Abstract
Converging lines of evidence support the use of environmental stimulation to ameliorate the symptoms of a variety of neurodevelopmental disorders. Applying these interventions at very early ages is critical to achieve a marked reduction of the pathological phenotypes. Here we evaluated the impact of early social enrichment in Fmr1-KO mice, a genetic mouse model of fragile X syndrome (FXS), a major developmental disorder and the most frequent monogenic cause of autism. Enrichment was achieved by providing male KO pups and their WT littermates with enhanced social stimulation, housing them from birth until weaning with the mother and an additional nonlactating female. At adulthood they were tested for locomotor, social, and cognitive abilities; furthermore, dendritic alterations were assessed in the hippocampus and amygdala, two brain regions known to be involved in the control of the examined behaviors and affected by spine pathology in Fmr1-KOs. Enrichment rescued the behavioral FXS-like deficits displayed in adulthood by Fmr1-KO mice, that is, hyperactivity, reduced social interactions, and cognitive deficits. Early social enrichment also eliminated the abnormalities shown by adult KO mice in the morphology of hippocampal and amygdala dendritic spines, namely an enhanced density of immature vs mature types. Importantly, enrichment did not induce neurobehavioral changes in WT mice, thus supporting specific effects on FXS-like pathology. These findings show that early environmental stimulation has profound and long-term beneficial effects on the pathological FXS phenotype, thereby encouraging the use of nonpharmacological interventions for the treatment of this and perhaps other neurodevelopmental diseases.
INTRODUCTION
Several lines of clinical and preclinical research support the use of behavioral/environmental stimulation to ameliorate the symptoms of a variety of neurological diseases (Singer et al, 2005). For neurodevelopmental disorders, applying these interventions at very early ages is critical to achieve a substantial reduction in the expression of behavioral abnormalities (Dawson, 2008). Several studies have demonstrated, for instance, marked beneficial effects of behavioral therapies in autistic children when started at 12 months of age, and have shown that stimulating parent–child interactions within the family context is necessary for the success of these early interventions (Dawson, 2008).
Mouse models have a crucial value for investigating the therapeutic impact of environmental stimulation because they allow manipulating selected components of the rearing environment at specific developmental phases (Nithianantharajah and Hannan, 2006). Nonetheless, providing additional stimulation during early life in laboratory mice is not always an easy task. The most common strategy to provide environmental stimulation consists of exposing animals to complex environments, that is, cages equipped with toys and running wheels (Renner and Rosenzweig, 1987). However, the exploitation of this kind of environment is limited in mouse pups owing to their motor and sensorial immaturity. In fact, the exposure of laboratory mice to this type of enrichment before weaning does not entail the neurobiological effects that are typically induced by the same postweaning intervention (Kohl et al, 2002).
Rather, as the main component of the environment of an infant laboratory mouse is represented by its mother, a useful alternative strategy to enhance stimulation during early life phases is to manipulate mother–infant interactions. This approach, by focusing on parent–infant relationships, models more closely human early behavioral interventions (Dawson, 2008). Our previous work has demonstrated that early environmental enrichment can be achieved by stimulating mouse pups with enhanced maternal care, housing them with an additional nonlactating female from birth until weaning (D'Amato et al, 2011). This enrichment has been shown to exert long-lasting beneficial brain and behavioral effects in outbred mice (D'Amato et al, 2011). Furthermore, the use of a nonlactating rather than a lactating additional dam allows a selective stimulation of mother–infant interactions without the confounding effects of supplementary nutrition (D'Amato et al, 2011; Heiderstadt et al, 2014).
Based on this background, here we used the experimental approach of the additional nonlactating dam to evaluate the therapeutic impact of early enrichment in a mouse model of a major neurodevelopmental disorder, that is, fragile X syndrome (FXS). FXS is the most common form of inherited intellectual disability (Crawford et al, 2001). It is caused by a mutation in the fragile X mental retardation 1 (FMR1) gene on the X chromosome, leading to a lack of the FMR protein (FMRP) (Pieretti et al, 1991), an RNA-binding protein that controls synaptic function/maturation (Greenough et al, 2001; Brennan et al, 2006) and regulates dendritic branching (Edbauer et al, 2010). Both human and animal studies have demonstrated that the lack of FMRP induces abnormal morphology of dendritic spines in several brain regions, thus leading to the predominance of an immature spine phenotype (Lauterborn et al, 2007; Qin et al, 2011; Portera-Cailliau, 2012; He and Portera-Cailliau, 2013; Lauterborn et al, 2013). Besides these brain abnormalities, FXS patients and mice are characterized by a constellation of behavioral symptoms, including hyperactivity, reduced social interest/interactions, and cognitive deficits (Tranfaglia, 2011; Pietropaolo and Subashi, 2014). Importantly, these behavioral abnormalities also represent autistic-like symptoms. In fact, FXS is the most common monogenic cause of autism, and hence increasing attention has been paid recently to the link between the two pathologies and to the possible implications of the Fmr1-KO mouse line for autism research (Bernardet and Crusio, 2006; Oddi et al, 2013).
Here Fmr1-KO mice and their WT littermates were exposed to environmental enrichment (ie, their lactating mother plus a nonlactating female) until weaning. At adulthood they were tested for locomotor, social, and cognitive behaviors; furthermore, dendritic abnormalities were assessed in the hippocampus and amygdala, two brain regions known to be involved in the control of the examined behaviors and affected by spine pathology in Fmr1-KOs (Lauterborn et al, 2007; Qin et al, 2011; Lauterborn et al, 2013). To demonstrate the impact of this environmental manipulation, we also evaluated the effects of enrichment on mother–infant interaction, thus assessing the amount of maternal care received by the pups and their response to maternal separation.
MATERIALS AND METHODS
Animals and Enrichment Procedure
Breeding trios obtained from The Jackson Laboratory were formed by two virgin heterozygous FVB.129P2-Fmr1tm1Cgr/J (FVB) females with a wild-type male from the FVB.129P2-Pde6b+Tyrc-ch/AntJ strain. The FVB background was used in our previous studies (Pietropaolo et al, 2011), in which it showed marked behavioral deficits, especially in social interaction with an adult female. Furthermore, this mouse strain has a larger litter size than others used as background for the Fmr1-KO line, thus allowing us to minimize the number of dams used for our experiments. The FVB.129P2-Pde6b+Tyrc-ch/AntJ strain was specifically chosen because it lacks the visual impairment typically affecting the FVB/N strain, thus making it more suitable for behavioral testing (Errijgers et al, 2007). After 2 weeks, the sire was removed and the heterozygous females were single caged and left either alone (standard group (STD)) or housed with a virgin NMRI adult female (enriched group (ENR)). NMRI 10 week-old females were purchased from Janvier (Le Genest-Saint-Isle, France).
Only male offspring (Fmr1-KOs and their WT littermates) were used for all experiments. Three cohorts of mice were employed. Cohort 1 (13 WT-STD, 9 KO-STD, 11 WT-ENR, and 13 KO-ENR) was evaluated for mother–infant interaction, locomotor activity, and social interaction at adulthood. Cohort 2 (7 WT-STD, 6 KO-STD, 10 WT-ENR, and 7 KO-ENR) underwent the cognitive tests of T-maze and context freezing at adulthood, and cohort 3 (7 WT-STD, 11 KO-STD, 8 WT-ENR, and 9 KO-ENR) was kept behaviorally naive and used for brain analyses. Cohort 1 was obtained from 19 litters (10 STD and 9 ENR), and cohorts 2 and 3 originated from 15 litters (7 STD and 8 ENR). The precise number of animals included in the analysis of each data set is specified in each figure legend. Small deviations from the original number are because of technical issues (ie, problems in data acquisition).
Litters were not culled at birth, and male mice were kept with their female littermates in their respective housing conditions until PND 21 when they were weaned, genotyped, and group-housed with their same-sex littermates (3–5/cage). All animals were maintained in a temperature-controlled (22 °C) and humidity-controlled (55%) vivarium, under a 12 : 12 h light–dark cycle (lights on at 0700 h).
All experimental procedures were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and local French legislation.
Behavioral Testing
Mother–infant interaction
Assessment of maternal behavior. Maternal behavior was assessed for a subset of 12 litters (6 STD and 6 ENR) from cohort 1. All mothers and nonlactating females were observed in their home cages twice a day for 1 h (at 1100 h and at 1500 h) from PND 1 to PND 7, using an instantaneous sampling method (one sampling/2 min, for a total of 30 sampling points/session). The following items were scored as frequencies (Champagne et al, 2007; Curley et al, 2012): (1) nursing postures, that is, arched-back nursing (the female is in an arched position over the nursing pups) and blanket nursing (the female is lying flat on top of the pups); (2) nonnursing postures (the female is in contact with the pups, but not nursing, ie, with no access to the nipples); (3) licking/grooming of the pups; and (4) nest building (the female is pushing and retrieving the nesting materials around the pups).
Assessment of pups' response to maternal separation. On PND 8, pups from cohort 1 were assessed for ultrasonic vocalizations (USVs) in response to maternal separation. Each subject was individually placed into a glass beaker (10 × 8 × 6.5 cm) containing fresh clean bedding and left alone for 5 min in a separate room. An ultrasound microphone (UltraSoundGate CM16, Avisoft Bioacoustics, Berlin, Germany) sensitive to frequencies of 15–180 kHz with a flat frequency response (±6 dB) between 25 and 140 kHz was placed 1 cm above the top of the beaker. Vocalizations were recorded at 250 kHz in 16-bit format through an external audio interface (USG 116, Avisoft Bioacoustics) connected to a personal computer. At the end of each recording session, each pup was weighted and returned to its home cage. Pups' body temperature was also measured immediately before and after testing via a rodent infrared thermometer (153 IRB, Bioseb, Vitrolles, France).
Analysis of ultrasonic calls. In addition to the number and mean duration of the USVs, a detailed qualitative analysis of USVs was conducted. This included peak amplitude, that is, the highest energy within the spectrum of the element, and peak frequency, that is, the frequency at the location of the peak amplitude. Peak amplitude and peak frequency at the start and the end of the calls were also measured, along with bandwidth, which is the difference between the highest and the lowest peak frequency within the element. Finally, the duration of intervals between subsequent calls was measured.
Adult behaviors
At 3 months of age, mice were assessed for locomotor activity and social interactions (cohort 1) or for cognition first in the T-maze and then in the context freezing paradigm (cohort 2) with 1-week interval between subsequent tests. All behavioral analyses were carried out by an experimenter who was unaware of the experimental condition of the animals.
Locomotor activity
The actimetry cages (Actimeter system, Imetronic, Pessac, France) have been described previously in detail (Pietropaolo et al, 2011). Locomotor activity was measured during 1 h test based on the number of breaks of the infrared captors mounted along the longer side walls of each actimetry cage.
Social interactions. Social interaction was assessed in a cage where male subjects were isolated for 24 h. An unfamiliar D2 female (3 months old, purchased from Janvier) was then introduced into the testing cage and left there for 6 min. D2 females were housed in unisexual groups in a female-only animal room and were in the nonestrous phase when tested (as assessed by the analysis of the vaginal smear). This specific procedure for assessing adult social interaction (short duration of the encounter and stimulus female in nonestrous phase) was chosen to maximize our chances to observe social investigation/interest, that is, the most relevant parameters of an autistic-like phenotype, thus minimizing confounding factors such as sexual and aggressive behaviors. Testing sessions were recorded and videos analyzed with Observer XT (version 7, Noldus, The Netherlands). Affiliative behaviors were evaluated, including sniffing the head and the snout of the partner, its anogenital region, or any other part of the body; allogrooming (grooming the partner); and traversing the partner's body by crawling over/under from one side to the other. This behavioral analysis has previously revealed a deficit in Fmr1-KO mice (Pietropaolo et al, 2011). During the test an ultrasonic microphone was suspended above the cage lid and used to record and analyze the USVs emitted by the male resident as previously described for pups' USVs.
Spontaneous alternation in the T-maze. Mice were assessed for spontaneous alternation in the T-maze using a 7-trial testing session (ITI=30 s) as previously described (Vandesquille et al, 2013). The presence/absence of alternation was scored on each trial starting from trial 2 using 1/0 values; the single values were combined in percent alternation values, with a chance level corresponding to 50% (alternating on 3 over the total of 6 possibilities). This experimental procedure is known to require intact hippocampal functionality (Vandesquille et al, 2013).
Fear conditioning. Conditioning and context test were conducted in 40 × 35 × 30 cm chambers (Imetronic) placed inside a sound-attenuating cubicle with a background noise of 55 dbA, controlled by the SkAAProg software (Imetronic). The floor consisted of stainless-steel bars allowing the delivery of electric foot shocks. During conditioning, mice received five 2-s electric shocks (0.4 mA, ITI=1 min) after 2 min of habituation. During the test phase (24 h after conditioning) mice were put back in the same chambers for 6 min, without receiving any shock; freezing time was manually scored as the absence of any body movement beside respiration and normalized by the test session length to obtain a percent measure.
Adult dendritic spine morphology
The 3-month-old mice never exposed to behavioral testing (cohort 3) were killed by decapitation. The brains were impregnated by immersion in Golgi-Cox solution (FD Rapid GolgiStain Kit, FD NeuroTechnologies, Columbia, MD) and stained according to the manufacture's protocol. Fully impregnated pyramidal neurons lying in the CA1 region of the dorsal hippocampus and in basolateral amygdala were identified under low magnification (20 × /0.5 NA) from sections mounted on gelatinized slides. The basolateral amygdala was chosen as it is known to be implicated in assigning affective value to stimuli, with a leading role in memory storage during fear conditioning, as demonstrated by a converging body of literature (reviewed in Sah et al, 2003). For each region of interest (ROI), 3 neurons displaying dendritic tree without obvious truncations and balanced per hemisphere were analyzed under higher magnification (100 × /0.75 NA). Spine analyses were carried out along all neurons by classifying individual spines into four distinct classes with growing levels of maturity/stability, that is, from the immature filopodia and chubby/stubby to the more mature thin and mushroom spines (Portera-Cailliau, 2012). Analyses were performed using a microscope (DMLB, Leica) equipped with a camera (resolution=2600 × 2600, Axiocam, Zeiss) and the KS300 3.0 system (Zeiss). A computer-based neuron tracing system (Neurolucida, Microbrightfield) was used to trace neurons. The average spine density (number of spines per 1-μm-long segment) for each individual spine category was calculated for group mean.
Statistical Analysis
Statistical analyses were carried out by ANOVA with genotype and enrichment as the between-subject factors. Additional within-subject factors such as spine category were included where appropriate. The post hoc comparisons were performed using Fisher's LSD test. For the T-maze data, an additional one-sample t test was further used for comparison with chance level (ie, 50%), as done in previous studies with this alternation testing procedure (Dorey et al, 2011; Vandesquille et al, 2013). All statistical analyses were carried out using SPSS for Windows (release 13.0, SPSS, Chicago, IL) and α was set at 0.05. Results are expressed as mean±SEM throughout the text. A square-root transformation was applied to the number of USVs to better conform to the assumptions of normality for ANOVA.
RESULTS
Specific Effects of Early Social Enrichment on Mother–Infant Interaction and Pup Behaviors
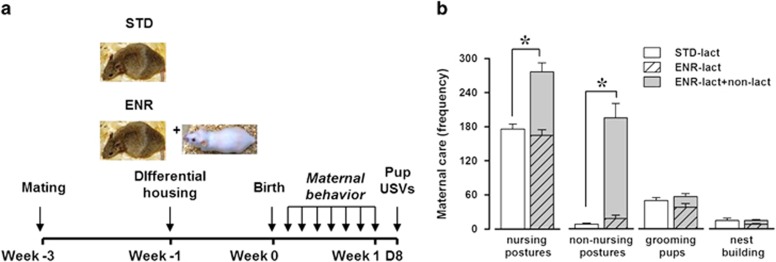
Maternal behavior was assessed during the first postnatal week (see Figure 1a for timing scheme). As expected, early social enrichment resulted in increased levels of total maternal care received by the pups, in particular on the time spent in nursing and nonnursing postures (enrichment effect, respectively: F(1, 10)=29.61 and 52.63, P<0.001 and 0.0001; Figure 1b, white vs gray bars); this was because of the presence of the additional nonlactating female, as no difference in the levels of maternal care provided only by the lactating mothers was observed between enriched and standard conditions on any of the considered behaviors (enrichment effect: NS; Figure 1b, white vs striped bars). Enrichment did not affect the litter size (mean±SEM: 7.33±1.26 (STD), 7.5±0.72 (ENR)) or litter sex composition as evaluated at PND 8 (mean±SEM of % males: 50.52±14.67 (STD), 61.88±8.96 (ENR)).
Figure 1.
Effects of early enrichment on maternal behaviors. Maternal care was assessed according to the timing schematized in (a) and expressed as a mean frequency score over 1-week observation (b). The presence of an additional nonlactating female resulted in a marked enhancement in the levels of total maternal care received by enriched pups (gray vs white bars, b). No difference existed in the maternal behavior of the lactating mothers between ENR and STD conditions (striped vs white bars, b). The behavior of the lactating mother only is represented by the white (STD) and the striped (ENR) bars, and the gray bars describe the sum of the maternal care of both the lactating and the nonlactating dams in the ENR condition. N=6/rearing condition. *Indicates P<0.05.
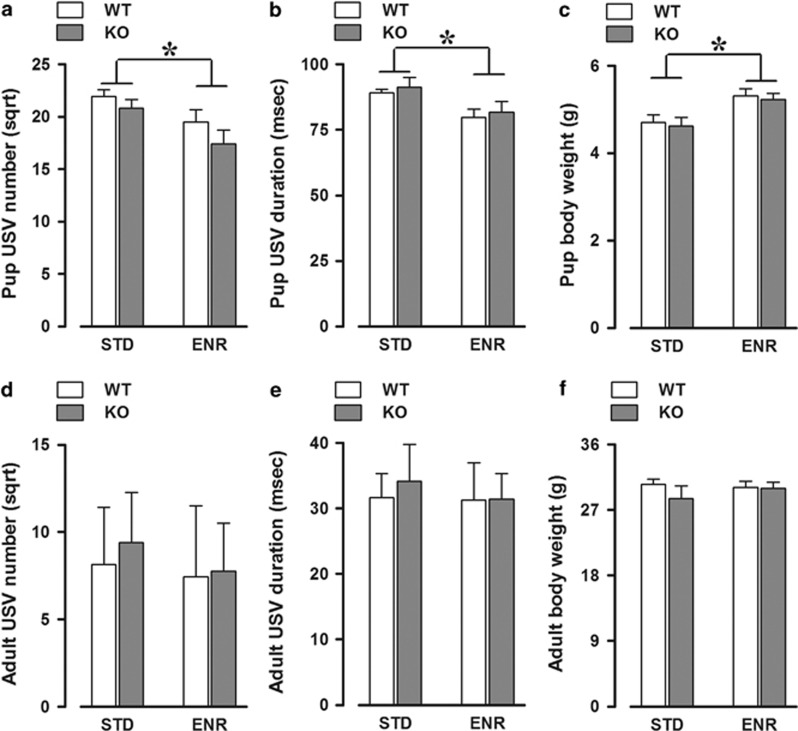
In parallel, at PND 8, enriched pups responded differently to maternal separation, emitting less USVs (enrichment effect: F(1, 39)=7.70, P<0.01; Figure 2a) and of shorter duration (enrichment effect: F(1, 39)=8.47, P<0.01; Figure 2b). This effect was equally observed in both WT and KO mice that did not differ in number as well as the duration of the USVs emitted (genotype effect and its interaction with enrichment, all NS). The effect of enrichment on pup USVs suggests a weaker emotional response to maternal separation, and was not confounded by differences in pups' body temperature loss (mean±SEM values expressed in °C: 2±0.2 and 2.49±0.22 (STD-WT and -KO), 1.93±0.31 and 2.11±0.16 (ENR-WT and -KO)). No difference was observed in other USV characteristics, as evaluated by spectrographic analysis (Table 1). Enriched pups also weighted more at PND 8 (enrichment effect: F(1, 39)=10.03, P<0.01; Figure 2c), suggesting that the enhanced maternal stimulation provided by the additional dam (although not lactating) promoted pup growth. Again, this effect of enrichment was observed in both WT and KO animals that did not differ on this parameter (genotype effect and its interaction with enrichment were both NS).
Figure 2.
Age-specific effects of early enrichment on ultrasonic communication and body weight. Early enrichment reduced the number and mean duration of ultrasonic calls (USVs) and increased body weight of both WT and KO pups (a–c), having no effect on the same parameters at adulthood (d–f). N=12 WT-STD, 8 KO-STD, 11 WT-ENR, and 12 KO-ENR. *Indicates P<0.05.
Table 1. Spectrographic Parameters (SPs) of Ultrasonic Vocalizations Not Significantly Differing among Experimental Groups.
| SP |
Standard |
Enriched |
||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| PND 8 | ||||
| Peak frequency (kHz) | 72.11±1.65 | 69.05±0.77 | 69.40±1.12 | 71.25±0.87 |
| Peak amplitude (dB) | −43.79±2.08 | −38.49±1.43 | −40.45±2.45 | −42.61±1.91 |
| Bandwidth (kHz) | 36.80±1.03 | 36.60±1.96 | 38.19±1.59 | 36.51±1.31 |
| Interval between calls (s) | 0.60±0.03 | 0.82±0.12 | 0.69±0.07 | 1.12±0.19 |
| PND 90 | ||||
| Peak frequency (kHz) | 78.48±1.84 | 75.31±2.00 | 70.92±1.62 | 75.29±2.21 |
| Peak amplitude (dB) | −61.03±1.76 | −59.73±1.51 | −54.51±2.20 | −58.23±2.57 |
| Bandwidth (kHz) | 18.06±3.66 | 21.06±3.99 | 12.39±1.94 | 15.27±2.21 |
| Interval between calls (s) | 0.76±0.15 | 2.63±0.70 | 3.55±1.21 | 2.09±0.46 |
Abbreviation: PND, postnatal day.
Values are mean±SEM.
These effects of enrichment on ultrasonic communication and body weight were both specifically observed at infancy only. No difference among experimental groups was observed at adulthood in both number and duration of USVs (all effects: NS; Figure 2d and e) emitted toward an adult WT female or in any other spectrographic parameters (Table 1). The effects of early enrichment on body weight observed in pups also disappeared at adulthood (all effects: NS; Figure 2f).
Long-Term Effects of Early Social Enrichment on Adult Behaviors
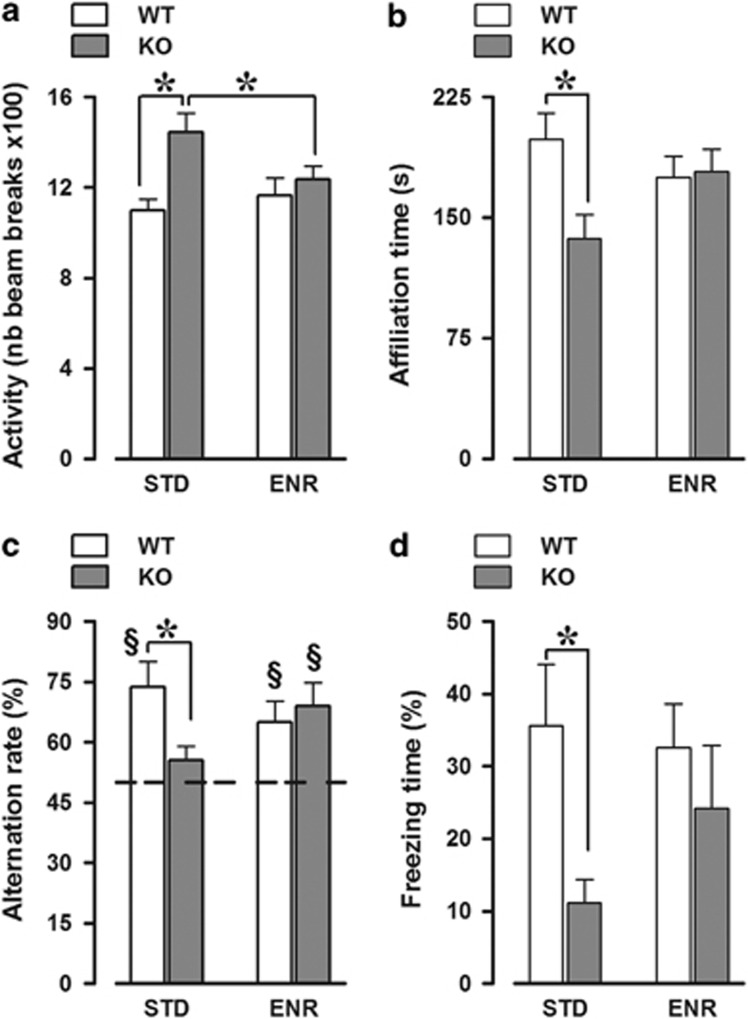
Early social enrichment eliminated the hyperactivity shown at adulthood by Fmr1-KO mice (genotype × enrichment: F(1, 39)=4.02, P=0.05; Figure 3a). Enrichment also rescued the social phenotype of adult Fmr1-KOs, that is, their reduced levels of investigative/affiliative behaviors towards a WT female (genotype × enrichment: F(1, 33)=4.78, P<0.05; Figure 3b). Furthermore, early enrichment eliminated the cognitive deficits displayed by adult Fmr1-KO mice in two hippocampal-dependent tasks. First, enrichment rescued the lack of spontaneous alternation of KO mice in the T-maze, where KO-STD animals showed markedly reduced alternation levels compared with the WT-STD (genotype × enrichment: F(1, 26)=4.05, P=0.06; genotype effect in STD mice: F(1, 11)=6.05, P<0.05, in ENR: F(1, 15)<1, NS; Figure 3c), with a performance that did not differ significantly from chance levels (t-test, P>0.05). Second, enrichment markedly reduced the deficits in context freezing of KO animals (overall genotype effect: F(1, 24)=5.24, P<0.05; Figure 3d), with separate analyses confirming a significant genotype effect in STD (F(1, 11)=6.31, P<0.05), but not in ENR mice (F(1, 13)<1, NS).
Figure 3.
Long-term behavioral effects of early enrichment. Early enrichment rescued the behavioral abnormalities displayed by adult Fmr1-KO mice, including their hyperactivity (a), reduced levels of social interactions with an adult WT female (b), and deficits in spontaneous alternation in the T-maze (c) and in context freezing (d). (a) N=12 WT-STD, 8 KO-STD, 11 WT-ENR, and 12 KO-ENR. (b) N=9 WT-STD, 7 KO-STD, 11 WT-ENR, and 10 KO-ENR. (c) N=7 WT-STD, 6 KO-STD, 10 WT-ENR, and 7 KO-ENR. (d) N=7 WT-STD, 6 KO-STD, 10 WT-ENR, and 5 KO-ENR. §Significantly different from chance level, represented by the dotted line (one-sample t-test was used for comparison with the chance level of 50%). *Indicates P<0.05.
Long-Term Effects of Early Social Enrichment on Adult Spine Morphology
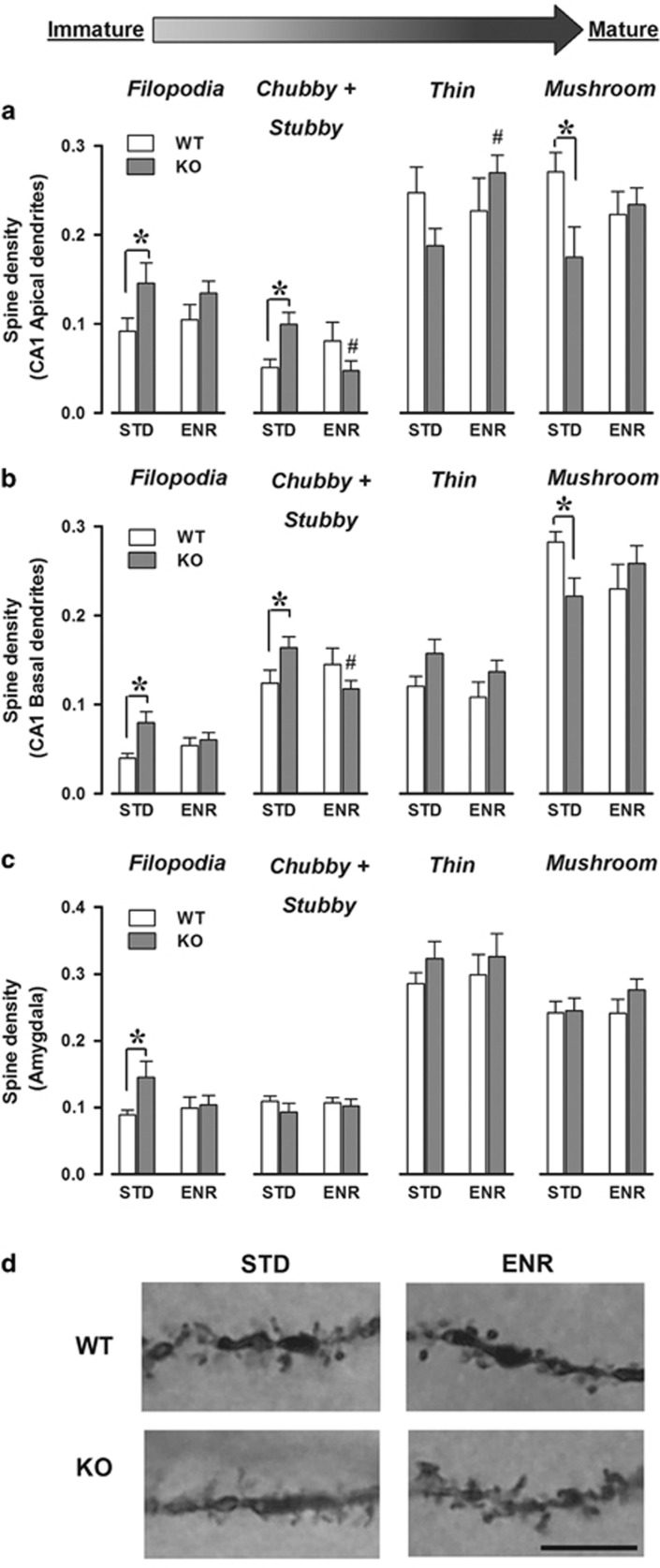
Fmr1-KO mice presented marked abnormalities on hippocampal spine morphology in both apical and basal dendrites and these abnormalities were corrected by enrichment (genotype × enrichment × spine category, respectively: F(3, 195)=4.99, F(3, 201)=5.76; P<0.01; Figure 4a and b). In apical dendrites, KOs reared in STD conditions have more immature filopodia and chubby/stubby and less mature thin and mushroom spines than their wild-type littermates (post hoc: P<0.05; Figure 4a). These abnormalities disappeared in enriched conditions. The same effects on filopodia, chubby/stubby, and mushroom types were observed in basal dendrites (post hoc: P<0.05; Figure 4b).
Figure 4.
Long-term brain effects of early enrichment. Density (ie, number of spines per 1-μm-long segment) of different spine categories in apical (a) and basal dendrites (b) along CA1 pyramidal neurons and in basolateral amygdala (c). #Versus STD-KOs. (a) N=21 WT-STD, 13 KO-STD, 12 WT-ENR, and 23 KO-ENR. (b) N=21 WT-STD, 14 KO-STD, 13 WT-ENR, and 23 KO-ENR. (c) N=15 WT-STD, 13 KO-STD, 13 WT-ENR, and 15 KO-ENR. Categories are ordered according to growing maturity/stability, that is, from the immature filopodia to the mature and stable mushroom types. Representative photomicrograph of a Golgi-stained section of CA1 dendrites (d) in wild-type (top) and FMR1-KO (bottom) mice under standard (left) and enriched (right) conditions. The images show the higher prevalence of immature filopodia-like spines in STD-KO mice, an effect eliminated by enrichment. Scale bar=10 μm. *Indicates P<0.05.
In amygdala neurons, subtle morphological differences were observed between WT and KO mice and they were eliminated by enrichment (Figure 4c). Although the genotype × enrichment × spine category interaction did not reach statistical significance (F(3, 156)=1.15, NS), separate analyses for each spine type revealed that STD-KO mice showed more filopodia-like spines, an effect that disappeared in ENR conditions (genotype effect: F(1, 52)=3.83, P=0.06; in STD: F(1, 24)=5.21, P<0.05, in ENR: NS; Figure 4c).
DISCUSSION
Our findings demonstrate that early environmental enrichment through enhanced maternal stimulation fully rescues the behavioral and brain morphology abnormalities displayed in adulthood by Fmr1-KO mice. The manipulation used clearly affected mother–infant interaction, thus resulting in enhanced maternal physical contact with the pups, that is, more time spent in nursing and nonnursing postures. Interestingly, our enrichment did not induce an increase in grooming/licking of the pups, a category of maternal behavior that is markedly influenced by maternal exposure to stressors (Champagne and Meaney, 2006) or by handling of the pups (Liu et al, 1997) and has been so far a main focus of the studies on maternal care in laboratory rodents, especially rats. Our results, instead, highlight the importance of the time spent in physical contact with the pups as a powerful index of maternal stimulation, as already suggested by previous mouse studies (Shoji and Kato, 2006). This finding agrees with some human data emphasizing the importance of maternal contact/involvement in early child stimulation: studies in preterm babies have shown, for example, a correlation between maternal involvement and the infants' cognitive development during the first year of life (Wijnroks, 1998).
Our data suggest that simply increasing maternal-like physical contact is sufficient to stimulate the pups, exerting several short- and long-term effects. First, early enrichment affected pup behavior, reducing the number and duration of the USVs emitted upon a short maternal separation. This enrichment effect could be surprising if a reduction in the USV number was interpreted as a communication deficit, as described in some proposed mouse models for autism (see Scattoni et al, 2009). Yet, the number of USVs emitted upon maternal separation is considered as an index of reduced emotional distress of the pups (D'Amato et al, 2005; Wohr and Schwarting, 2008), an interpretation that seems the most appropriate here, and also because it fits with the enhanced levels of maternal care induced by our enrichment procedure. Importantly, no alterations in USV response were detected in KO pups in STD conditions compared with their WT littermates, in line with previous studies (Roy et al, 2012), thus suggesting that enrichment was not correcting any preexisting alteration in mother–infant interaction. Indeed, the effect of enrichment on USVs was also observed in WT pups. Here, social enrichment also promoted pup body growth in both genotypes. This enrichment-induced increase in body weight disappeared in adulthood, in contrast to a persistent one that has been observed if the additional mother is lactating (D'Amato et al, 2011; Heiderstadt et al, 2014). Similarly, the effect of enrichment on USV communication was specific to the infant phase, disappearing in adult mice. It is worth noting that, at adulthood, KOs did not differ from WT mice in USV emission toward an adult female, as expected from previous data (Pietropaolo et al, 2011).
Second, early enrichment exerted long-term effects, rescuing the hyperactivity and the social and cognitive deficits displayed by adult KO mice as well as their alterations in dendritic spine morphology. The neurobehavioral abnormalities observed here in KO animals were mostly expected based on previous data, and resemble those of FXS patients (Pietropaolo and Subashi, 2014): in particular, the abnormally high proportion of immature (filopodia, chubby, and stubby) vs mature (thin and mushroom) spines is a major pathological hallmark of FXS (Portera-Cailliau, 2012). This morphological alteration was more pronounced in the hippocampus than in the amygdala, where it was limited to one type of spines. Importantly, the morphological abnormalities in both brain regions were not accompanied by changes in total spine density; in fact, alterations in spine density are known as a nonconsistent FXS-associated phenotype in mice (Segal et al, 2003; de Vrij et al, 2008; Gross et al, 2010; Levenga et al, 2011; Pop et al, 2012) and humans (Rudelli et al, 1985; Hinton et al, 1991; Irwin et al, 2001). Although the precise molecular mechanisms involved in the immature-spine FXS phenotype are still under investigation, it is known that FMRP, as an mRNA-binding protein, directly regulates local synthesis of proteins that are important for spine morphology and synaptic function (Zalfa et al, 2006). Within dendritic spines, FMRP participates in actin dynamics that determines spine shape, receptor trafficking, and changes in synaptic strength (Bassell and Warren, 2008). Consistently, deficits in long-term potentiation and depression as well as in neuronal activity have been described in several brain areas of Fmr1-KO mice, including the hippocampus and the amygdala (see, eg, (Lauterborn et al, 2007; Suvrathan et al, 2010; Michalon et al, 2014)).
It is likely that the rescue of spine abnormalities has induced the behavioral improvements observed here in enriched animals. In fact, it has been recently proposed that the aberrant spine morphology observed in Fmr1-KO mice may be responsible for the deficiencies in synaptic function observed in the same brain areas and, in turn, for the FXS-like behavioral alterations (Portera-Cailliau, 2012). It is instead unlikely that the effect on spine morphology may be a consequence of the better behavioral performance of enriched KO mice, as spine evaluation was conducted on naive animals. In line with this notion of a link between spine morphology and behavioral rescue, the long-term neurobehavioral effects of early enrichment, that is, on adult brain and behaviors, occurred exclusively in pathological conditions, that is, in KO animals. This genotype specificity was detected despite an equivalent effect of early enrichment on mother–infant interactions in WT and KO mice, as demonstrated by the similar pup USV response to maternal separation. The lack of enrichment effect observed here in adult WT animals enhances the value of our environmental manipulation, thus supporting a specific long-term compensatory effect of the environment on FXS pathology rather than a general stimulating effect.
Altogether, this evidence supports that the addition of a nonlactating female represents a valid early enrichment tool to selectively manipulate mother–infant interactions without the nutritional confounds inherent to models based on an additional lactating female. Our enrichment procedure also does not directly alter interactions among peers, as intended by other interventions, such as communal nesting (Branchi, 2009). Our form of enrichment has also a straightforward translational value, as it follows the more recently suggested lines of behavioral interventions for developmental diseases that focus on promoting parent–infant interactions (Dawson, 2008). It is important to underline that our data do not point to a causal role of parental style in the etiopathology of developmental disorders, an hypothesis that in the past has been suggested for autism (Bettelheim, 1967) and has been abandoned since then. Rather, our findings highlight the therapeutic impact of providing additional parental stimulation on a genetically induced syndrome, thus allowing the environmental compensation of a genetic defect, an approach that is indeed receiving increasing clinical attention (Elsabbagh and Johnson, 2009).
In line with clinical data (Dawson, 2008), our findings strengthen the importance of early interventions to prevent/correct the pathological phenotypes in mouse models of FXS. Indeed, later postweaning exposure to ‘classical' enriched environments (ie, large cages with running wheels and toys) induced limited behavioral effects in adult Fmr1-KO mice, and did not rescue their alterations in locomotor activity and exploration (Restivo et al, 2005). A similar type of postweaning enrichment also failed to normalize the morphological abnormalities in hippocampal spines in the same KOs (Lauterborn et al, 2013). Our data therefore strongly support the use of early stimulation through the experimental protocol of the additional nonlactating mother in genetic mouse models of neurodevelopmental diseases beyond FXS, starting with autism. FXS represents in fact a major genetic risk factor for autism that is easily and clearly diagnosed and could therefore facilitate the early implementation of environmental therapeutic interventions for autistic children. Furthermore, the presence of autistic symptoms in FXS patients encourages the use of this disease and its models to study the impact of gene–environment interactions in autism that are known to play a key role in the complexity of the autistic pathology (Elsabbagh and Johnson, 2009).
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the March of Dimes (12-FY05-1198), Conseil Régional d'Aquitaine, CNRS, and the University of Bordeaux I to WE Crusio. S Pietropaolo, D Oddi, F D'Amato, S Middei, and WE Crusio were supported by the bilateral grant CNRS-CNR 2010 (#23779). L Bellocchio was funded by an EMBO long-term fellowship (ALTF975-2011). M Guzmán was funded by a grant from MINECO (SAF2012-35759). We thank Dr Martine Amassari-Teule for her help and expertise with the assessment of spine morphology and Dr Daniel Beracochea, Dr Etienne Coutureau, and Fabienne Naneix for their experimental assistance. We also thank Raphael Pineau and Marie-Paule Algeo for their expert animal care. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardet M, Crusio WE. Fmr1 KO mice as a possible model of autistic features. ScientificWorldJournal. 2006;6:1164–1176. doi: 10.1100/tsw.2006.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelheim B. The Empty Fortress: Infantile Autism and the Birth of the Self. Free Press: New York; 1967. [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Albeck DS, Paylor R. Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 2006;5:467–471. doi: 10.1111/j.1601-183X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP, Keverne EB, Bateson PP. Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav. 2007;91:325–334. doi: 10.1016/j.physbeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Franks B, Champagne FA. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm Behav. 2012;61:454–461. doi: 10.1016/j.yhbeh.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups. Behav Genet. 2005;35:103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- D'Amato FR, Zanettini C, Sgobio C, Sarli C, Carone V, Moles A, et al. Intensification of maternal care by double-mothering selectively boosts cognitive function and hippocampal morphology in the adult offspring. Hippocampus. 2011;21:298–308. doi: 10.1002/hipo.20750. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dorey R, Piérard C, Shinkaruk S, Tronche C, Chauveau F, Baudonnat M, et al. Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology. 2011;36:2639–2649. doi: 10.1038/npp.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends Cogn Sci. 2009;14:81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D'Hooge R, et al. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007;6:552–557. doi: 10.1111/j.1601-183X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci USA. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CX, Portera-Cailliau C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience. 2013;251:120–128. doi: 10.1016/j.neuroscience.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiderstadt KM, Vandenbergh DJ, Gyekis JP, Blizard DA. Communal nesting increases pup growth but has limited effects on adult behavior and neurophysiology in inbred mice. J Am Assoc Lab Anim Sci. 2014;53:152–160. [PMC free article] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kohl Z, Kuhn HG, Cooper-Kuhn CM, Winkler J, Aigner L, Kempermann G. Preweaning enrichment has no lasting effects on adult hippocampal neurogenesis in four-month-old mice. Genes Brain Behav. 2002;1:46–54. doi: 10.1046/j.1601-1848.2001.00009.x. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Jafari M, Babayan AH, Gall CM.2013Environmental enrichment reveals effects of genotype on hippocampal spine morphologies in the mouse model of fragile X syndrome Cereb Cortexdoi: 10.1093/cercor/bht249 [DOI] [PMC free article] [PubMed]
- Lauterborn JC, Rex CS, Kramár E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenga J, de Vrij FM, Buijsen RA, Li T, Nieuwenhuizen IM, Pop A, et al. Subregion-specific dendritic spine abnormalities in the hippocampus of Fmr1 KO mice. Neurobiol Learn Mem. 2011;95:467–472. doi: 10.1016/j.nlm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Michalon A, Bruns A, Risterucci C, Honer M, Ballard TM, Ozmen L, et al. Chronic metabotropic glutamate receptor 5 inhibition corrects local alterations of brain activity and improves cognitive performance in fragile X mice. Biol Psychiatry. 2014;75:189–197. doi: 10.1016/j.biopsych.2013.05.038. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Oddi D, Crusio WE, D'Amato FR, Pietropaolo S. Monogenic mouse models of social dysfunction: implications for autism. Behav Brain Res. 2013;251:75–84. doi: 10.1016/j.bbr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Guilleminot A, Martin B, D'Amato FR, Crusio WE. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS One. 2011;6:e17073. doi: 10.1371/journal.pone.0017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo S, Subashi E.2014Mouse models of Fragile X syndromeIn: Pietropaolo S, Sluyter F, Crusio WE, (eds)Behavioral Genetics of the Mouse Vol. 2Cambridge University Press: Cambridge; 2014. pp 146–163. [Google Scholar]
- Pop AS, Levenga J, de Esch CE, Buijsen RA, Nieuwenhuizen IM, Li T, et al. Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant. Psychopharmacology (Berl) 2012;231:1227–1235. doi: 10.1007/s00213-012-2947-y. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C. Which comes first in fragile X syndrome, dendritic spine dysgenesis or defects in circuit plasticity. Neuroscientist. 2012;18:28–44. doi: 10.1177/1073858410395322. [DOI] [PubMed] [Google Scholar]
- Qin M, Xia Z, Huang T, Smith CB. Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience. 2011;194:282–290. doi: 10.1016/j.neuroscience.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner MJ, Rosenzweig MR. Enriched and Impoverished Environments. Springer-Verlag: New York; 1987. [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, et al. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci USA. 2005;102:11557–11562. doi: 10.1073/pnas.0504984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Watkins N, Heck D. Comprehensive analysis of ultrasonic vocalizations in a mouse model of fragile x syndrome reveals limited, call type specific deficits. PLoS One. 2012;7:e44816. doi: 10.1371/journal.pone.0044816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, et al. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathol. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez de Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorder. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Kreher U, Greenberger V, Braun K. Is fragile X mental retardation protein involved in activity-induced plasticity of dendritic spines. Brain Res. 2003;972:9–15. doi: 10.1016/s0006-8993(03)02410-7. [DOI] [PubMed] [Google Scholar]
- Shoji H, Kato K. Maternal behavior of primiparous females in inbred strains of mice: a detailed descriptive analysis. Physiol Behav. 2006;89:320–328. doi: 10.1016/j.physbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Singer B, Friedman E, Seeman T, Fava GA, Ryff CD. Protective environments and health status: cross-talk between human and animal studies. Neurobiol Aging. 2005;26 (Suppl 1:113–118. doi: 10.1016/j.neurobiolaging.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA. 2010;107:11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranfaglia MR. The psychiatric presentation of fragile X: evolution of the diagnosis and treatment of the psychiatric comorbidities of fragile X syndrome. Dev Neurosci. 2011;33:337–348. doi: 10.1159/000329421. [DOI] [PubMed] [Google Scholar]
- Vandesquille M, Baudonnat M, Decorte L, Louis C, Lestage P, Beracochea D. Working memory deficits and related disinhibition of the cAMP/PKA/CREB are alleviated by prefrontal alpha4beta2*-nAChRs stimulation in aged mice. Neurobiol Aging. 2013;34:1599–1609. doi: 10.1016/j.neurobiolaging.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Wijnroks L. Early maternal stimulation and the development of cognitive competence and attention of preterm infants. Early Dev Parent. 1998;7:19–30. [Google Scholar]
- Wohr M, Schwarting RK. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav Neurosci. 2008;122:310–330. doi: 10.1037/0735-7044.122.2.310. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Achsel T, Bagni C. mRNPs, polysomes or granules: FMRP in neuronal protein synthesis. Curr Opin Neurobiol. 2006;16:265–269. doi: 10.1016/j.conb.2006.05.010. [DOI] [PubMed] [Google Scholar]