Abstract
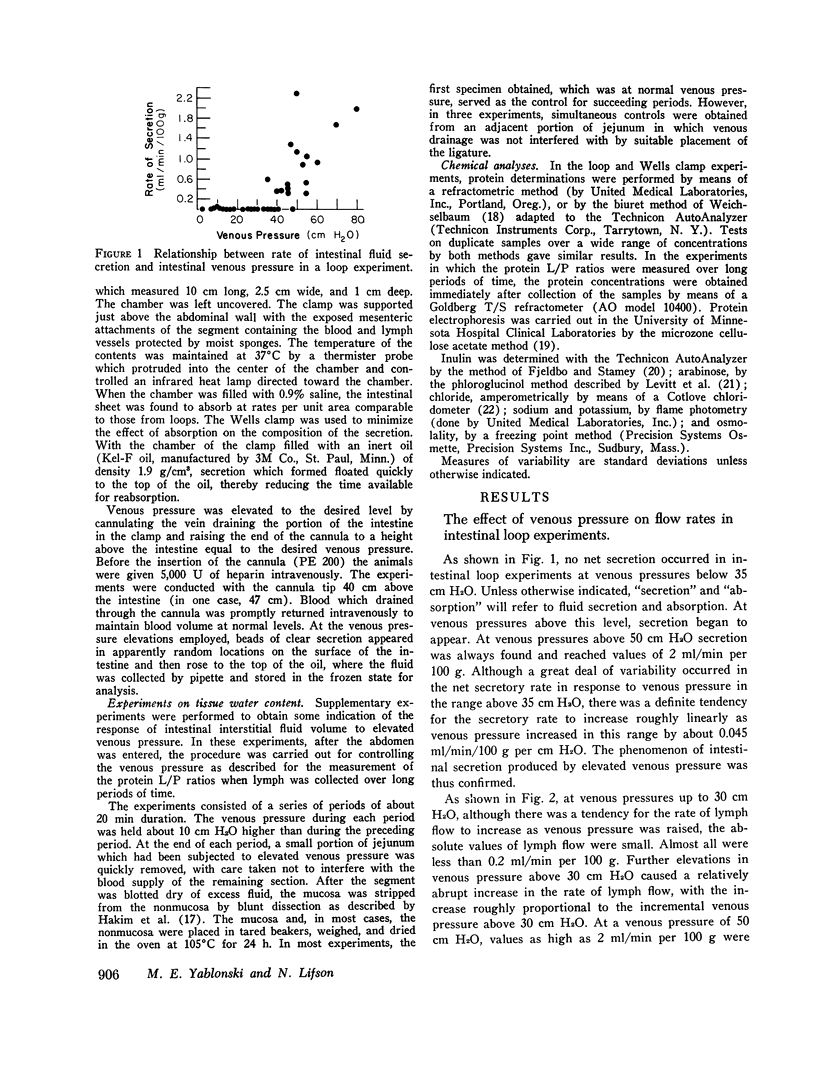
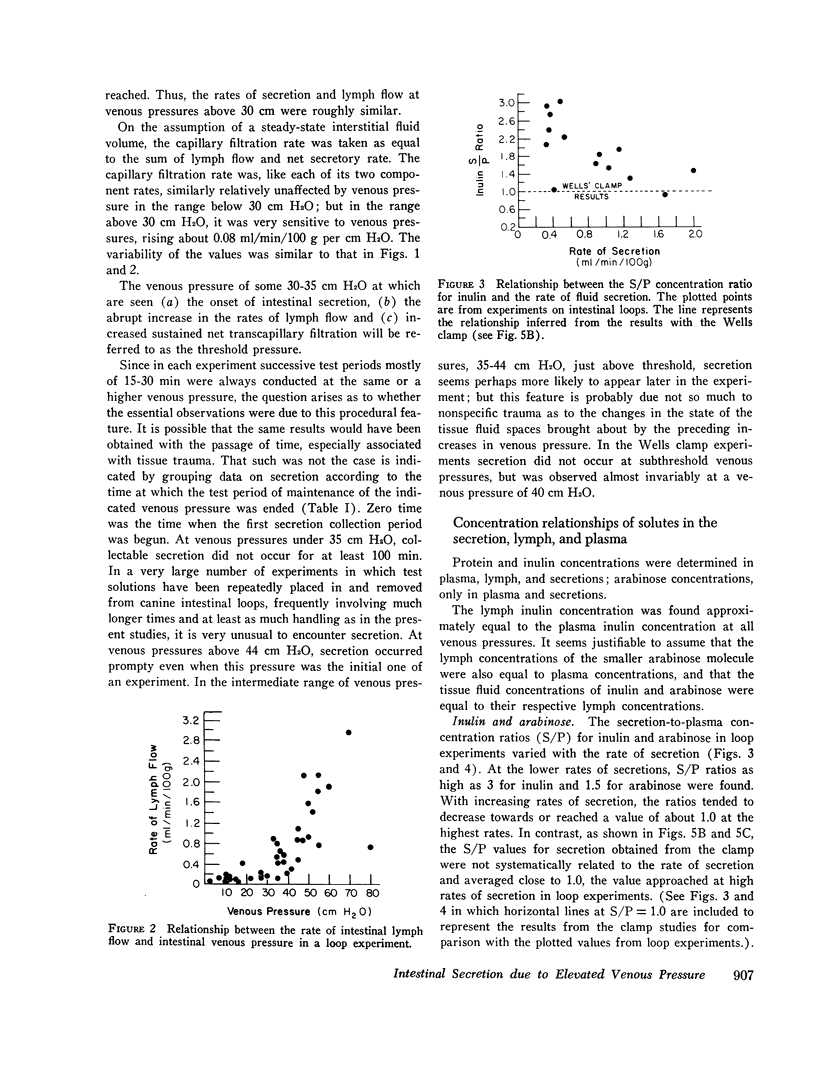
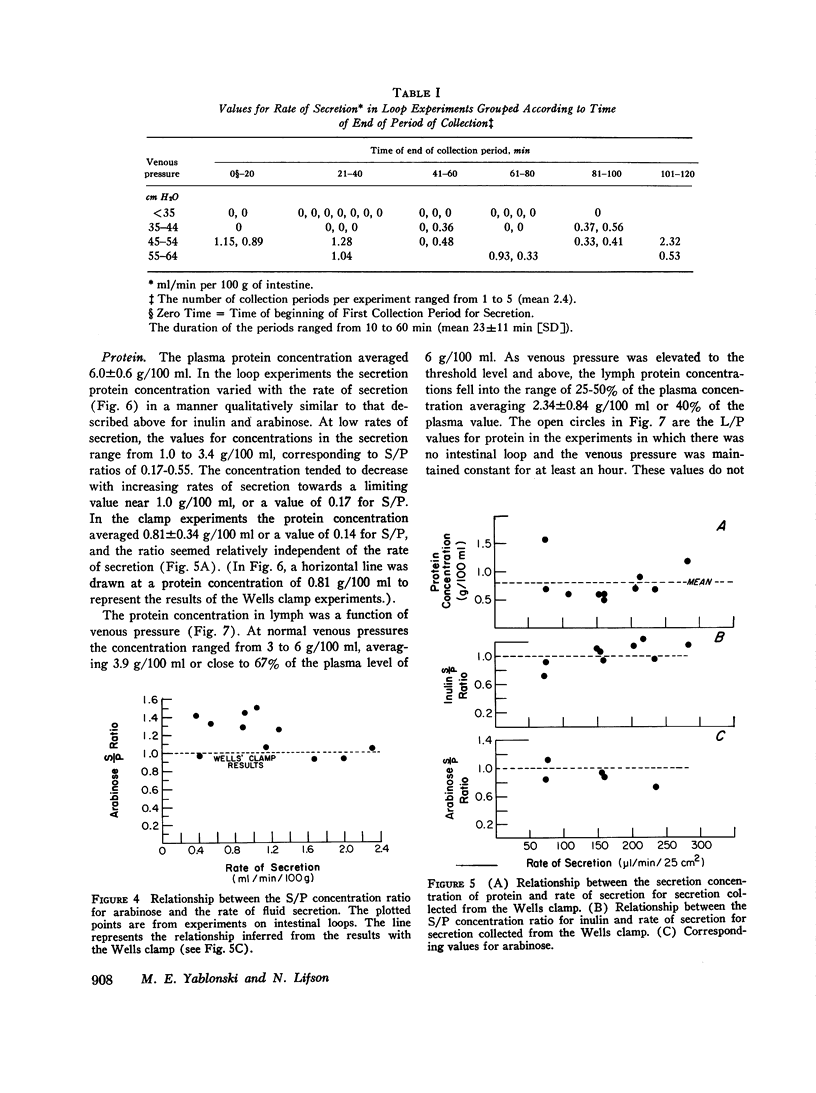
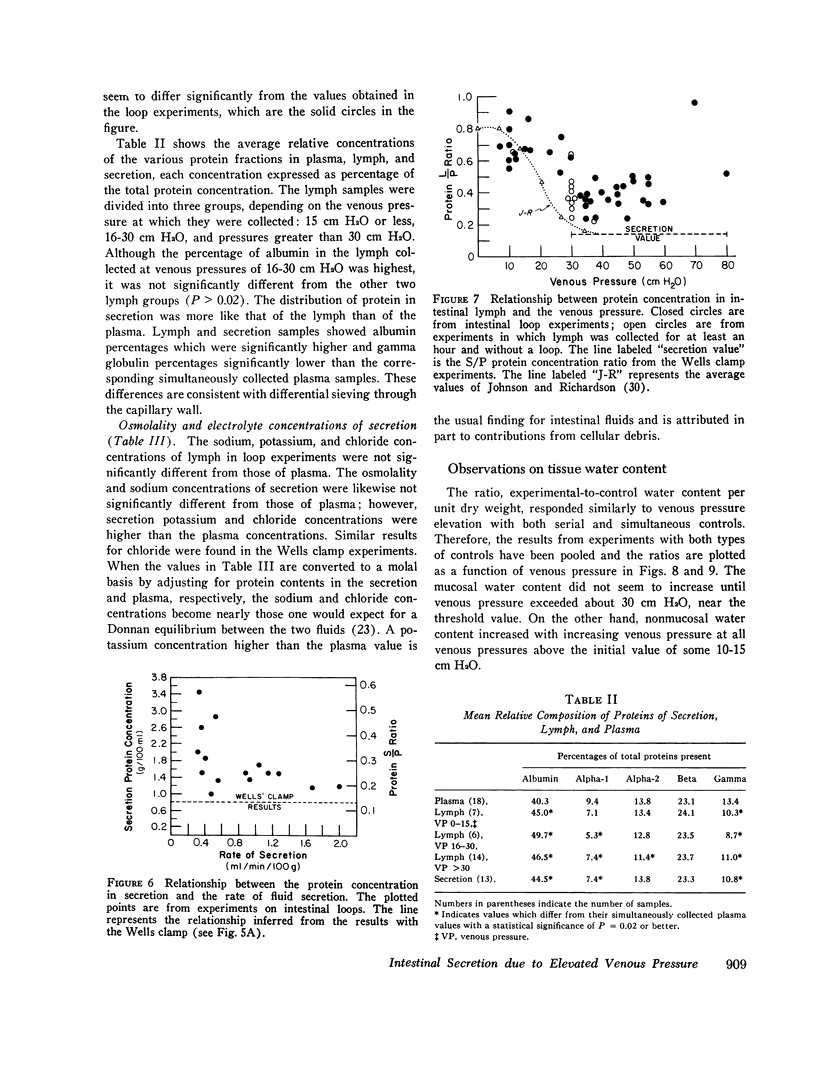
A study was carried out to elucidate the physiological mechanisms responsible for the intestinal secretion produced by venous pressure elevation. In dogs, measurements were made of the rate and composition of small intestinal secretion, rate of flow and composition of intestinal lymph, plasma composition, and mucosal water content, all in response to elevations of intestinal venous pressure. Venous pressure elevations above a threshold value of 30-35 cm H2O produce secretion at a rate of approximately proportional to the value of the pressure minus the threshold value. Above the threshold value, there were large increases in the rates of lymph flow and net sustained transcapillary filtration. These rates were also roughly proportional to the incremental venous pressure. It is concluded that intestinal secretion produced by elevated venous pressure is almost surely secretory filtration, a passive process with the driving force for secretion an increase in mucosal tissue fluid pressures to values of only some 4-6 cm H2O. The increased tissue fluid pressure not only provides the driving force but also produces an increase in the hydraulic permeability of the epithelium without which the driving force would be ineffective. The transepithelial channels are large enough to permit insulin to pass freely and even plasma protein to pass in large amounts, and hence are most probably intercellular. Secretory filtration probably represents a general pathophysiological response of transporting epithelia to elevated tissue fluid pressure. It is proposed that the threshold value for secretion and associated changes is explained by dilution of the tissue fluid protein colloid osmotic pressure in a small subepithelial, juxtacapillary compartment.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTALDI G., STROSSELLI E. Peroral biopsy of the intestinal mucosa in hepatic cirrhosis. Am J Dig Dis. 1960 Jul;5:603–612. doi: 10.1007/BF02290193. [DOI] [PubMed] [Google Scholar]
- Altamirano M., Requena M., Durbin R. P. Effects of gastric arterial and venous pressures on gastric secretion in the dog. Am J Physiol. 1974 Jul;227(1):152–160. doi: 10.1152/ajplegacy.1974.227.1.152. [DOI] [PubMed] [Google Scholar]
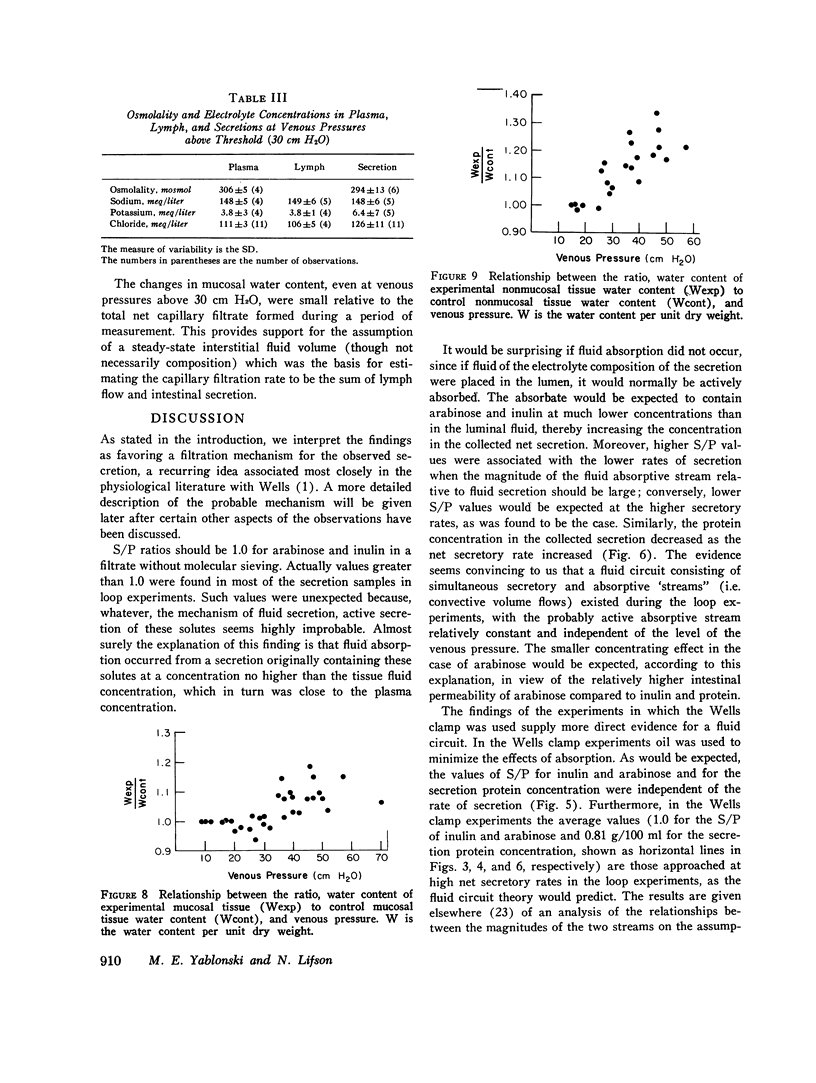
- Bank N., Yarger W. E., Aynedjian H. S. A microperfusion study of sucrose movement across the rat proximal tubule during renal vein constriction. J Clin Invest. 1971 Feb;50(2):294–302. doi: 10.1172/JCI106494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTLOVE E., TRANTHAM H. V., BOWMAN R. L. An instrument and method for automatic, rapid, accurate, and sensitive titration of chloride in biologic samples. J Lab Clin Med. 1958 Mar;51(3):461–468. [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi F., Palade G. E. Intestinal capillaries. I. Permeability to peroxidase and ferritin. J Cell Biol. 1969 Apr;41(1):33–58. doi: 10.1083/jcb.41.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook B. H., Wilson E. R., Jr, Taylor A. E. Intestinal fluid loss in hemorrhagic shock. Am J Physiol. 1971 Nov;221(5):1494–1498. doi: 10.1152/ajplegacy.1971.221.5.1494. [DOI] [PubMed] [Google Scholar]
- DIETSCHY J. M. WATER AND SOLUTE MOVEMENT ACROSS THE WALL OF THE EVERTED RABBIT GALL BLADDER. Gastroenterology. 1964 Oct;47:395–408. [PubMed] [Google Scholar]
- DiBona D. R., Chen L. C., Sharp G. W. A study of intercellular spaces in the rabbit jejunum during acute volume expansion and after treatment with cholera toxin. J Clin Invest. 1974 May;53(5):1300–1307. doi: 10.1172/JCI107677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeldbo W., Stamey T. A. Adapted method for determination of inulin in serum and urine with an AutoAnalyzer. J Lab Clin Med. 1968 Aug;72(2):353–358. [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Ewton M. F., Soter N., Kinney J. Permeability characteristics of the human small intestine. J Clin Invest. 1965 Dec;44(12):1935–1944. doi: 10.1172/JCI105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKIM A., LESTER R. G., LIFSON N. Absorption by an in vitro preparation of dog intestinal mucosa. J Appl Physiol. 1963 Mar;18:409–413. doi: 10.1152/jappl.1963.18.2.409. [DOI] [PubMed] [Google Scholar]
- Hakim A. A., Lifson N. Effects of pressure on water and solute transport by dog intestinal mucosa in vitro. Am J Physiol. 1969 Feb;216(2):276–284. doi: 10.1152/ajplegacy.1969.216.2.276. [DOI] [PubMed] [Google Scholar]
- Higgins J. T., Jr, Blair N. P. Intestinal transport of water and electrolytes during extracellular volume expansion in dogs. J Clin Invest. 1971 Dec;50(12):2569–2579. doi: 10.1172/JCI106757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann E. Uber das Endothel der Zottenkapillaren im Dünndarm des Meerschweinchens und des Menschen. Z Zellforsch Mikrosk Anat. 1966;72(3):364–369. [PubMed] [Google Scholar]
- Humphreys M. H., Earley L. E. The mechanism of decreased intestinal sodium and water absorption after acute volume expansion in the rat. J Clin Invest. 1971 Nov;50(11):2355–2367. doi: 10.1172/JCI106734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON P. C. EFFECT OF VENOUS PRESSURE ON MEAN CAPILLARY PRESSURE AND VASCULAR RESISTANCE IN THE INTESTINE. Circ Res. 1965 Mar;16:294–300. doi: 10.1161/01.res.16.3.294. [DOI] [PubMed] [Google Scholar]
- Johnson P. C., Hanson K. M. Capillary filtration in the small intestine of the dog. Circ Res. 1966 Oct;19(4):766–773. doi: 10.1161/01.res.19.4.766. [DOI] [PubMed] [Google Scholar]
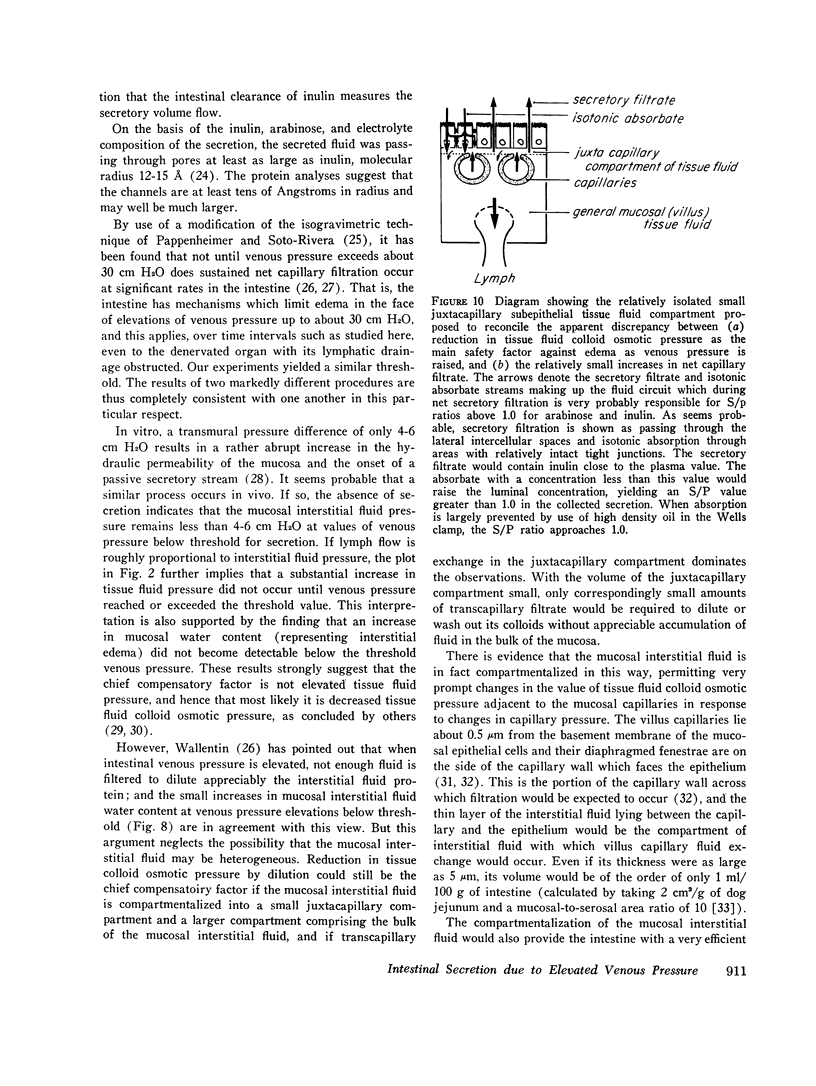
- Johnson P. C., Richardson D. R. The influence of venous pressure on filtration forces in the intestine. Microvasc Res. 1974 May;7(3):296–306. doi: 10.1016/0026-2862(74)90017-x. [DOI] [PubMed] [Google Scholar]
- KEDEM O., KATCHALSKY A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958 Feb;27(2):229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- Lee J. S. Effects of pressures on water absorption and secretion in rat jejunum. Am J Physiol. 1973 Jun;224(6):1338–1344. doi: 10.1152/ajplegacy.1973.224.6.1338. [DOI] [PubMed] [Google Scholar]
- Levitt D. G., Hakim A. A., Lifson N. Evaluation of components of transport of sugars by dog jejunum in vivo. Am J Physiol. 1969 Sep;217(3):777–783. doi: 10.1152/ajplegacy.1969.217.3.777. [DOI] [PubMed] [Google Scholar]
- Lifson N., Hakim A. A., Lender E. J. Effects of cholera toxin on intestinal permeability and transport interactions. Am J Physiol. 1972 Jun;222(6):1479–1487. doi: 10.1152/ajplegacy.1972.222.6.1479. [DOI] [PubMed] [Google Scholar]
- Nusbaum M., Baum S., Rajatapiti B., Blakemore W. S. Intestinal lymphangiography in vivo. J Cardiovasc Surg (Torino) 1967 Jan-Feb;8(1):62–68. [PubMed] [Google Scholar]
- Parsons D. S., Powis G. Some properties of a preparation of rat colon perfused in vitro through the vascular bed. J Physiol. 1971 Sep;217(3):641–663. doi: 10.1113/jphysiol.1971.sp009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R. The absorption and secretion of fluid and electrolytes by the obstructed bowel. Br J Surg. 1965 Oct;52(10):774–779. doi: 10.1002/bjs.1800521019. [DOI] [PubMed] [Google Scholar]
- Soergel K. H., Whalen G. E., Harris J. A. Passive movement of water and sodium across the human small intestinal mucosa. J Appl Physiol. 1968 Jan;24(1):40–48. doi: 10.1152/jappl.1968.24.1.40. [DOI] [PubMed] [Google Scholar]



