Abstract
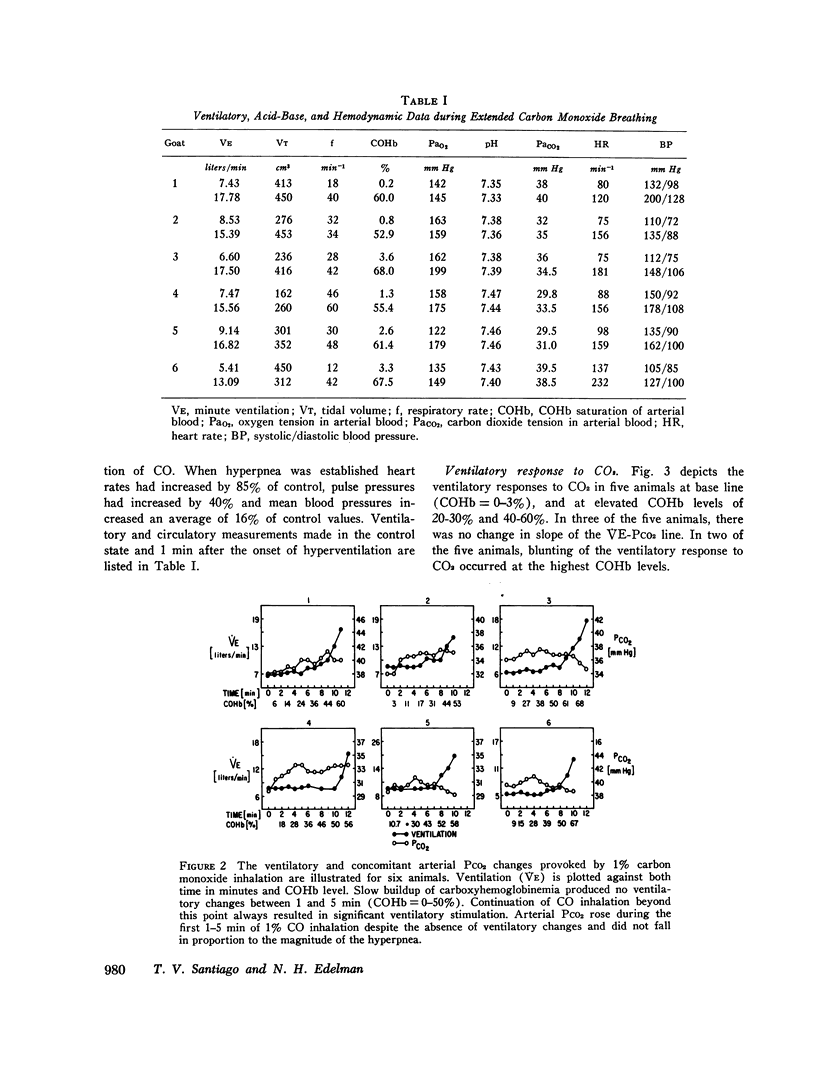
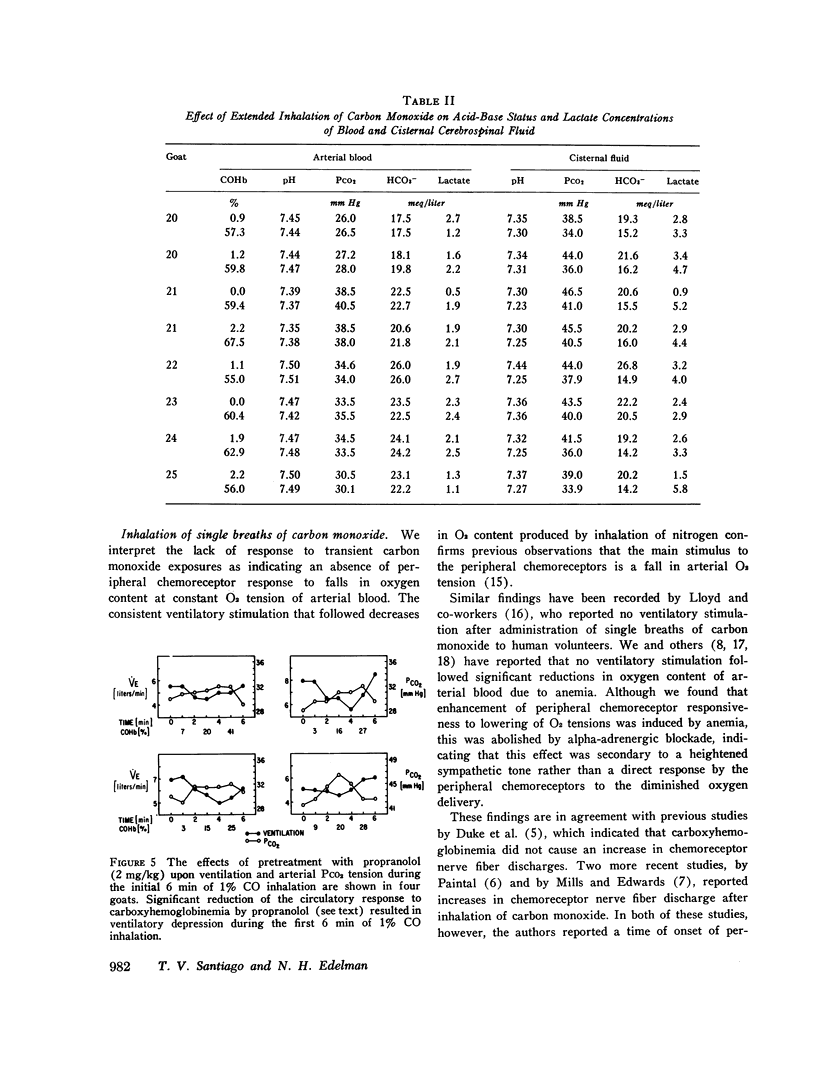
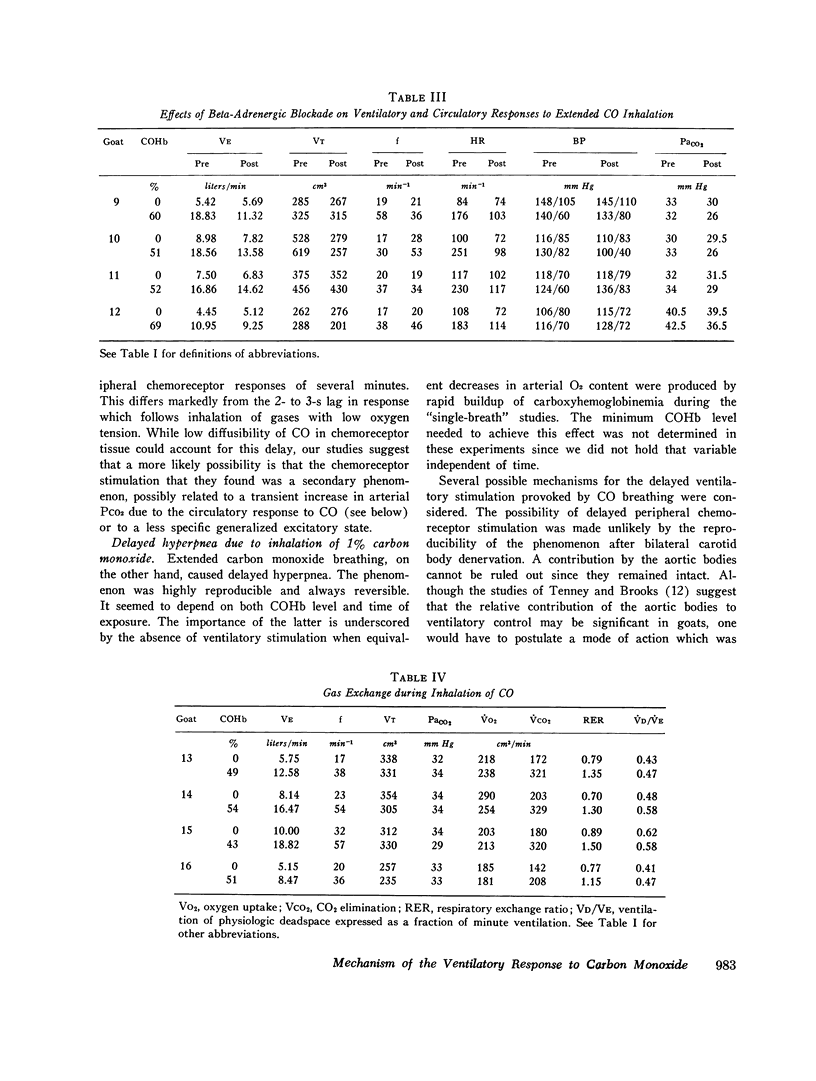
The effects of carbon monoxide on ventilation were studied in unanesthetized goats. Responses to single breaths of 10-25% CO in O2, which rapidly raised carboxyhemoglobin (COHb) from 5 to 60%, were considered to reflect peripheral chemoreceptor-mediated reflexes whereas responses to continuous inhalation of 1% CO in O2, which slowly raised COHb from 0 to 60%, were considered to reflect both peripheral chemoreceptor and nonperipheral chemoreceptor mechanisms. In each of six goats, single breaths of CO failed to elicit any immediate ventilatory response. However, slow buildup of carboxyhemoglobinemia in the same animals always elicited ventilatory stimulation (from a mean of 7.43 to 16.02 liter/min, P less than 0.001) beginning 5-6 min after onset of 1% CO in O2 inhalation when COHb saturation reached 50-60%. In eight studies of six animals HCO3- concentration fell (from 21.3 to 15.8 meq/liter; P less than 0.001) and lactate concentration rose (from 2.5 to 4.2 meq/liter; P less than 0.05) in the cisternal cerebrospinal fluid during the CO-induced hyperpnea. Additional studies ruled out ventilatory stimulation from left heart failure or enhanced chemo-sensitivity to carbon dioxide. Although the delayed hyperpnea was associated with a hyperdynamic cardiovascular response to CO, blockade of these circulatory effects with propranolol (2 mg/kg) failed to abolish the delayed hyperpnea; however, the propranolol did unmask an element of ventilatory depression which preceded the hyperpnea. Conclusions were: (a) hyperventilation in response to CO inhalation is not mediated by the carotid bodies; (b) the delayed hyperpnea in response to CO inhalation is primarily due to brain-cerebrospinal fluid acidosis; (c) mobilization of body CO2 stores due to the circulatory response to CO may obscure an initial depression of ventilation by CO.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres S. M., Giannelli S., Jr, Mueller H. Myocardial and systemic responses to carboxyhemoglobin. Ann N Y Acad Sci. 1970 Oct 5;174(1):268–293. doi: 10.1111/j.1749-6632.1970.tb49795.x. [DOI] [PubMed] [Google Scholar]
- COHEN M. I. RESPIRATORY PERIODICITY IN THE PARALYZED, VAGOTOMIZED CAT: HYPOCAPNIC POLYPNEA. Am J Physiol. 1964 Apr;206:845–854. doi: 10.1152/ajplegacy.1964.206.4.845. [DOI] [PubMed] [Google Scholar]
- DUKE H. N., GREEN J. H., NEIL E. Carotid chemoceptor impulse activity during inhalation of carbon monoxide mixtures. J Physiol. 1952 Dec;118(4):520–527. doi: 10.1113/jphysiol.1952.sp004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman N. H., Cherniack N. S., Lahiri S., Fishman A. P. Response of goats to acute, chronic and life-long hypoxia. Fed Proc. 1969 May-Jun;28(3):1223–1227. [PubMed] [Google Scholar]
- Haldane J. The Action of Carbonic Oxide on Man. J Physiol. 1895 Nov 16;18(5-6):430–462. doi: 10.1113/jphysiol.1895.sp000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner P. I., Uther J. B., White S. W. Central nervous integration of the circulatory and respiratory responses to arterial hypoxemia in the rabbit. Circ Res. 1969 Jun;24(6):757–776. doi: 10.1161/01.res.24.6.757. [DOI] [PubMed] [Google Scholar]
- Lugliani R., Whipp B. J., Seard C., Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med. 1971 Nov;285(20):1105–1111. doi: 10.1056/NEJM197111112852002. [DOI] [PubMed] [Google Scholar]
- Marbach E. P., Weil M. H. Rapid enzymatic measurement of blood lactate and pyruvate. Use and significance of metaphosphoric acid as a common precipitant. Clin Chem. 1967 Apr;13(4):314–325. [PubMed] [Google Scholar]
- Miller M. J., Tenney S. M. Hypoxia-induced tachypnea in carotid-deafferented cats. Respir Physiol. 1975 Jan;23(1):31–39. doi: 10.1016/0034-5687(75)90069-9. [DOI] [PubMed] [Google Scholar]
- Mills E., Edwards M. W., Jr Stimulation of aortic and carotid chemoreceptors during carbon monoxide inhalation. J Appl Physiol. 1968 Nov;25(5):494–502. doi: 10.1152/jappl.1968.25.5.494. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., FENCL V., HEISEY S. R., HELD D. ROLE OF CEREBRAL FLUIDS IN CONTROL OF RESPIRATION AS STUDIED IN UNANESTHETIZED GOATS. Am J Physiol. 1965 Mar;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Mechanism of stimulation of aortic chemoreceptors by natural stimuli and chemical substances. J Physiol. 1967 Mar;189(1):63–84. doi: 10.1113/jphysiol.1967.sp008155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D. W., Strauss M. L. CIRCULATORY ADJUSTMENT IN ANEMIA. J Clin Invest. 1928 Feb;5(2):161–180. doi: 10.1172/JCI100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago T. V., Edelman N. H., Fishman A. P. The effect of anemia on the ventilatory response to transient and steady-state hypoxia. J Clin Invest. 1975 Feb;55(2):410–418. doi: 10.1172/JCI107945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen S. C. Ventilatory acclimatization to hypoxia in rabbits after denervation of peripheral chemoreceptors. J Appl Physiol. 1970 Jun;28(6):836–839. doi: 10.1152/jappl.1970.28.6.836. [DOI] [PubMed] [Google Scholar]
- Tenney S. M., Brooks J. G., 3rd Carotid bodies, stimulus interaction, and ventilatory control in unanesthetized goats. Respir Physiol. 1966;1(2):211–224. doi: 10.1016/0034-5687(66)90018-1. [DOI] [PubMed] [Google Scholar]
- Wade J. G., Larson C. P., Jr, Hickey R. F., Ehrenfeld W. K., Severinghaus J. W. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970 Apr 9;282(15):823–829. doi: 10.1056/NEJM197004092821501. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. J., Castagna J. Cardiodynamic hyperpnea: hyperpnea secondary to cardiac output increase. J Appl Physiol. 1974 Apr;36(4):457–464. doi: 10.1152/jappl.1974.36.4.457. [DOI] [PubMed] [Google Scholar]


