Background: Separase, the trigger protease of eukaryotic anaphase, remains regulated in the absence of its inhibitor, securin.
Results: Cdk1-cyclin B1 triggers precipitation of separase by phosphorylation but stabilizes it by inhibitory binding.
Conclusion: Only separase that is first complexed by Cdk1-cyclin B1 can later be activated by cyclin B1 degradation.
Significance: These minimal requirements of separase regulation could explain the faithful execution of anaphase in the absence of securin.
Keywords: Chromosomes, Cyclin-dependent Kinase (CDK), Cysteine Protease, Mitosis, Phosphorylation, Cdk1-Cyclin B1, Centriole Disengagement, Securin, Separase, Sister Chromatid Separation
Abstract
Sister chromatid cohesion is established during replication by entrapment of both dsDNAs within the cohesin ring complex. It is dissolved in anaphase when separase, a giant cysteine endopeptidase, cleaves the Scc1/Rad21 subunit of cohesin, thereby triggering chromosome segregation. Separase is held inactive by association with securin until this anaphase inhibitor is destroyed at the metaphase-to-anaphase transition by ubiquitin-dependent degradation. The relevant ubiquitin ligase, the anaphase-promoting complex/cyclosome, also targets cyclin B1, thereby causing inactivation of Cdk1 and mitotic exit. Although separase is essential, securin knock-out mice are surprisingly viable and fertile. Capitalizing on our previous finding that Cdk1-cyclin B1 can also bind and inhibit separase, we investigated whether this kinase might be suitable to maintain faithful timing and execution of anaphase in the absence of securin. We found that, similar to securin, Cdk1-cyclin B1 regulates separase in both a positive and negative manner. Although securin associates with nascent separase to co-translationally assist proper folding, Cdk1-cyclin B1 acts on native state separase. Upon entry into mitosis, Cdk1-cyclin B1-dependent phosphorylation of Ser-1126 renders separase prone to inactivation by aggregation/precipitation. Stable association of Cdk1-cyclin B1 with phosphorylated separase counteracts this tendency and stabilizes separase in an inhibited yet activatable state. These opposing effects are suited to prevent premature cleavage of cohesin in early mitosis while ensuring timely activation of separase by anaphase-promoting complex/cyclosome-dependent degradation of cyclin B1. Coupling sister chromatid separation with subsequent exit from mitosis by this simplified mode might have been the common scheme of mitotic control prior to the evolution of securin.
Introduction
Separase, a giant cysteine endopeptidase, triggers all eukaryotic anaphases (1). It cleaves the Scc1/Rad21 subunit of the ring-shaped cohesin complex, which, up to this point, maintains cohesion by entrapping the two sister chromatids of each chromosome (2, 3). Prior to anaphase, separase is held in check by association with securin (4). When the sister kinetochores of every chromosome have acquired a proper amphitelic attachment to microtubules from opposite spindle poles, separase is unleashed in its proteolytically active form by ubiquitin-dependent degradation of securin (4). The relevant E3 ligase is the anaphase-promoting complex/cyclosome, which also sees to the destruction of cyclin B1, the activating subunit of Cdk1 (cyclin-dependent kinase 1) (5). The resulting concomitant activation of separase and inactivation of the master regulatory kinase of mitosis couple sister chromatid separation with mitotic exit.
Securin and separase also influence each other in a positive way. This is illustrated by identical loss-of-function phenotypes for securin and separase in species in which securin is essential and by elevated levels of securin as a result of separase overexpression (6–8). Phosphorylation turns vertebrate securin into a better anaphase-promoting complex/cyclosome substrate. This is counteracted by separase, which mediates the PP2A (protein phosphatase 2A)-dependent dephosphorylation and hence stabilization of associated securin (9). Although this provides an explanation for the positive effect of separase on securin, data are lacking that address how vertebrate securin positively affects separase.
Given the essentiality of separase, it is a surprise that mice and cultured human cells are largely unaffected by the knock-out of securin (1, 10, 11). Could it be that, under these circumstances, regulation of the substrate rather than the protease takes center stage? Indeed, Plk1 (Polo-like kinase 1)-dependent phosphorylation of Scc1 enhances its cleavage by separase (12, 13). However, this phosphorylation is unlikely to be restricted to just the small window of metaphase-to-anaphase transition and does not constitute an absolute requirement for the cleavage of mitotic cohesin by separase. Therefore, regulation at the level of the substrate seems unfit to explain how securin-free mammalian cells maintain proper timing and fidelity of sister chromatid separation in mitosis. Instead, it is likely that, in the absence of securin, at least the inhibitory aspect of separase regulation is taken over by Cdk1-cyclin B1 (14).
This securin-independent inhibition of human separase requires that Ser-1126 and several residues within a Cdc6-like domain (CLD)2 centered around position 1370 are phosphorylated by Cdk1-cyclin B1 (and possibly other mitotic kinases) (15, 16). Although necessary, these phosphorylations are not sufficient for inhibition of separase, which additionally requires Cdk1-cyclin B1 to stably associate, via its regulatory cyclin B1 subunit, with the phosphorylated CLD of separase (17). Within the Cdk1-cyclin B1-separase complex, Ser-1126 is probably not in direct contact with the kinase because this residue is dispensable for binding of Cdk1-cyclin B1 to separase fragments (16). However, Ser-1126 phosphorylation is absolutely required for Cdk1-cyclin B1 to associate with full-length separase. Interestingly, securin and Cdk1-cyclin B1 bind to separase in a mutually exclusive manner (17). At the level of an individual separase molecule, they therefore represent alternative rather than synergistic inhibitory mechanisms.
Although most of separase is typically controlled by securin, Cdk1-cyclin B1 can compensate for missing or limited securin. This is illustrated by the fact that murine embryonic stem cells with a combined securin knock-out and (heterozygote) knock-in of a Cdk1-cyclin B1-resistant separase allele quickly lose cohesion in a prometaphase arrest, whereas the isolated defects do not cause such a phenotype (18). Similarly, S1126A and ΔCLD variants, but not WT separase, cause premature separation of sister chromatids upon overexpression in HEK293 cells (8). Although Cdk1-cyclin B1 seems able to always compensate for the loss of securin, securin cannot always substitute for Cdk1-cyclin B1 in separase regulation. For unknown reasons, murine early embryonic and post-migratory primordial germ cells express only little securin and fully rely on Cdk1-cyclin B1-dependent control of separase (19, 20). Despite the importance of this securin-independent control of anaphase, it remains enigmatic whether Cdk1-cyclin B1, similar to securin, might exert not only a negative but simultaneously also a positive effect on separase.
Here, we investigated the positive effect of securin on separase and demonstrate that co-translational association of securin with nascent separase coincides with increased solubility of the giant protease, indicating that securin assists separase in achieving a natively folded state. We unraveled two novel and opposing effects of Cdk1-cyclin B1 on separase. Upon entry into mitosis, Cdk1-cyclin B1-dependent phosphorylation of free separase further enhances the protease's tendency to become insoluble and catalytically inactive. This effect is counteracted by a stabilizing association of Cdk1-cyclin B1 with phosphorylated separase. Thus, much like securin, Cdk1-cyclin B1 is both a positive and negative regulator of separase at the same time. Together, these effects of Cdk1-cyclin B1 ensure that only separase, which has been in complex with its inhibitor in early mitosis, can later be activated at anaphase onset. Our findings help explain how anaphase and mitotic exit might have been coupled prior to the evolution of securin and are still coupled today in a securin knock-out.
EXPERIMENTAL PROCEDURES
Antibodies
The following antibodies were used for immunoblotting according to standard protocols: rabbit anti-separase (15), mouse anti-securin (1:1000; MBL International Corp.), mouse anti-Myc (1:50, clone 9E10, hybridoma supernatant; Developmental Studies Hybridoma Bank), rabbit anti-phospho-Ser-10 histone H3 (1:1000; Millipore), mouse anti-cyclin B1 (1:1000; Millipore), goat anti-Cdc27 (1:1000; gift from Thomas U. Mayer), and mouse anti-α-tubulin (1:200, clone 12G10, hybridoma supernatant; Developmental Studies Hybridoma Bank). For immunoprecipitation experiments, the following affinity matrices and antibodies were used: mouse anti-Myc antibody-agarose (Sigma-Aldrich) and protein A-Sepharose (GE Healthcare) coupled to rabbit anti-securin antibody (raised against His6-tagged full-length human securin).
Cell Lines and Treatments
For stable inducible expression of Myc6-tobacco etch virus (TEV)2-separase (WT, S1126A, and ΔCLD (amino acids 1342–1400 deleted), the corresponding transgenes were stably integrated into an HEK293 Flp-In T-REx cell line. Clones were selected with 150 μg/ml hygromycin B (Roth). Induction of transgenic myc6-TEV2-separase was done using 0.2–1 μg/ml doxycycline (Sigma-Aldrich) for 10–14 h. All cells were cultured in DMEM (GE Healthcare) supplemented with 10% FCS (Sigma-Aldrich) at 37 °C and 5% CO2. For transient overexpression, HEK293T cells were transfected using the calcium phosphate-based method with pCS2-based plasmids encoding the following proteins: Myc6-TEV2-separase (WT and S1126A) and untagged WT securin (see Figs. 1B and 2B). For synchronization at the G1/S boundary, cells were treated with 2 mm thymidine (Sigma-Aldrich) for 20 h and released into fresh medium. Synchronization of cells in prometaphase was done by addition of nocodazole (Sigma-Aldrich) or Taxol (Calbiochem) at 0.2 μg/ml each 6 h after release from a single thymidine block or for 14 h to asynchronous cells. Synchronization in interphase was achieved by addition of 10 μm roscovitine (Calbiochem) for 20 h, followed by thymidine treatment for 12 h.
FIGURE 1.
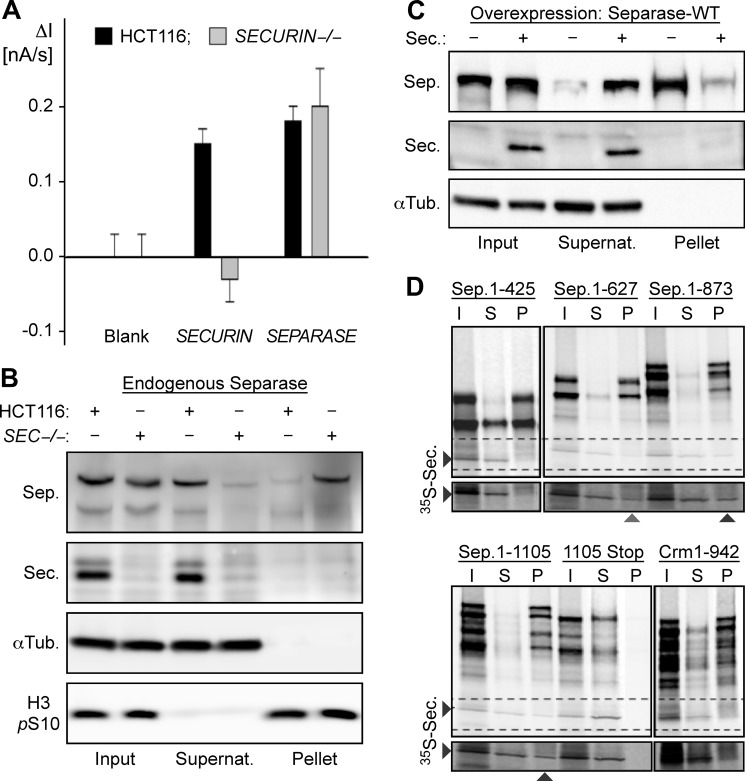
Studying the positive effect of securin on separase. A, securin does not influence the mRNA level of separase. The relative amounts of securin and separase mRNAs in securin−/− and parental HCT116 cells were quantified on an electrochemical biochip. B, endogenous separase (Sep.) is prone to precipitation in the absence of securin (Sec.). Lysates (input) of mitotically arrested securin−/− (SEC−/−) and parental HCT116 cells were centrifuged to assess the solubility of separase by Western analysis of the resulting supernatant (Supernat.) and pellet fractions. α-Tubulin (αTub.) and Ser-10-phosphorylated histone H3 (H3 pS10) served as loading controls. C, association with securin prevents precipitation of transiently overexpressed separase. Following its overexpression in HEK293T cells with or without co-overexpression of securin, the solubility of separase was determined by centrifugation. D, co-translational association of securin with nascent separase. mRNAs lacking or containing a stop codon and coding for amino acids 1–425, 1–627, 1–873, or 1–1105 of human separase or amino acids 1–942 of human exportin-1 (Crm1) were translated in vitro in the presence of [35S]methionine and 35S-labeled securin. Input (I) samples were fractionated by ultracentrifugation into supernatant (S) and RNC-containing pellet (P) fractions prior to SDS-PAGE and autoradiography. Crm1 was chosen as a negative control because, similar to the N-terminal half of separase, it contains a superhelical structure.
FIGURE 2.
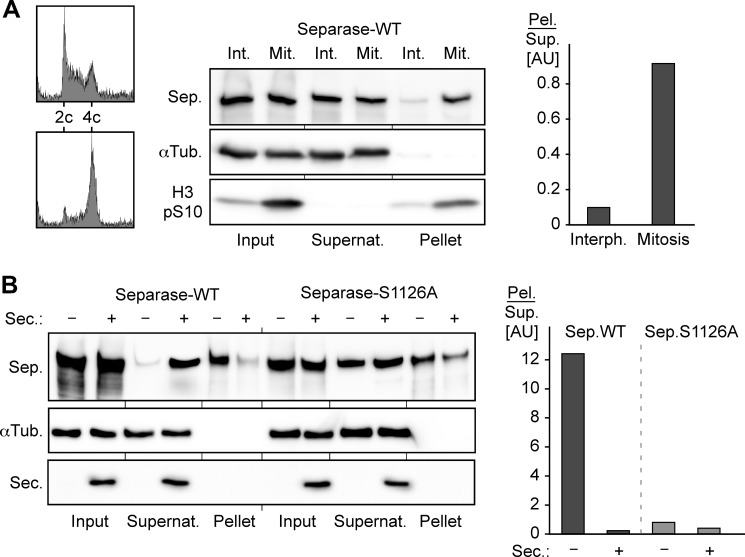
Mitosis-specific phosphorylation of Ser-1126 decreases the solubility of separase. A, aggravated aggregation tendency of separase (Sep.) in mitosis. Synchronized interphase (Int., Interph.) or prometaphase (Mit.) transgenic HEK293 cells induced to express WT separase were stained with propidium iodide and analyzed by flow cytometry or lysed and subjected to ultracentrifugation and Western analysis. Signals for pelleted (Pel.) versus soluble (Sup.) separase were quantified by densitometry and are depicted as quotients (gray bars). αTub., α-tubulin; H3 pS10, Ser-10-phosphorylated histone H3; Supernat., supernatant; AU, arbitrary units. B, preventing phosphorylation of Ser-1126 stabilizes securin-less separase. HEK293T cells transiently overexpressing securin (Sec.) and/or Myc-tagged WT separase or S1126A were arrested in prometaphase before the solubility of separase was determined by pelleting assay and quantified as described for A.
Separase Pelleting and Activity Assays
Myc6-TEV2-separase was expressed by transient transfection or induction of a stably integrated transgene in the presence or absence of securin co-overexpression. Securin-dependent solubility of endogenous separase (see Fig. 1B) was studied by comparing securin−/− with the corresponding parental HCT116 cells. Approximately 1 × 107 prometaphase-arrested cells were lysed with a Dounce homogenizer in 1 ml of lysis buffer (20 mm Tris-HCl (pH 7.7), 100 mm NaCl, 10 mm NaF, 20 mm β-glycerophosphate, 5 mm MgCl2, 0.1% Triton X-100, and 5% glycerol) supplemented with complete protease inhibitor mixture (Roche Applied Science) and incubated for 10 min at 4 °C. Crude lysate (referred to as input) was ultracentrifuged at 66,000 × g for 35 min and the corresponding supernatant was harvested. The pellet was washed twice with 1× PBS, combined with 1 ml of fresh denaturation buffer (8 m urea, 20 mm Tris-HCl (pH 6.8), and 2 mm DTT), and resolubilized by sonification (6 min, 20% power, 50% duty cycle; BANDELIN SONOPULS) on ice. For the separase activity assay (see Fig. 3A), crude lysates were treated with Benzonase nuclease (30 units/liters; Santa Cruz Biotechnology) for 1 h at 4 °C. Crude lysate were incubated with anti-Myc beads for 4 h without prior centrifugation. Beads were washed and incubated three times for 5 min in 10 mm Hepes-KOH (pH 7.7), 100 mm KCl, 0.1 mm CaCl2, 2 mm MgCl2, 50 mm sucrose, 5 mm EGTA, and 0.02% Triton X-100 additionally supplemented with 400 mm NaCl (unless specified otherwise) and subsequently equilibrated in cleavage buffer (10 mm Hepes-KOH (pH 7.7), 50 mm NaCl, 25 mm NaF, 1 mm EGTA, and 20% glycerol) before separase was eluted by incubation with TEV protease. Concomitant with elution in a volume of 30 μl for 30 min at room temperature, separase activity was measured by addition of 2 μl of 35S-labeled Scc1-GFP. Samples were analyzed by SDS-PAGE, followed by Western blotting or autoradiography.
FIGURE 3.
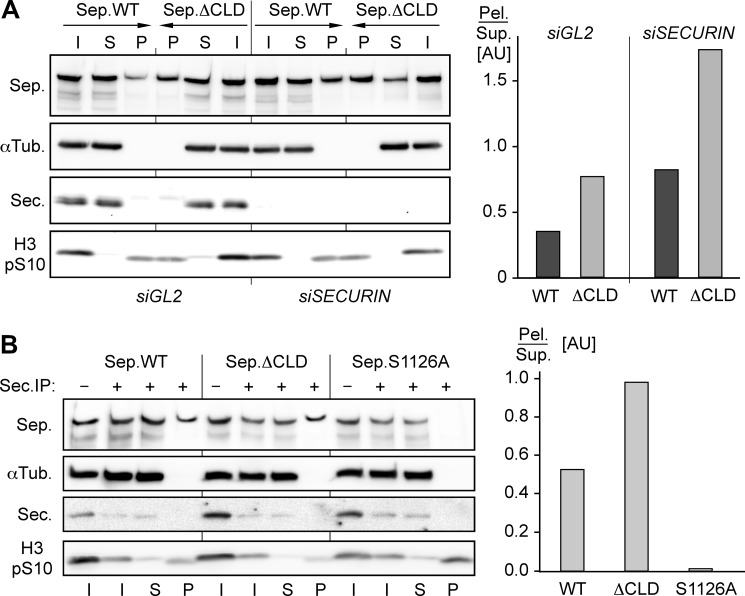
Ser-1126 phosphorylation and binding of Cdk1-cyclin B1 have opposing effects on separase solubility. A, mutational inactivation of its cyclin B1-binding site decreases the solubility of separase (Sep.). Transgenic HEK293 cells transfected with securin (siSECURIN) or GL2 (siGL2) siRNA and expressing WT separase or ΔCLD were arrested in prometaphase before the solubility of separase was determined by pelleting assay and quantified as described for Fig. 2A. I, input; S, supernatant; P, pellet; αTub., α-tubulin; Sec., securin; H3 pS10, Ser-10-phosphorylated histone H3; AU, arbitrary units; Pel., pelleted separase; Sup., soluble separase. B, precipitation of separase in mitosis is alleviated by the S1126A mutation but aggravated by CLD deletion. Lysates of transgenic, prometaphase-arrested HEK293 cells expressing the indicated separase variants were immunodepleted of securin before the solubility of securin-free separase was determined by quantitative pelleting assay as described for Fig. 2A. IP, immunoprecipitation.
RNA Interference and Flow Cytometry
For knockdown of human securin or separase, 70 nm siRNA duplex (securin, 5′-UCUUAGUGCUUCAGAGUUUGUGUGUAU-3′; and separase, 5′-AUAAGUGCCUGGCUUCACCAAACCC-3′) was transfected either with RNAiMAX (Invitrogen) according to the manufacturer's instructions or following a calcium phosphate-based method. Luciferase siRNA (GL2) was used as a negative control. Cells were grown for at least 24 h before synchronization procedures were applied. Analysis of DNA content by flow cytometry was performed as described (9).
Ribosome-Nascent Chain Complex Formation and Isolation
The generation of ribosome-nascent chain complexes (RNCs) was based on the translation of truncated synthetic mRNA coding for at least 425 N-terminal amino acids of separase. Plasmids coding for separase or Crm1 (both pCS2-based) were linearized by restriction enzyme digestion at different positions within the coding sequence to create an open reading frame without a stop signal. To create a construct for the transcription of the separase 1–1105 Stop mRNA, a pCS2-based vector containing the coding sequence for full-length separase was linearized with XbaI, filled in with Klenow polymerase (New England Biolabs), and then religated to create a frameshift mutation. For generation of runoff transcripts, plasmids were linearized with a suitable restriction endonuclease, purified by phenol/chloroform extraction and ethanol precipitation, and redissolved in diethylpyrocarbonate/H2O. Transcription was carried out with 1 μg of pure linear DNA using an SP6 mMESSAGE mMACHINE kit (Ambion) according to the manufacturer's instructions. TnT rabbit reticulocyte lysate (Promega) was used for the generation of RNCs. A translation reaction was conducted in a volume of 20 μl in the presence of 1 μl of 35S-labeled securin at 30 °C for 90 min and terminated (when no stop codon was present) by addition of 0.4 μl of 10 mg/ml cycloheximide. Aliquots were taken (referred to as input) before RNCs were further purified as described (21). Translation reaction mixtures were spun through a 80-μl cushion of buffer A (50 mm Tris-HCl (pH 7.0), 500 mm potassium acetate, 25 mm magnesium acetate, 2 mm DTT, 1 m sucrose, 10 μg/ml cycloheximide, and 0.1% Nonidet P-40) supplemented with complete protease inhibitor mixture at 180,000 × g for 90 min. Supernatants were harvested before the RNC pellets were washed twice with buffer B (32.5 mm Tris-HCl (pH 7.0), 125 mm potassium acetate, 26.25 mm magnesium acetate, 1.5 mm DTT, 250 mm sucrose, 100 μg/ml cycloheximide, 0.1% Nonidet P-40, and 0.4 units/μl RNasin) to remove residual soluble contaminants and then resuspended in 20 μl of buffer B. Input, supernatant, and resuspended pellet fractions were finally analyzed by SDS-PAGE and autoradiography.
Electrochemical Biochip Analysis of mRNA Levels
Electrochemical biochip analysis of mRNA levels was performed as described previously (22, 23). Capture oligonucleotides were as follows: securin, ACGGTTCCCGAAGGCACATTCTCATTTTTTTTT (3′-thiol); and separase, TCTGCCCCCGAAGGGGACGTCCTATTTTTTTTT (3′-thiol). The detector oligonucleotide was TTTTTTGGTTGCGCTCGTTGCGGGACTTAACCCAACAT (5′-amino). Oligonucleotides used for preparation of complementary mRNA probes (with the T7 RNA polymerase promoter sequence underlined) were as follows: Securin_1, GGTTGGTAATACGACTCACTATAGGTGAGAATGTGCCTT; Securin_2, GATGGGCACGGTTCCCGAAGGCACATTCTCAC; Securin_3, CGGGAACCGTGCCCATCCTTAGCAACCACACG; Securin_4, CTTAACCCAACATGGCACCCGTGTGGTTGCTA; Securin_5, TGCCATGTTGGGTTAAGTCCCGCAACGAGCGC; and Securin_6, GGTTGCGCTCGTTGCGGGA; Separase_1, GGTTGGTAATACGACTCACTATAGGTAGGACGTCCCCTT; Separase_2, CAGCAGCTCTGCCCCCGAAGGGGACGTCCTAC; Separase_3, CGGGGGCAGAGCTGCTGGTTGCAAGCCCTCAG; Separase_4, CTTAACCCAACATGCCATCCTGAGGGCTTGCA; Separase_5, TGGCATGTTGGGTTAAGTCCCGCAACGACGGC; and Separase_6, GGTTGCGCTCGTTGCGGGA. An untreated gold electrode served as a negative control (blank).
Total RNA was isolated from human securin−/− and the parental HCT116 cells using an RNeasy mini kit (Qiagen) following the manufacturer's instructions. The integrity of ribosomal RNA was confirmed by agarose gel electrophoresis, and the quantity and purity of total RNA were spectrophotometrically confirmed. Deviating from the described procedure for microRNA analysis (22), total RNA was fragmented in the presence of 30 mm magnesium acetate, 100 mm potassium acetate, and 40 mm Tris-HCl (pH 8.1) at 95 °C for 15 min before hybridization. Fragmented RNA in 450 mm NaCl, 0.025% Tween 20, 1 mg/ml BSA, 25 mm EDTA, 30 mm NaH2PO4 (pH 7.4), 1 μg of each complementary RNA probe, and 0.2 μm esterase 2-detector oligodeoxynucleotide conjugate was applied to each electrode. Electrode arrays were then incubated at 65 °C for 20 min in a humidity chamber, and the chip was kept at 20 °C for 5 min. After washing with 75 mm NaCl, 0.5 mm EDTA, 0.05% Tween 20, and 5 mm NaH2PO4 (pH 7.4) for 1 min at 25 °C, the chip was inserted onto a multi-potentiostat. Background current reached steady state after flow through 100 mm NaCl and 10 mm sodium phosphate (pH 7.0) for ∼1 min. Specific mRNA was detected by Alicyclobacillus acidocaldarius esterase 2 molecules bound to the electrode by hybridization. The enzyme substrate p-aminophenyl butyrate was delivered through the flow chamber at a flow rate of 250 μl/min. After the flow was stopped, the esterase 2 activity was measured as a change in current intensity (dI) per time (dt) within the first 5 s (22, 23).
Immunofluorescence Staining of Pelleted Centrosomes
Stable transgenic HEK293 Flp-In T-REx cells were transfected with separase or GL2 siRNA 24 h prior to synchronization at G1/S by addition of thymidine. 20 h thereafter, cells were released into fresh medium, and where indicated, transgene expression was doxycycline-induced 8 h later. 16 h after release into fresh medium, cells were harvested and subjected to immunoblotting, flow cytometry, and centrosome purification. To assess centriole engagement status, centrosomes were isolated from 4 × 106 cells and stained as described previously (24).
RESULTS
Several publications have proposed roles for securin in transcriptional regulation (25–28). To address the possibility that securin might stimulate transcription of separase, the levels of separase mRNA in human securin−/− cells and the parental HCT116 line were compared using an electrochemical biochip (22, 23, 29). Here, the complementary binding of the target mRNA fragment stabilizes the hybridization complex between a capture and a detector oligodeoxynucleotide, thereby bringing a reporter enzyme into the vicinity of an electrode to produce an electrochemical signal. This gap hybridization assay unambiguously revealed that separase mRNA levels are the same in both cell lines (Fig. 1A). This result strongly argues against a positive role of securin in transcription of separase. Immunoblotting consistently showed that the total amounts of separase in securin−/− and HCT116 cells are very similar (Fig. 1B, first and second lanes). However, the two cell lines greatly differ in the separase solubility as determined by simple centrifugation of total cell lysates. Although most separase from securin-containing HCT116 cells stayed in the supernatant, most separase from the securin-free knock-out cells pelleted (Fig. 1B, third through sixth lanes). Similarly, the overall level of separase in transiently transfected HEK293T cells was unaffected by co-overexpression of securin, whereas separase solubility was strongly stimulated (Fig. 1C). In summary, we propose that securin has no effect on transcription, mRNA stability, or translation efficiency of separase. Instead, it appears to prevent aggregation/precipitation of separase presumably to keep it in a conformational state, from which it can later be activated by proteasomal degradation of ubiquitylated securin.
To fulfill its dual function as a separase specific chaperone and inhibitor, securin might bind to the protease co-translationally. To clarify this issue, we tested whether recombinant securin added to in vitro translation mixtures would associate with RNCs (21) if and only if separase was being translated. Indeed, 35S-labeled securin co-purified with ribosomes stalled in the process of translation on an mRNA that codes for the first 1105 amino acids of separase but lacks a stop codon (Fig. 1D). However, it could not be pelleted if ribosomes were stalled on a control mRNA or if a stop codon within the mRNA allowed for dissociation of the separase fragment from ribosomes. The use of stop codon-less mRNAs that encode increasingly shorter N-terminal separase fragments revealed that securin cannot interact with RNCs displaying the first 425 amino acids but starts to bind to nascent separase 627 amino acids in length (Fig. 1D). These observations suggest that co-translational association of securin with separase serves two purposes: assisting the giant protease in achieving a native fold and inhibiting it at the same time.
Given that vertebrate securin is dispensable for life, a sufficient amount of separase apparently reaches a natively folded state even in the absence of securin. But who controls separase under these conditions to prevent premature separation of sister chromatids? An obvious candidate is Cdk1-cyclin B1 because, next to securin, this kinase constitutes the only other known inhibitor of vertebrate separase (15, 17). Interestingly, the tendency of overexpressed separase to be spun out from a cell lysate by centrifugation is enhanced in mitosis compared with interphase, consistent with the idea that mitotic phosphorylation renders separase particularly prone to aggregation/precipitation (Fig. 2A). On the basis of this observation and our previous work (16), we speculated that Ser-1126 phosphorylation by Cdk1-cyclin B1 induces a conformational change in native state separase with two opposing consequences. On the one hand, it favors misfolding/aggregation, but on the other hand, it renders the CLD accessible to association with cyclin B1, and this complex formation stabilizes the native fold of separase. This suggestion was easily testable because it predicted that changing Ser-1126 to Ala should prevent the conformational change, thereby rendering separase resistant to precipitation in mitosis. In contrast, preserving Ser-1126 but deleting the CLD should still allow for the conformational switch to occur but abrogate the downstream association with Cdk1-cyclin B1, thereby enhancing the tendency of separase to precipitate.
HEK293T cells were transiently transfected to overexpress WT separase or an S1126A variant and then arrested in mitosis. As before, corresponding lysates were fractionated by centrifugation into a soluble supernatant and insoluble pellet. Subsequent immunoblotting revealed that the profound aggregation of separase in mitosis was indeed greatly suppressed by changing Ser-1126 to Ala (Fig. 2B). Conversely, a ΔCLD variant exhibited aggravated insolubility relative to WT separase as revealed by analysis of the corresponding transgenic HEK293 lines by the same pelleting assay (Fig. 3A). As expected, siRNA-mediated depletion of securin enhanced the insolubility of overexpressed separase in general and of the ΔCLD variant in particular (Fig. 3A). Using transiently transfected, nocodazole-arrested HEK293T cells, we also directly compared the aggregation tendencies of WT separase and mutants S1126A and ΔCLD after removal of the securin-associated pool. To this end, securin was immunodepleted prior to centrifugation of the lysates. Confirming the previous results, the insolubility of S1126A was again reduced relative to the WT protease, whereas that of the ΔCLD variant was again increased (Fig. 3B).
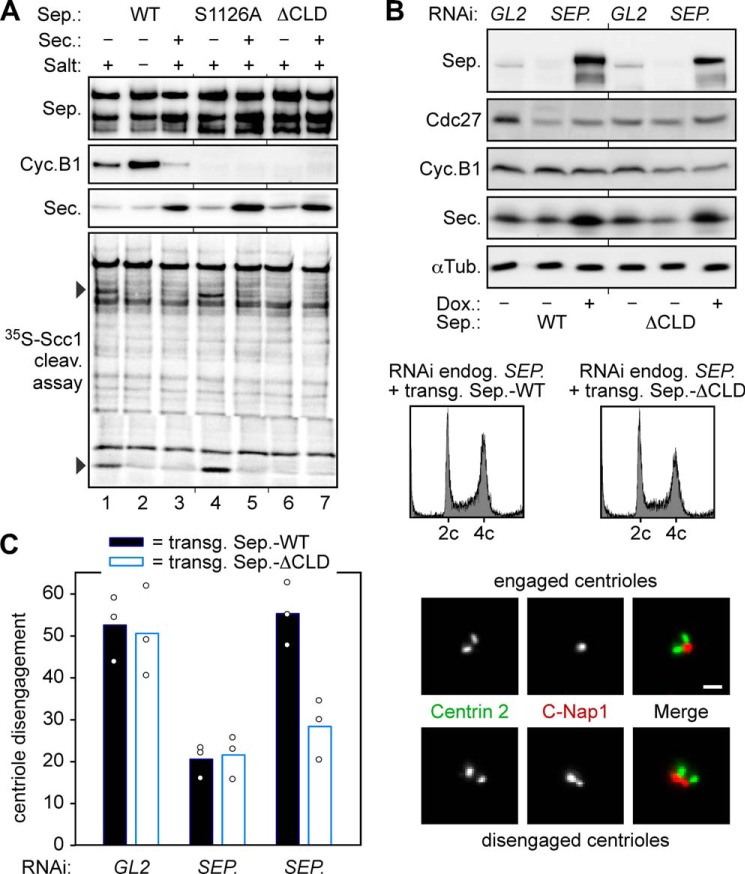
The centrifugation of cell lysates quickly determined solubility of separase but did not allow us to draw conclusions about its proteolytic activity. To clarify this issue, Myc-tagged WT separase and mutants S1126A and ΔCLD were transiently overexpressed in HEK293T cells with or without simultaneous overexpression of securin. Following cell synchronization in prometaphase, the corresponding lysates were subjected to anti-Myc immunoprecipitation without prior centrifugation to avoid removal of insoluble separase fractions. Finally, the immunoaffinity-purified separase variants were assayed for their ability to cleave cohesin (Fig. 4A). When produced under conditions of limiting amounts of securin, WT separase and S1126A exhibited proteolytic activity toward 35S-labeled Scc1, but ΔCLD did not (Fig. 4A, lanes 1, 4, and 6). In the case of WT separase, cohesin cleavage activity became apparent only upon displacement of co-purified Cdk1-cyclin B1 by washing with a high ionic strength buffer (compare lanes 1 and 2) (17). As expected, none of the protease variants cleaved Scc1 when securin had been co-overexpressed (lanes 3, 5, and 7). In combination with the pelleting assays, this experiment demonstrates good accordance of solubility and proteolytic activity for securin-less separase. In summary, we interpret our observations as follows. Under conditions of limiting securin, WT separase and ΔCLD are switched by Ser-1126 phosphorylation into a precipitation-prone conformation. Although WT separase is protected by association with Cdk1-cyclin B1 and stays soluble and activatable, the ΔCLD variant, which cannot form this complex, aggregates/misfolds and quickly becomes inactive. S1126A also cannot bind to Cdk1-cyclin B1, but for another reason. It is already protected from the destabilizing conformational change and hence retains proteolytic activity despite the lack of any binding partner.
FIGURE 4.
Positive and negative effects of Cdk1-cyclin B1 define the minimal requirements of separase regulation in mitosis. A, WT separase and mutants S1126A and ΔCLD exhibit some, high, or no cohesin cleavage activity upon isolation from prometaphase-arrested cells, respectively. Taxol-treated HEK293T cells transiently overexpressing securin (Sec.) and/or Myc6-TEV2-tagged WT separase (Sep.), S1126A, or ΔCLD were lysed and subjected to anti-Myc immunoprecipitation without prior centrifugation to avoid the removal of insoluble separase fractions. Where indicated, separase-associated Cdk1-cyclin B1 (Cyc.B1) was washed away with a high salt buffer. TEV protease-eluted separases were analyzed by immunoblotting and 35S-labeled Scc1 cleavage assay/autoradiography. B and C, in ΔCLD-expressing cells, the short half-life of proteolytic activity correlates with reduced centriole disengagement. Transgenic HEK293 cells transfected with separase or GL2 siRNA were thymidine-arrested and, where indicated, induced to express WT separase or ΔCLD. Cells were harvested 16 h after release into fresh medium and analyzed by immunoblotting, propidium iodide flow cytometry (B), and immunofluorescence microscopy of purified centrosomes (C). Fixed centrosomes were co-stained for centrin-2 and C-Nap1. Depending on a centrin-2 to C-Nap1 signal ratio of 2:1 or 2:2, centrioles were classified as engaged or disengaged, respectively. In three independent experiments (dots), the engagement status of at least 200 centrosomes per cell line and condition was analyzed and blotted as mean values (bars). Scale bar = 1 μm. αTub., α-tubulin; Dox., doxycycline.
Separase also has a chromosome-independent function within the centrosome cycle (30). By cleaving centrosomal cohesin and kendrin/pericentrin B, separase triggers centriole disengagement, which represents a licensing step for subsequent centriole duplication (24, 30–32). In the absence of separase function, centriole disengagement is profoundly delayed, but eventually still occurs by an unresolved mechanism that requires Plk1 activity (33). Because centriole disengagement normally takes place after sister chromatid separation at the end of mitosis or in early G1 phase and because the proteolytic activity of ΔCLD seems to exhibit a short half-life, centriole disengagement might be delayed upon replacement of endogenous separase by this deregulated variant. To test this prediction, stable transgenic HEK293 cells depleted of endogenous separase by RNAi were analyzed by immunofluorescence microscopy for their centriole engagement status 16 h after release from G1/S arrest and 8 h after induction of WT separase or ΔCLD expression by addition of doxycycline. Mock siRNA (GL2)-transfected, uninduced cells served as an additional control. All separase-expressing cultures exhibited highly similar cell cycle distributions as judged by flow cytometry and immunoblotting (Fig. 4B and data not shown). However, although centrioles were disengaged in ∼55% of all WT separase-containing cells, only 28% of ΔCLD-expressing cells displayed centriole disengagement on average (Fig. 4C). The above loss-of-function phenotypes do not contradict the observation that overexpression of ΔCLD causes premature separation of sister chromatids in prometaphase-arrested cells (8, 16). ΔCLD is a hypermorph in that it can no longer be bound and inhibited by Cdk1-cyclin B1, but at the same time, it is a hypomorph because Ser-1126 phosphorylation renders it aggregation-prone, thus limiting its half-life as an active protease.
DISCUSSION
Considering its central role in chromosome segregation, it is not surprising that hypo- and hyperactivity of separase both cause aneuploidy and cancer (34–38). Thus, activation of this essential but dangerous protease must be exactly controlled and timed. Here, we found that, in vitro, securin associates with nascent separase polypeptides while they are still on ribosomes and that securin-less separase tends to aggregate/precipitate as determined by pelleting assay. Based on these results, the co-translational association of securin with separase both assists the giant protease in achieving a natively folded state and inhibits it at the same time. Interestingly, caspase-activated DNase, another dangerous enzyme that needs tight controlling, also misfolds and aggregates during translation if it does not associate co-translationally with its inhibitor (39, 40). Merging in one protein (securin or caspase-activated DNase inhibitor) the functions of a specific inhibitor and of a specific chaperone therefore appears ideally suited to prevent the unscheduled unleashing of enzymatic activities that would otherwise threaten genome integrity.
We also analyzed the effects of Cdk1-cyclin B1 on separase. By phosphorylating Ser-1126, this chief mitotic kinase increases the tendency of securin-free separase to aggregate and become inactive. Interestingly, this inactivation mechanism involves a conformational change as a result of cis/trans-isomerization at Pro-1127, which is catalyzed by the phospho-Ser/Pro-specific peptidyl-prolyl isomerase Pin1.3 However, although Ser-1126 phosphorylation limits the half-live of the proteolytic activity of separase, Cdk1-cyclin B1 simultaneously counteracts this inactivation mechanism by stable association with the protease in a second step (Fig. 5). Thus, like securin, Cdk1-cyclin B1 also unites the functions of a specific inhibitor and a specific chaperone. Unlike securin, however, it does not assist in folding but rather protects from a “phosphorylation shock” (by analogy to “heat shock”) that it inflicts on separase as the cell enters mitosis (15). In this manner, it is ensured that, even in the absence of securin, any separase, which can later be activated, is first held inactive by association with Cdk1-cyclin B1. This mechanism, plus the fact that chromosomes become accessible to separase only when the nuclear envelope breaks down at the onset of mitosis (41), could explain why vertebrate securin is not essential (10). Our findings furthermore suggest that, in a primordial eukaryotic cell, the control of sister chromatid separation and mitotic exit might have been both mastered by Cdk1-cyclin B1. Then, with the invention of securin, sister chromatid separation was gradually handed over to this folding helper and superior inhibitor but stayed linked to mitotic exit via the simultaneous anaphase-promoting complex/cyclosome-mediated degradation of securin and cyclin B1. In some organisms such as yeast, the Cdk1-cyclin B1-dependent separase regulation appears to have been totally lost during evolution. However, as mouse genetics teach us, this form of anaphase control remains essential in mammals until today (19, 20).
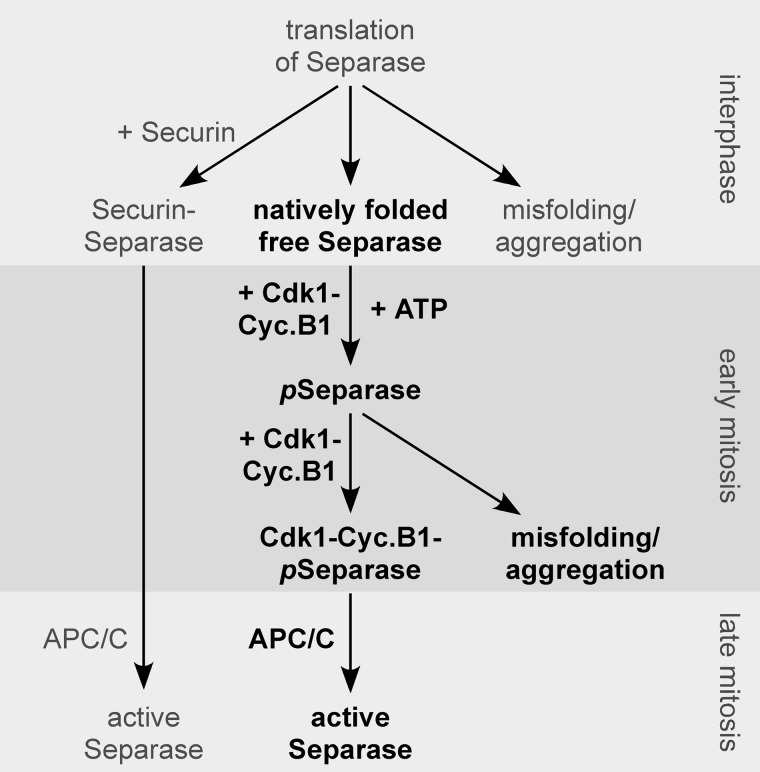
FIGURE 5.
Model of how phosphorylation and mutually exclusive association with securin or Cdk1-cyclin B1 determine the fate of separase across the cell cycle. The minimal module of separase control (shown in boldface) relies only on the positive and negative effects of Cdk1-cyclin B1 (Cyc.B1) on separase to maintain faithful chromosome segregation in the absence of securin. pSeparase, phosphorylated separase; APC/C, anaphase-promoting complex/cyclosome.
Acknowledgments
We thank Stefan Heidmann for critical reading of the manuscript and Dorothea Karalus for helping establish the RNC assay.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grants STE 997/3-2 (within the priority program SPP1384) and STE 997/4-1 (to O. S.).
S. Hellmuth, S. Rata, A. Brown, S. Heidmann, B. Novak, and O. Stemmann, submitted for publication.
- CLD
- Cdc6-like domain
- TEV
- tobacco etch virus
- RNC
- ribosome-nascent chain complex.
REFERENCES
- 1. Wirth K. G., Wutz G., Kudo N. R., Desdouets C., Zetterberg A., Taghybeeglu S., Seznec J., Ducos G. M., Ricci R., Firnberg N., Peters J. M., Nasmyth K. (2006) Separase: a universal trigger for sister chromatid disjunction but not chromosome cycle progression. J. Cell Biol. 172, 847–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uhlmann F., Wernic D., Poupart M. A., Koonin E. V., Nasmyth K. (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375–386 [DOI] [PubMed] [Google Scholar]
- 3. Haering C. H., Farcas A. M., Arumugam P., Metson J., Nasmyth K. (2008) The cohesin ring concatenates sister DNA molecules. Nature 454, 297–301 [DOI] [PubMed] [Google Scholar]
- 4. Zou H., McGarry T. J., Bernal T., Kirschner M. W. (1999) Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285, 418–422 [DOI] [PubMed] [Google Scholar]
- 5. King R. W., Peters J. M., Tugendreich S., Rolfe M., Hieter P., Kirschner M. W. (1995) A 20 S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279–288 [DOI] [PubMed] [Google Scholar]
- 6. Funabiki H., Kumada K., Yanagida M. (1996) Fission yeast Cut1 and Cut2 are essential for sister chromatid separation, concentrate along the metaphase spindle and form large complexes. EMBO J. 15, 6617–6628 [PMC free article] [PubMed] [Google Scholar]
- 7. Jäger H., Herzig A., Lehner C. F., Heidmann S. (2001) Drosophila separase is required for sister chromatid separation and binds to PIM and THR. Genes Dev. 15, 2572–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holland A. J., Taylor S. S. (2006) Cyclin-B1-mediated inhibition of excess separase is required for timely chromosome disjunction. J. Cell Sci. 119, 3325–3336 [DOI] [PubMed] [Google Scholar]
- 9. Hellmuth S., Böttger F., Pan C., Mann M., Stemmann O. (2014) PP2A delays APC/C-dependent degradation of separase-associated but not free securin. EMBO J. 33, 1134–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mei J., Huang X., Zhang P. (2001) Securin is not required for cellular viability, but is required for normal growth of mouse embryonic fibroblasts. Curr. Biol. 11, 1197–1201 [DOI] [PubMed] [Google Scholar]
- 11. Pfleghaar K., Heubes S., Cox J., Stemmann O., Speicher M. R. (2005) Securin is not required for chromosomal stability in human cells. PLoS Biol. 3, e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alexandru G., Uhlmann F., Mechtler K., Poupart M. A., Nasmyth K. (2001) Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105, 459–472 [DOI] [PubMed] [Google Scholar]
- 13. Hauf S., Roitinger E., Koch B., Dittrich C. M., Mechtler K., Peters J. M. (2005) Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 3, e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stemmann O., Gorr I. H., Boos D. (2006) Anaphase topsy-turvy: Cdk1 a securin, separase a CKI. Cell Cycle 5, 11–13 [DOI] [PubMed] [Google Scholar]
- 15. Stemmann O., Zou H., Gerber S. A., Gygi S. P., Kirschner M. W. (2001) Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715–726 [DOI] [PubMed] [Google Scholar]
- 16. Boos D., Kuffer C., Lenobel R., Körner R., Stemmann O. (2008) Phosphorylation-dependent binding of cyclin B1 to a Cdc6-like domain of human separase. J. Biol. Chem. 283, 816–823 [DOI] [PubMed] [Google Scholar]
- 17. Gorr I. H., Boos D., Stemmann O. (2005) Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol. Cell 19, 135–141 [DOI] [PubMed] [Google Scholar]
- 18. Huang X., Hatcher R., York J. P., Zhang P. (2005) Securin and separase phosphorylation act redundantly to maintain sister chromatid cohesion in mammalian cells. Mol. Biol. Cell 16, 4725–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang X., Andreu-Vieyra C. V., York J. P., Hatcher R., Lu T., Matzuk M. M., Zhang P. (2008) Inhibitory phosphorylation of separase is essential for genome stability and viability of murine embryonic germ cells. PLoS Biol. 6, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang X., Andreu-Vieyra C. V., Wang M., Cooney A. J., Matzuk M. M., Zhang P. (2009) Preimplantation mouse embryos depend on inhibitory phosphorylation of separase to prevent chromosome missegregation. Mol. Cell. Biol. 29, 1498–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beckmann R., Spahn C. M., Eswar N., Helmers J., Penczek P. A., Sali A., Frank J., Blobel G. (2001) Architecture of the protein-conducting channel associated with the translating 80 S ribosome. Cell 107, 361–372 [DOI] [PubMed] [Google Scholar]
- 22. Pöhlmann C., Sprinzl M. (2010) Electrochemical detection of microRNAs via gap hybridization assay. Anal Chem. 82, 4434–4440 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y., Stanzel M., Gumbrecht W., Humenik M., Sprinzl M. (2007) Esterase 2-oligodeoxynucleotide conjugates as sensitive reporter for electrochemical detection of nucleic acid hybridization. Biosens. Bioelectron. 22, 1798–1806 [DOI] [PubMed] [Google Scholar]
- 24. Schöckel L., Möckel M., Mayer B., Boos D., Stemmann O. (2011) Cleavage of cohesin rings coordinates the separation of centrioles and chromatids. Nat. Cell Biol. 13, 966–972 [DOI] [PubMed] [Google Scholar]
- 25. Bernal J. A., Luna R., Espina A., Lázaro I., Ramos-Morales F., Romero F., Arias C., Silva A., Tortolero M., Pintor-Toro J. A. (2002) Human securin interacts with p53 and modulates p53-mediated transcriptional activity and apoptosis. Nat. Genet. 32, 306–311 [DOI] [PubMed] [Google Scholar]
- 26. Hamid T., Kakar S. S. (2004) PTTG/securin activates expression of p53 and modulates its function. Mol. Cancer 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pei L. (2001) Identification of c-myc as a down-stream target for pituitary tumor-transforming gene. J. Biol. Chem. 276, 8484–8491 [DOI] [PubMed] [Google Scholar]
- 28. Tong Y., Eigler T. (2009) Transcriptional targets for pituitary tumor-transforming gene-1. J. Mol. Endocrinol. 43, 179–185 [DOI] [PubMed] [Google Scholar]
- 29. Jallepalli P. V., Waizenegger I. C., Bunz F., Langer S., Speicher M. R., Peters J. M., Kinzler K. W., Vogelstein B., Lengauer C. (2001) Securin is required for chromosomal stability in human cells. Cell 105, 445–457 [DOI] [PubMed] [Google Scholar]
- 30. Tsou M. F., Stearns T. (2006) Mechanism limiting centrosome duplication to once per cell cycle. Nature 442, 947–951 [DOI] [PubMed] [Google Scholar]
- 31. Matsuo K., Ohsumi K., Iwabuchi M., Kawamata T., Ono Y., Takahashi M. (2012) Kendrin is a novel substrate for separase involved in the licensing of centriole duplication. Curr. Biol. 22, 915–921 [DOI] [PubMed] [Google Scholar]
- 32. Lee K., Rhee K. (2012) Separase-dependent cleavage of pericentrin B is necessary and sufficient for centriole disengagement during mitosis. Cell Cycle 11, 2476–2485 [DOI] [PubMed] [Google Scholar]
- 33. Tsou M. F., Wang W. J., George K. A., Uryu K., Stearns T., Jallepalli P. V. (2009) Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev. Cell 17, 344–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shepard J. L., Amatruda J. F., Finkelstein D., Ziai J., Finley K. R., Stern H. M., Chiang K., Hersey C., Barut B., Freeman J. L., Lee C., Glickman J. N., Kutok J. L., Aster J. C., Zon L. I. (2007) A mutation in separase causes genome instability and increased susceptibility to epithelial cancer. Genes Dev. 21, 55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mukherjee M., Ge G., Zhang N., Huang E., Nakamura L. V., Minor M., Fofanov V., Rao P. H., Herron A., Pati D. (2011) Separase loss of function cooperates with the loss of p53 in the initiation and progression of T- and B-cell lymphoma, leukemia and aneuploidy in mice. PLoS ONE 6, e22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mukherjee M., Ge G., Zhang N., Edwards D. G., Sumazin P., Sharan S. K., Rao P. H., Medina D., Pati D. (2014) MMTV-Espl1 transgenic mice develop aneuploid, estrogen receptor alpha (ERα)-positive mammary adenocarcinomas. Oncogene 33, 5511–5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mukherjee M., Byrd T., Brawley V. S., Bielamowicz K., Li X. N., Merchant F., Maitra S., Sumazin P., Fuller G., Kew Y., Sun D., Powell S. Z., Ahmed N. M., Zhang N., Pati D. (2014) Overexpression and constitutive nuclear localization of cohesin protease Separase protein correlates with high incidence of relapse and reduced overall survival in glioblastoma multiforme. J. Neurooncol. 119, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meyer R., Fofanov V., Panigrahi A., Merchant F., Zhang N., Pati D. (2009) Overexpression and mislocalization of the chromosomal segregation protein separase in multiple human cancers. Clin. Cancer Res. 15, 2703–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakahira H., Nagata S. (2002) Co-translational folding of caspase-activated DNase with Hsp70, Hsp40, and inhibitor of caspase-activated DNase. J. Biol. Chem. 277, 3364–3370 [DOI] [PubMed] [Google Scholar]
- 40. Sakahira H., Iwamatsu A., Nagata S. (2000) Specific chaperone-like activity of inhibitor of caspase-activated DNase for caspase-activated DNase. J. Biol. Chem. 275, 8091–8096 [DOI] [PubMed] [Google Scholar]
- 41. Sun Y., Yu H., Zou H. (2006) Nuclear exclusion of separase prevents cohesin cleavage in interphase cells. Cell Cycle 5, 2537–2542 [DOI] [PubMed] [Google Scholar]