitrite.
FIGURE 1.
Sensitivity of β-cells to ROS- and RNS-induced death. A–D, INS832/13 cells were exposed to increasing concentrations of the nitric oxide donor DPTA/NO (A), the peroxynitrite donor SIN-1 (B), the superoxide generator menadione (C), and H2O2 (D) for 4 h. Cell viability was determined using the neutral red assay. The results are the averages ± S.D. of three independent experiments. Statistically significant differences in viability were made in comparison with untreated control cells and are indicated (*, p < 0.05).
Activation of Signaling Pathways in Response to ROS and RNS
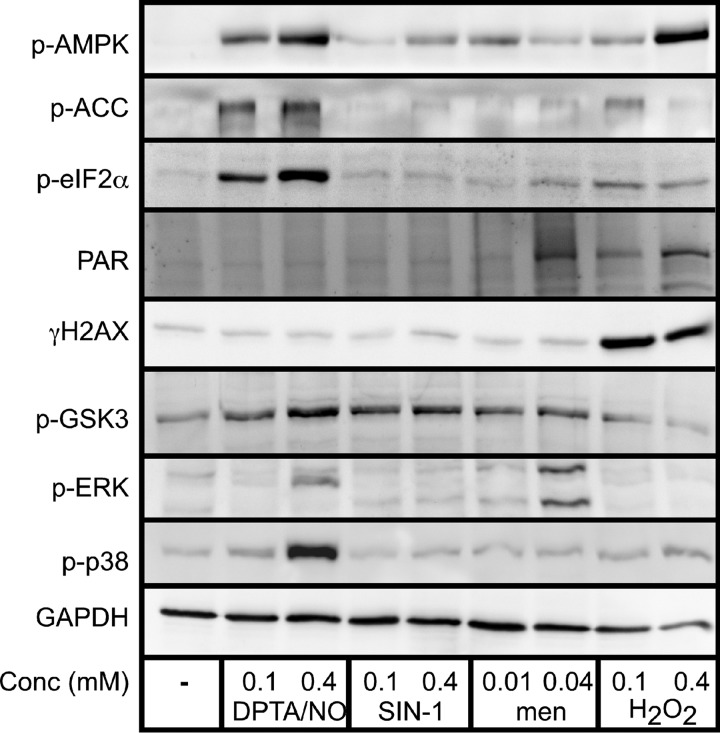
Because β-cells display differential sensitivity to reactive oxygen and nitrogen species, the signaling cascades activated in response to these oxidants were examined. INS832/13 cells were exposed to the nitric oxide donor DPTA/NO, peroxynitrite donor SIN-1, superoxide donor menadione, and hydrogen peroxide for 30 min, and the activation of signaling pathways that are known to be involved in the response of β-cells to cytokines was evaluated (Fig. 2). Nitric oxide and hydrogen peroxide activate AMPK as evidenced by enhanced phosphorylation of AMPK and its substrate acetyl-CoA carboxylase. At higher concentrations of hydrogen peroxide (400 μm), the phosphorylation of acetyl-CoA carboxylase is diminished as compared with the levels observed at 100 μm. This effect is most likely due to the high level of cell death observed at this concentration of hydrogen peroxide (>80%, Fig. 1D). Consistent with previous studies (36), AMPK activation in response to hydrogen peroxide is associated with the activation of PARP-1 as demonstrated by the accumulation of poly(ADP-ribose) (PAR) (37). Also, hydrogen peroxide induces the rapid formation of γH2AX (the phosphorylated form of H2AX) indicative of DNA double-strand breaks and activation of the DNA damage response (38). Nitric oxide and peroxynitrite donors do not stimulate PAR accumulation or γH2AX formation within 30 min of treatment, indicating that AMPK activation in response to nitric oxide is triggered by mechanisms that differ from the processes induced by hydrogen peroxide. Exposure of β-cells to a high concentration of menadione (40 μm) also results in PAR formation. Consistent with many studies (39–41), nitric oxide stimulates the phosphorylation of eukaryotic initiation factor 2α (eIF2α), an indicator of endoplasmic reticulum stress induction. GSK3 is the only cellular target, among those tested, that is phosphorylated in response to nitric oxide, peroxynitrite, and superoxide. Although GSK3 can be phosphorylated during unfolded protein response activation (42, 43), we show that it is also phosphorylated in response to superoxide and peroxynitrite, reactive species that do not appear to activate the unfolded protein response (absence of eIF2α phosphorylation). This finding suggests that superoxide and peroxynitrite activate GSK3 through pathways independent of the unfolded protein response. Consistent with this interpretation, superoxide (44, 45) and peroxynitrite (46) have been shown to induce GSK3 phosphorylation through a PI3K/Akt-dependent pathway. MAPK are differentially activated by ROS and RNS. Nitric oxide stimulates p38 and ERK phosphorylation, and this activation is concentration-related as phosphorylation of these MAPK only occurs at higher concentrations of DPTA/NO (400 μm). Superoxide also stimulates ERK phosphorylation at high concentrations, but it does not stimulate p38 phosphorylation. These findings indicate that the activation of signaling pathways is dependent on the type and concentration of reactive species. Nitric oxide stimulates the unfolded protein response, MAPK, and AMPK activation, whereas hydrogen peroxide only activates AMPK, and this is due to the induction of DNA double-strand breaks (γH2AX formation) and the overactivation of PARP (PAR formation), leading to ATP depletion (47, 48). Superoxide can dismutate to hydrogen peroxide spontaneously or in a reaction catalyzed by superoxide dismutase; however, we show that pathways activated by menadione (superoxide treatment) are selective and do not overlap with those activated by hydrogen peroxide. Overall, these findings indicate that the individual forms of ROS and RNS leave unique footprints of signaling pathway activation in β-cells.
FIGURE 2.
The activation of signaling pathways by ROS and RNS. INS832/13 cells were treated with DPTA/NO (100 and 400 μm), SIN-1 (100 and 400 μm), menadione (men, 10 and 40 μm), and hydrogen peroxide (100 and 400 μm) for 30 min. The cells were harvested, and the activation of selective signaling pathways was examined by Western blot analysis using antibodies specific for the indicated proteins. Results are representative of three independent experiments. Protein targets include the phosphorylated (P) forms of AMPK, acetyl-CoA carboxylase (ACC), eIF2α, H2AX, GSK-3, ERK, and p38. Also shown are PAR and GAPDH. Conc, concentration.
ROS and RNS Effects on Metabolic Function
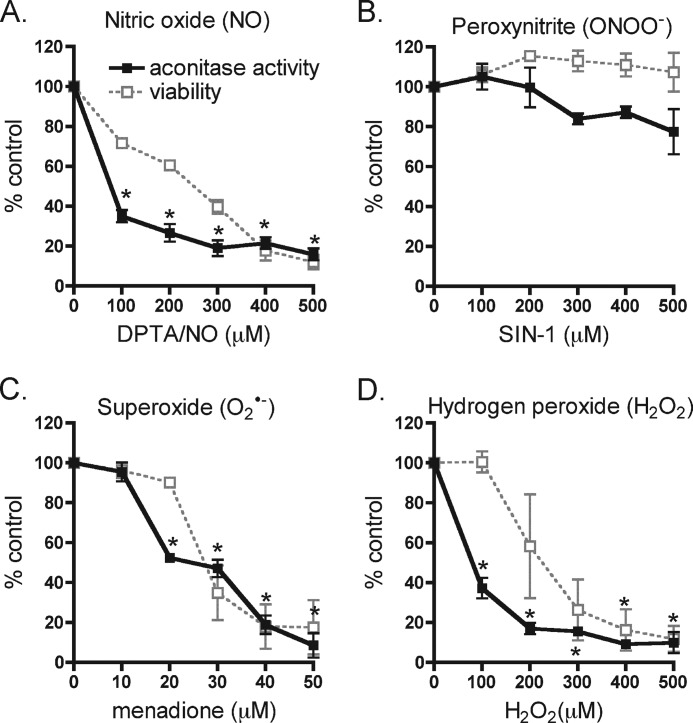
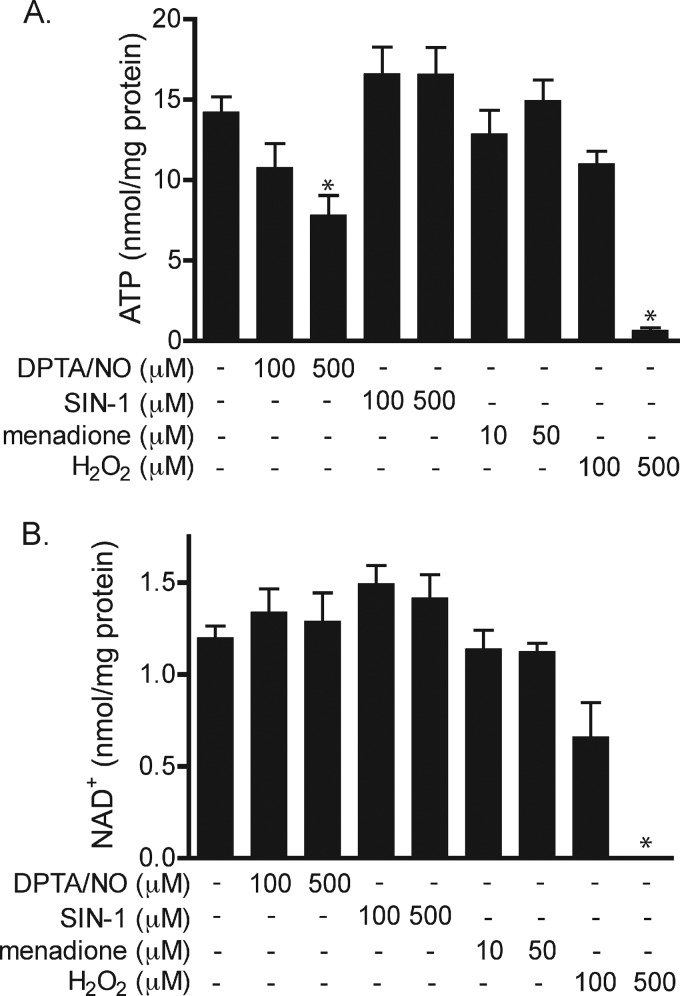
Because the inhibitory actions of cytokines on β-cell function and viability are associated with decreases in the activity of the Krebs cycle enzyme aconitase, the effects of ROS and RNS on aconitase activity were evaluated. Nitric oxide provided by DPTA/NO (100 μm) treatment results in ∼60% reduction in the activity of INS832/13 cell mitochondrial aconitase, and greater than 80% inhibition at 400 μm donor (Fig. 3A). In agreement with the lack of an inhibitory effect on cell viability, the peroxynitrite donor SIN-1 does not significantly decrease aconitase activity at concentrations up to 500 μm (Fig. 3B). In contrast, superoxide inhibits aconitase activity in a concentration-dependent manner, with half-maximal inhibition at 25 μm. Hydrogen peroxide at 100 μm inhibits aconitase activity by ∼60% (Fig. 3, C and D). At this concentration, hydrogen peroxide does not decrease INS832/13 cell viability, suggesting that mitochondrial aconitase in β-cells is sensitive to hydrogen peroxide at concentrations lower than the levels needed to reduce cell viability. To determine whether ROS and RNS inhibition of mitochondrial aconitase activity correlates with decreases in the generation of high energy intermediates, ATP levels were determined (Fig. 4A). DPTA/NO decreases cellular ATP levels in a concentration-related fashion that correlates with the concentration-dependent inhibition of aconitase activity and loss in INS832/13 cell viability in response to this nitric oxide donor. This inhibition of mitochondrial aconitase activity and decreased levels of ATP also correlate with the enhanced phosphorylation of AMPK (Fig. 2). Peroxynitrite generation by SIN-1 increases steady-state levels of ATP; however, this effect does not achieve statistical significance. It is somewhat surprising that exposure to superoxide does not modify ATP levels in INS832/13 cells as 50 μm menadione inhibits aconitase and decreases the viability of INS832/13 cells. Like nitric oxide, hydrogen peroxide causes a reduction in ATP levels with almost complete depletion at 500 μm. Because both nitric oxide and hydrogen peroxide decrease ATP levels and stimulate AMPK phosphorylation (Fig. 2), the effects of nitric oxide and hydrogen peroxide on INS832/13 cell NAD+ levels were determined by HPLC (Fig. 4B). Consistent with the overactivation of PARP-1, hydrogen peroxide was the only species of ROS and RNS to decrease steady-state levels of NAD+ in INS832/13 cells. In response to 100 μm hydrogen peroxide, there is a 50% decrease in NAD+ levels and a complete depletion of NAD+ following a 1-h culture at 500 μm. These observations are consistent with PARP-1 overactivation in response to hydrogen peroxide (Fig. 2). When hyperactivated, PARP-1 catalyzes the cycling of NAD+-dependent ribosylation of proteins, including itself, and deribosylation, resulting in the depletion of NAD+ and ATP (47, 48). These findings suggest that the decrease in β-cell ATP levels in response to nitric oxide is the result of an inhibition of mitochondrial function, whereas hydrogen peroxide-dependent loss of ATP results from excessive PARP-1 activation.
FIGURE 3.
Inhibition of mitochondrial aconitase activity by ROS and RNS. A–D, INS832/13 cells were treated with the indicated concentrations of the nitric oxide donor DPTA/NO (A), peroxynitrite donor SIN-1 (B), superoxide generator menadione (C), and H2O2 (D) for 1 h, and mitochondrial aconitase activity was measured. The results are the averages ± S.D. of three independent experiments. Viability data from Fig. 1 were plotted together with aconitase activity to show the concordance between these two parameters. Statistically significant changes from the untreated control are indicated (*, p < 0.05).
FIGURE 4.
The effects of ROS and RNS on β-cell ATP and NAD+ levels. A and B, INS832/13 cells were treated with DPTA/NO, SIN-1, menadione, and H2O2 for 1 h, and ATP (A) and NAD+ (B) levels were measured by HPLC and normalized to total protein. Values represent means ± S.D. of three independent experiments. Statistically significant changes as compared with the untreated control are indicated (*, p < 0.05).
Overactivation of PARP-1 Selectively Contributes to Hydrogen Peroxide Toxicity
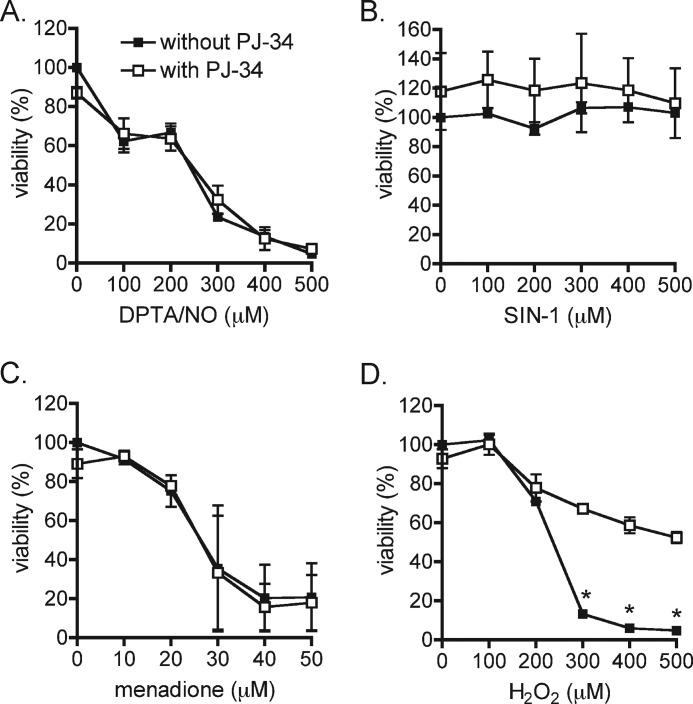
The selective PARP-1 inhibitor, PJ-34, was used to evaluate the role of PARP-1 in the loss of INS832/13 cell viability in response to ROS and RNS treatment. PJ-34 attenuates hydrogen peroxide-mediated killing of INS832/13 cells (Fig. 5D); however, this PARP-1 inhibitor does not modify the toxicity of nitric oxide, superoxide, or peroxynitrite on the insulinoma cells. Consistent with protection against the loss of viability, PJ-34 attenuates the depletion of ATP and NAD+ in INS832/13 cells treated with 500 μm hydrogen peroxide, preserving 90% of the cellular ATP pool (12.8 ± 0.6 nmol ATP/mg of protein, p < 0.01) and 66% of the cellular NAD+ pool (0.79 ± 0.01 nmol NAD+/mg of protein, p < 0.01). Although the dismutation of superoxide results in the production of hydrogen peroxide, PJ-34 does not modify the effects of menadione on INS832/13 cell viability. These findings indicate that the cytotoxic effects of hydrogen peroxide on β-cells are partially regulated by the overactivation of PARP-1 and the depletion of cellular levels of ATP and NAD+. The toxicity of superoxide does not appear to be due to the dismutation to hydrogen peroxide as PARP-1 inhibitors do not influence the levels of INS832/13 cell death in response to menadione. Like superoxide, nitric oxide-mediated toxicity is not associated with PARP-1 overactivation.
FIGURE 5.
PARP-1 inhibition prevents hydrogen peroxide-dependent β-cell death. A–D, INS832/13 cells were pretreated without (closed symbols) or with (open symbols) PJ-34 (5 μm) for 30 min and exposed to DPTA/NO (A), SIN-1 (B), menadione (C), and hydrogen peroxide (D) in the absence or presence of PJ-34 for 4 h. Cell viability was determined using the neutral red assay. The results are the average ± S.D. of three independent experiments with statistically significant difference between samples treated with and without PJ-34 as indicated (*, p < 0.05).
The Effects of the Location of Superoxide Generation on Nitric Oxide-dependent Toxicity in β-Cells
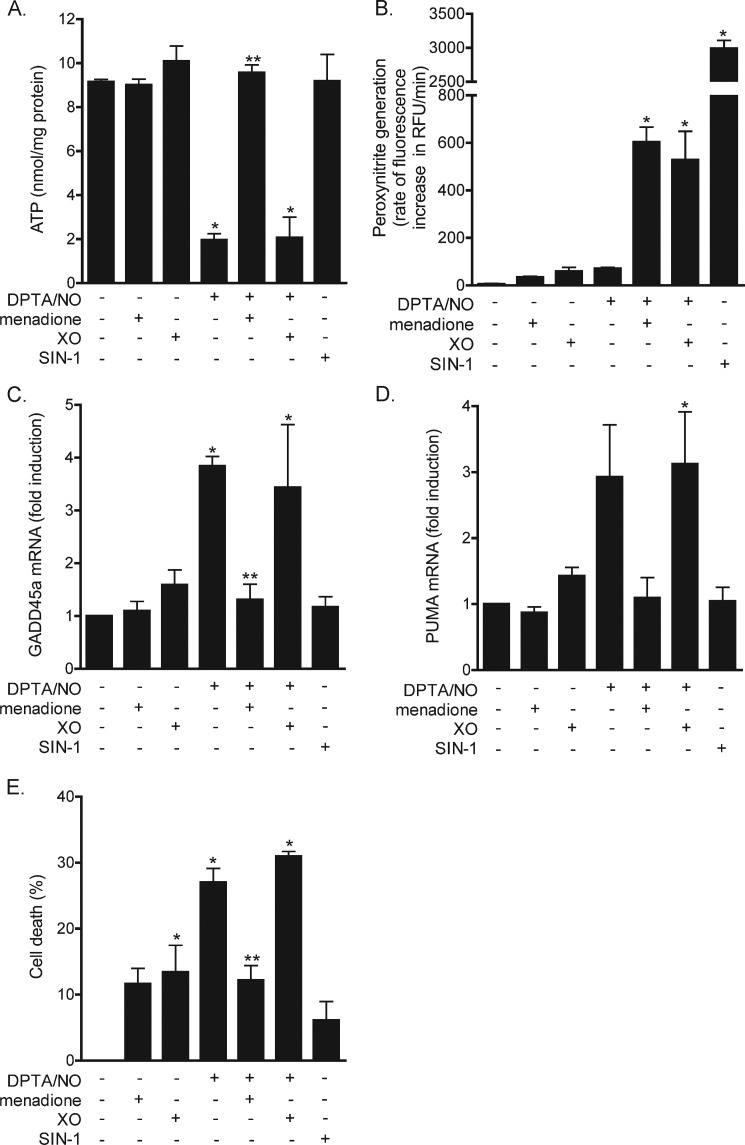
In response to cytokine treatment, β-cells produce micromolar levels of nitric oxide, but due to the inability to produce superoxide, they do not have the capacity to generate peroxynitrite (26). Further, activators of NADPH oxidase (superoxide-generating enzyme) fail to stimulate superoxide production by β-cells (26). To our surprise, when nitric oxide-producing β-cells are forced to generate superoxide (when treated with a redox cycler), superoxide scavenges nitric oxide, forming peroxynitrite, and this results in the attenuation of nitric oxide-mediated toxicity in INS832/13 cells (26). Consistent with this interpretation, peroxynitrite produced in response to SIN-1 is not toxic to β-cells. To examine whether the location of superoxide generation contributes to the response of β-cells to nitric oxide, the effects of superoxide produced within INS832/13 cells using the redox cycling agent menadione were compared with the production of superoxide outside of the INS832/13 cells using hypoxanthine and xanthine oxidase. In response to the nitric oxide donor DPTA/NO, intracellular ATP levels decrease by nearly 80% within 2 h (Fig. 6A). Superoxide produced within INS832/13 cells using menadione prevents this depletion of ATP (Fig. 6A). In contrast, the generation of superoxide in the extracellular space following treatment with xanthine/xanthine oxidase does not modify the loss of ATP in DPTA/NO-exposed INS832/13 cells. The differences in responses are not due to differences in peroxynitrite production as superoxide produced within or outside nitric oxide-treated INS832/13 cells results in the formation of similar levels of peroxynitrite (Fig. 6B). SIN-1 was used as a positive control for the detection of peroxynitrite, and concentrations of 100 μm generate peroxynitrite at rates severalfold higher than the levels produced following treatment with DPTA/NO and menadione or DPTA/NO and xanthine oxidase.
FIGURE 6.
The effects of the location of superoxide generation on its interactions with nitric oxide. A–E, INS832/13 cells were exposed to DPTA/NO (100 μm), menadione (10 μm), hypoxanthine (250 μm) + xanthine oxidase (XO, 2 milliunits/ml), alone or in combination, and to SIN-1 (100 μm). A, following a 2-h incubation, the cells were harvested, and ATP levels, normalized to total protein, were determined by HPLC. B, peroxynitrite generation was determined by the oxidation of coumarin-7-boronate. RFU, relative fluorescence units. C and D, INS832/13 cells were treated with indicated compounds for 3 h, total RNA was isolated, and the accumulation of GADD45α (C) and PUMA (D) mRNA was determined by quantitative real-time PCR. E, cell viability was determined in a parallel set of experiments in cells treated with the indicated compounds for 4 h. The results are the averages of three independent experiments ± S.D. Statistically significant changes from the untreated control are indicated by *, p < 0.05, and changes as compared with the DPTA/NO-treated cells are indicated by **, p < 0.05.
The results described above suggest that the site of superoxide production is a primary determinant in the β-cell response to nitric oxide. Specifically, when superoxide is produced inside β-cells, it attenuates nitric oxide-mediated cellular events but not when it is generated outside of β-cells. To explore this hypothesis further, we examined the effects of superoxide produced within and outside of β-cells on nitric oxide-stimulated gene expression. Consistent with previous studies (49, 50), treatment with the nitric oxide donor DPTA/NO results in ∼3–4-fold increase in GADD45α and PUMA mRNA levels (Fig. 6, C and D). Alone, intracellular (menadione) or extracellular (xanthine oxidase) superoxide production does not stimulate GADD45α and PUMA mRNA accumulation in INS832/13 cells (Fig. 6, C and D). However, in combination with nitric oxide, menadione attenuates the stimulatory actions of nitric oxide on GADD45α and PUMA mRNA accumulation. Much like the effects on cellular ATP levels, extracellular production of superoxide does not modify GADD45α and PUMA mRNA accumulation in response to DPTA/NO treatment. To confirm that the location of superoxide generation affects the biological response, the viability of nitric oxide-treated INS832/13 was examined in the presence or absence of superoxide generated within or outside of β-cells. Approximately 30% of β-cells are killed following a 4-h incubation with DPTA/NO (Fig. 6E). Alone, menadione is slightly toxic to INS832/13 cells as there is ∼10% death following a 4-h incubation. In the presence of DPTA/NO, menadione attenuates nitric oxide-mediated toxicity to levels observed with menadione alone. When superoxide is generated in the extracellular space using xanthine oxidase, there is no protection from nitric oxide toxicity. These findings are consistent with the effects of superoxide on nitric oxide-mediated decreases in INS832/13 cell ATP levels (Fig. 6A).
DISCUSSION
Cytokines released from inflammatory cells during islet inflammation have been proposed to participate in the development of autoimmune diabetes by impairing β-cell function and inducing β-cell destruction (1). The damaging actions of cytokines on β-cell function are mediated by nitric oxide, produced in micromolar quantities following the induction of iNOS in β-cells (2–4). Inhibitors of iNOS prevent the damaging actions of cytokines on insulin secretion, oxidative metabolism, protein synthesis, and DNA strand breaks, as well as the loss of β-cell viability (5, 33, 49). In addition, cytokines fail to impair β-cell function or reduce the viability of islets isolated from iNOS-deficient mice (6). Nitric oxide is known to interact with superoxide, resulting in the formation of the powerful oxidant peroxynitrite, and it has been generally assumed that this oxidant is the form of RNS that mediates cytokine-induced β-cell damage (17, 19). In addition to RNS, excessive production of ROS may contribute to the destruction of pancreatic islets during diabetes development as oxidants such as hydrogen peroxide inhibit insulin secretion by a mechanism associated with impaired oxidative metabolism and decreased ATP levels in β-cells (51).
The methodological development of coumarin-7-boronate as a selective peroxynitrite probe has allowed for the direct measurement of this powerful oxidant in cells (52). We have used this probe to address whether β-cells have the capacity to produce peroxynitrite in response to cytokine treatment. Unlike macrophages, which generate peroxynitrite in response to LPS (iNOS inducer) and PMA (NADPH oxidase activator), cytokines fail to stimulate β-cell production of peroxynitrite alone, or in combination with PMA (26). The lack of peroxynitrite generation by β-cells is associated with an inability to generate superoxide. Although cytokines stimulate iNOS expression and nitric oxide production, alone or in combination with PMA, cytokines do not stimulate superoxide generation by β-cells (26). These findings are consistent with the absence of any changes in the response to cytokines of islets isolated from NADPH oxidase-deficient mice as compared with wild type controls (14). Although β-cells do not appear to endogenously produce peroxynitrite, it is possible to generate this oxidant by treating islets with superoxide-generating redox cycling agents and nitric oxide donors. Under these conditions, we have shown that superoxide attenuates the inhibitory actions of nitric oxide on oxidative metabolism (26). In contrast to the β-cell response, concomitant generation of superoxide and nitric oxide results in rapid killing of endothelial cells to levels that exceed the toxicity of either agent alone (29). These findings suggest that the response to various reactive species is cell type-dependent.
To address the β-cell response to different reactive species, the effects of chemical donors that liberate nitric oxide (DPTA/NO), peroxynitrite (SIN-1), superoxide (menadione), and hydrogen peroxide on β-cell viability, metabolism, signaling, and gene expression were examined. In response to nitric oxide and hydrogen peroxide, there is a concentration-dependent decrease in cell viability that is associated with decreases in aconitase activity and ATP content. Although the functional responses to these reactive species are similar, the mechanisms of toxicity differ in that hydrogen peroxide toxicity is associated with double-strand DNA breaks (γH2AX formation), PARP-1 overactivation, and the subsequent depletion of ATP. Although nitric oxide causes DNA double-strand breaks, we have shown that PARP-1 is not activated by nitric oxide and does not participate in cytokine-induced death of islet and insulinoma cells (53). Menadione is also toxic to INS832/13 cells in a concentration-related manner that is first evident at 20 μm and maximal at 40 μm. The toxicity to superoxide differs from hydrogen peroxide as PARP-1 inhibitors do not modify menadione-induced cell death and menadione does not stimulate γH2AX formation or decrease ATP or NAD+ levels in INS832/13 cells. Although NAD+ and ATP levels are maintained, menadione treatment inhibits aconitase activity in a concentration-related manner that is similar to the loss of INS832/13 cell viability. Peroxynitrite at concentrations up to 1 mm is not toxic to β-cells and does not modify aconitase activity. Together, the direct comparison performed in this study reveals that the responses of insulin-producing cells are selective for the individual reactive species. Nitric oxide, superoxide, and hydrogen peroxide are toxic to β-cells; however, the mechanisms of cell death as well as the effects on oxidative metabolism differ. Further, the signaling cascades activated by each form of ROS and RNS are selective and mechanism-dependent. Most surprising is the resistance or lack of response of insulin-producing cells to the powerful oxidant peroxynitrite.
It has been suggested that β-cells are highly sensitive to free radicals because they express low levels of antioxidant defense enzymes such as glutathione peroxidase and catalase (20–22). This statement is based on a comparison of enzymatic activity and expression levels of these antioxidant defense enzymes in pancreatic islets with other tissues. In addition, β-cells are sensitive to ROS and RNS as compounds such as menadione, hydrogen peroxide, and nitric oxide donors inhibit oxidative metabolism, reduce ATP levels, and result in toxicity to β-cells (this study and Ref. 26). The primary cellular sources of ROS include NADPH oxidase and leakage during uncoupled mitochondrial oxidation (10, 12). In this regard, oxidative metabolism in β-cells is highly coupled such that 80–90% of the carbons from glucose are oxidized to CO2, a percentage that is much higher than in most other cell types (54). This oxidation is essential for the generation of ATP, a second messenger that inhibits potassium channel activity, allowing for membrane depolarization and insulin secretion in response to glucose (55). Although studies suggest that superoxide is produced by β-cells in response to elevated concentrations of glucose and that it participates in glucose-stimulated insulin secretion (56), no differences were found in the response of islets isolated from mice deficient in NADPH oxidase activity as compared with islets from wild type mice following treatment with cytokines or elevated levels of glucose (14). These findings suggest that β-cells may not be weak in their antioxidant defense but may express antioxidant enzymes to levels appropriate for the levels of ROS and RNS that are produced.
It could be argued that β-cells are more sensitive to ROS generated from activated inflammatory cells migrating into islets during autoimmune-mediated damage (57). This would suggest that the location of radical formation is an important factor controlling the individual cellular response. To address the role of the location of ROS generation, we examined the effects of superoxide produced within (menadione) and outside of β-cells (xanthine oxidase) on ATP levels and gene expression. We show that superoxide generated within β-cells prevents the decreases in ATP levels induced by nitric oxide, but fails to modify the nitric oxide-dependent decrease in cellular ATP pool when generated extracellularly. This is also the case for nitric oxide-dependent gene expression as superoxide generated in β-cells attenuates DPTA/NO-stimulated GADD45α and PUMA mRNA accumulation, whereas xanthine oxidase does not modify this expression. Importantly, under both conditions (sites of superoxide generation), similar levels of peroxynitrite are produced. These findings indicate that the location of superoxide generation may have dramatic effects on the β-cell response to ROS and RNS. When produced within cells, it is capable of scavenging nitric oxide and attenuating nitric oxide-dependent depletion in ATP levels and changes in gene expression. When made outside of β-cells, conditions that would mimic extracellular production by macrophages, it does not modify β-cell function. Although β-cells are often described to express low levels of antioxidant defense enzymes, resulting in enhanced sensitivity to free radicals, these findings suggest that they may indeed express antioxidant defense enzymes to levels required for the type and amount of reactive species produced. Consistent with this interpretation is the location of radical production, which seems to determine the cellular response. When forced to produce superoxide, β-cells are protected from nitric oxide-mediated effects; however, when superoxide is formed outside of the cell, it does not modify the β-cell response to nitric oxide. These findings support nitric oxide as the primary mediator of the actions of cytokines on β-cell function, and the location of reactive species generation is a key determinant in the biological response elicited.
Acknowledgment
We thank Dr. Kalyanaraman for providing the coumarin-7-boronate probe.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-052194 and AI-44458 (to J. A. C) and DK-074656 (to C. E. M.) This work was also supported by a grant from the American Diabetes Association (to C. E. M.); American Heart Association Postdoctoral Fellowship 13POST16940076 (to K. A. B); American Heart Association Predoctoral Fellowship 14PRE20380585 (to B. J. O.); and a gift from the Forest County Potawatomi Foundation.
- iNOS
- inducible nitric oxide synthase
- DPTA/NO
- dipropylenetriamine NONOate
- RNS
- reactive nitrogen species
- ROS
- reactive oxygen species
- GADD45α
- growth arrest and DNA damage 45α
- PUMA
- p53-upregulated modulator of apoptosis
- PARP
- poly(ADP-ribose) polymerase
- PAR
- poly(ADP-ribose)
- AMPK
- AMP-activated protein kinase
- GSK3
- glycogen synthase kinase 3
- eIF2α
- eukaryotic initiation factor 2α
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1. Mandrup-Poulsen T. (1996) The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 39, 1005–1029 [DOI] [PubMed] [Google Scholar]
- 2. Corbett J. A., Wang J. L., Sweetland M. A., Lancaster J. R., Jr., McDaniel M. L. (1992) Interleukin 1 β induces the formation of nitric oxide by β-cells purified from rodent islets of Langerhans: evidence for the β-cell as a source and site of action of nitric oxide. J. Clin. Invest. 90, 2384–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eizirik D. L., Björklund A., Welsh N. (1993) Interleukin-1-induced expression of nitric oxide synthase in insulin-producing cells is preceded by c-fos induction and depends on gene transcription and protein synthesis. FEBS Lett. 317, 62–66 [DOI] [PubMed] [Google Scholar]
- 4. Southern C., Schulster D., Green I. C. (1990) Inhibition of insulin secretion by interleukin-1 β and tumour necrosis factor-α via an l-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 276, 42–44 [DOI] [PubMed] [Google Scholar]
- 5. Corbett J. A., McDaniel M. L. (1994) Reversibility of interleukin-1 β-induced islet destruction and dysfunction by the inhibition of nitric oxide synthase. Biochem. J. 299, 719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu D., Pavlovic D., Chen M. C., Flodström M., Sandler S., Eizirik D. L. (2000) Cytokines induce apoptosis in β-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS−/−). Diabetes 49, 1116–1122 [DOI] [PubMed] [Google Scholar]
- 7. Gusdon A. M., Votyakova T. V., Reynolds I. J., Mathews C. E. (2007) Nuclear and mitochondrial interaction involving mt-Nd2 leads to increased mitochondrial reactive oxygen species production. J. Biol. Chem. 282, 5171–5179 [DOI] [PubMed] [Google Scholar]
- 8. Mathews C. E., Dunn B. D., Hannigan M. O., Huang C. K., Leiter E. H. (2002) Genetic control of neutrophil superoxide production in diabetes-resistant ALR/Lt mice. Free Radic. Biol. Med. 32, 744–751 [DOI] [PubMed] [Google Scholar]
- 9. Newsholme P., Gaudel C., Krause M. (2012) Mitochondria and diabetes: an intriguing pathogenetic role. Adv. Exp. Med. Biol. 942, 235–247 [DOI] [PubMed] [Google Scholar]
- 10. Oliveira H. R., Verlengia R., Carvalho C. R., Britto L. R., Curi R., Carpinelli A. R. (2003) Pancreatic β-cells express phagocyte-like NAD(P)H oxidase. Diabetes 52, 1457–1463 [DOI] [PubMed] [Google Scholar]
- 11. Vásquez-Vivar J., Kalyanaraman B., Martásek P., Hogg N., Masters B. S., Karoui H., Tordo P., Pritchard K. A., Jr. (1998) Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. U.S.A. 95, 9220–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bindokas V. P., Kuznetsov A., Sreenan S., Polonsky K. S., Roe M. W., Philipson L. H. (2003) Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J. Biol. Chem. 278, 9796–9801 [DOI] [PubMed] [Google Scholar]
- 13. Leloup C., Tourrel-Cuzin C., Magnan C., Karaca M., Castel J., Carneiro L., Colombani A. L., Ktorza A., Casteilla L., Pénicaud L. (2009) Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 58, 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thayer T. C., Delano M., Liu C., Chen J., Padgett L. E., Tse H. M., Annamali M., Piganelli J. D., Moldawer L. L., Mathews C. E. (2011) Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes. Diabetes 60, 2144–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sumoski W., Baquerizo H., Rabinovitch A. (1989) Oxygen free radical scavengers protect rat islet cells from damage by cytokines. Diabetologia 32, 792–796 [DOI] [PubMed] [Google Scholar]
- 16. Huie R. E., Padmaja S. (1993) The reaction of NO with superoxide. Free Radic. Res. Commun. 18, 195–199 [DOI] [PubMed] [Google Scholar]
- 17. Delaney C. A., Tyrberg B., Bouwens L., Vaghef H., Hellman B., Eizirik D. L. (1996) Sensitivity of human pancreatic islets to peroxynitrite-induced cell dysfunction and death. FEBS Lett. 394, 300–306 [DOI] [PubMed] [Google Scholar]
- 18. Lakey J. R., Suarez-Pinzon W. L., Strynadka K., Korbutt G. S., Rajotte R. V., Mabley J. G., Szabó C., Rabinovitch A. (2001) Peroxynitrite is a mediator of cytokine-induced destruction of human pancreatic islet β cells. Lab. Invest. 81, 1683–1692 [DOI] [PubMed] [Google Scholar]
- 19. Suarez-Pinzon W. L., Szabó C., Rabinovitch A. (1997) Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet β-cells. Diabetes 46, 907–911 [DOI] [PubMed] [Google Scholar]
- 20. Lenzen S., Drinkgern J., Tiedge M. (1996) Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 20, 463–466 [DOI] [PubMed] [Google Scholar]
- 21. Mathews C. E., Suarez-Pinzon W. L., Baust J. J., Strynadka K., Leiter E. H., Rabinovitch A. (2005) Mechanisms underlying resistance of pancreatic islets from ALR/Lt mice to cytokine-induced destruction. J. Immunol. 175, 1248–1256 [DOI] [PubMed] [Google Scholar]
- 22. Mathews C. E., Leiter E. H. (1999) Constitutive differences in antioxidant defense status distinguish alloxan-resistant and alloxan-susceptible mice. Free Radic. Biol. Med. 27, 449–455 [DOI] [PubMed] [Google Scholar]
- 23. Bensellam M., Van Lommel L., Overbergh L., Schuit F. C., Jonas J. C. (2009) Cluster analysis of rat pancreatic islet gene mRNA levels after culture in low-, intermediate- and high-glucose concentrations. Diabetologia 52, 463–476 [DOI] [PubMed] [Google Scholar]
- 24. Godoy J. R., Funke M., Ackermann W., Haunhorst P., Oesteritz S., Capani F., Elsässer H. P., Lillig C. H. (2011) Redox atlas of the mouse. Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim. Biophys. Acta 1810, 2–92 [DOI] [PubMed] [Google Scholar]
- 25. Brunmark A., Cadenas E. (1989) Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic. Biol. Med. 7, 435–477 [DOI] [PubMed] [Google Scholar]
- 26. Broniowska K. A., Mathews C. E., Corbett J. A. (2013) Do β-cells generate peroxynitrite in response to cytokine treatment? J. Biol. Chem. 288, 36567–36578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Repetto G., del Peso A., Zurita J. L. (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 3, 1125–1131 [DOI] [PubMed] [Google Scholar]
- 28. Steer S. A., Scarim A. L., Chambers K. T., Corbett J. A. (2006) Interleukin-1 stimulates β-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 3, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Broniowska K. A., Diers A. R., Corbett J. A., Hogg N. (2013) Effect of nitric oxide on naphthoquinone toxicity in endothelial cells: role of bioenergetic dysfunction and poly (ADP-ribose) polymerase activation. Biochemistry 52, 4364–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stocchi V., Cucchiarini L., Canestrari F., Piacentini M. P., Fornaini G. (1987) A very fast ion-pair reversed-phase HPLC method for the separation of the most significant nucleotides and their degradation products in human red blood cells. Anal. Biochem. 167, 181–190 [DOI] [PubMed] [Google Scholar]
- 31. Perez J., Hill B. G., Benavides G. A., Dranka B. P., Darley-Usmar V. M. (2010) Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet-derived growth factor. Biochem. J. 428, 255–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 33. Scarim A. L., Heitmeier M. R., Corbett J. A. (1997) Irreversible inhibition of metabolic function and islet destruction after a 36-hour exposure to interleukin-1β. Endocrinology 138, 5301–5307 [DOI] [PubMed] [Google Scholar]
- 34. Khan P., Idrees D., Moxley M. A., Corbett J. A., Ahmad F., von Figura G., Sly W. S., Waheed A., Hassan M. I. (2014) Luminol-based chemiluminescent signals: clinical and non-clinical application and future uses. Appl. Biochem. Biotechnol. 173, 333–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zielonka J., Zielonka M., Sikora A., Adamus J., Joseph J., Hardy M., Ouari O., Dranka B. P., Kalyanaraman B. (2012) Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J. Biol. Chem. 287, 2984–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meares G. P., Fontanilla D., Broniowska K. A., Andreone T., Lancaster J. R., Jr., Corbett J. A. (2013) Differential responses of pancreatic β-cells to ROS and RNS. Am. J. Physiol. Endocrinol. Metab. 304, E614–E622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schreiber V., Dantzer F., Ame J. C., de Murcia G. (2006) Poly(ADP-ribose): novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 7, 517–528 [DOI] [PubMed] [Google Scholar]
- 38. Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 [DOI] [PubMed] [Google Scholar]
- 39. Chambers K. T., Unverferth J. A., Weber S. M., Wek R. C., Urano F., Corbett J. A. (2008) The role of nitric oxide and the unfolded protein response in cytokine-induced β-cell death. Diabetes 57, 124–132 [DOI] [PubMed] [Google Scholar]
- 40. Oyadomari S., Takeda K., Takiguchi M., Gotoh T., Matsumoto M., Wada I., Akira S., Araki E., Mori M. (2001) Nitric oxide-induced apoptosis in pancreatic β cells is mediated by the endoplasmic reticulum stress pathway. Proc. Natl. Acad. Sci. U.S.A. 98, 10845–10850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chambers K. T., Weber S. M., Corbett J. A. (2007) PGJ2-stimulated β-cell apoptosis is associated with prolonged UPR activation. Am. J. Physiol. Endocrinol. Metab. 292, E1052–E1061 [DOI] [PubMed] [Google Scholar]
- 42. Huang S., Zhu M., Wu W., Rashid A., Liang Y., Hou L., Ning Q., Luo X. (2014) Valproate pretreatment protects pancreatic β-cells from palmitate-induced ER stress and apoptosis by inhibiting glycogen synthase kinase-3β. J. Biomed. Sci. 21, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meares G. P., Mines M. A., Beurel E., Eom T. Y., Song L., Zmijewska A. A., Jope R. S. (2011) Glycogen synthase kinase-3 regulates endoplasmic reticulum (ER) stress-induced CHOP expression in neuronal cells. Exp. Cell Res. 317, 1621–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Son Y. O., Pratheeshkumar P., Wang L., Wang X., Fan J., Kim D. H., Lee J. Y., Zhang Z., Lee J. C., Shi X. (2013) Reactive oxygen species mediate Cr(VI)-induced carcinogenesis through PI3K/AKT-dependent activation of GSK-3β/β-catenin signaling. Toxicol. Appl. Pharmacol. 271, 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiara F., Gambalunga A., Sciacovelli M., Nicolli A., Ronconi L., Fregona D., Bernardi P., Rasola A., Trevisan A. (2012) Chemotherapeutic induction of mitochondrial oxidative stress activates GSK-3α/β and Bax, leading to permeability transition pore opening and tumor cell death. Cell Death Dis. 3, e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klotz L. O., Schieke S. M., Sies H., Holbrook N. J. (2000) Peroxynitrite activates the phosphoinositide 3-kinase/Akt pathway in human skin primary fibroblasts. Biochem. J. 352, 219–225 [PMC free article] [PubMed] [Google Scholar]
- 47. David K. K., Andrabi S. A., Dawson T. M., Dawson V. L. (2009) Parthanatos, a messenger of death. Front Biosci. (Landmark Ed.) 14, 1116–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zong W. X., Ditsworth D., Bauer D. E., Wang Z. Q., Thompson C. B. (2004) Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 18, 1272–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hughes K. J., Meares G. P., Chambers K. T., Corbett J. A. (2009) Repair of nitric oxide-damaged DNA in β-cells requires JNK-dependent GADD45α expression. J. Biol. Chem. 284, 27402–27408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hughes K. J., Meares G. P., Hansen P. A., Corbett J. A. (2011) FoxO1 and SIRT1 regulate β-cell responses to nitric oxide. J. Biol. Chem. 286, 8338–8348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maechler P., Jornot L., Wollheim C. B. (1999) Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic β cells. J. Biol. Chem. 274, 27905–27913 [DOI] [PubMed] [Google Scholar]
- 52. Zielonka J., Sikora A., Joseph J., Kalyanaraman B. (2010) Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J. Biol. Chem. 285, 14210–14216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andreone T., Meares G. P., Hughes K. J., Hansen P. A., Corbett J. A. (2012) Cytokine-mediated β-cell damage in PARP-1-deficient islets. Am. J. Physiol. Endocrinol. Metab. 303, E172–E179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schuit F., De Vos A., Farfari S., Moens K., Pipeleers D., Brun T., Prentki M. (1997) Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in β cells. J. Biol. Chem. 272, 18572–18579 [DOI] [PubMed] [Google Scholar]
- 55. Wollheim C. B., Maechler P. (2002) β-Cell mitochondria and insulin secretion: messenger role of nucleotides and metabolites. Diabetes 51, Suppl. 1, S37–S42 [DOI] [PubMed] [Google Scholar]
- 56. Syed I., Kyathanahalli C. N., Jayaram B., Govind S., Rhodes C. J., Kowluru R. A., Kowluru A. (2011) Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 60, 2843–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schwizer R. W., Leiter E. H., Evans R. (1984) Macrophage-mediated cytotoxicity against cultured pancreatic islet cells. Transplantation 37, 539–544 [DOI] [PubMed] [Google Scholar]