Abstract
The hypocretin/orexin (HCRT) system has been associated with both positive and negative drug reinforcement, implicating HCRT receptor 1 (HCRT-R1) signaling in drug-related behaviors for all major drug classes, including opioids. However, to date there are limited studies investigating the role of HCRT receptor 2 (HCRT-R2) signaling in compulsive-like drug seeking. Escalation of drug intake with extended access has been suggested to model the transition from controlled drug use to compulsive-like drug seeking/taking. The current study examined the effects of a HCRT-R2 antagonist, NBI-80713, on heroin self-administration in rats allowed short- (1 h; ShA) or long- (12 h; LgA) access to intravenous heroin self-administration. Results indicate that systemically administered NBI-80713 dose-dependently decreased heroin self-administration in LgA, but not in ShA, animals. Quantitative PCR analyses showed an increase in Hcrtr2 mRNA levels in the central amygdala, a stress-related brain region, of LgA rats. These observations suggest a functional role for HCRT-R2 signaling in compulsive-like heroin self-administration associated with extended access and indicate HCRT-R2 antagonism as a potential pharmacological target for the treatment of heroin dependence.
Introduction
Opioid abuse and dependence are major public health problems and the number of people dependent on or abusing opioids is rising (Substance Abuse & Mental Health Services Administration, 2013). Heroin has been argued to be the second most harmful psychoactive drug, behind only alcohol (Nutt et al, 2010). Opioid addiction is characterized by patterns of excessive/repetitive drug seeking and taking, including a preoccupation with obtaining the drug, a loss of control over drug intake, and the development of somatic and/or affective withdrawal symptoms (Koob et al, 2014). Treatment options to date are mainly based on substitution therapy with the use of long-lasting opioid agonists such as methadone (Camí et al, 1985; Kleber et al, 1987). Therefore, effective non-opioid therapies would be a valuable addition to the pharmacological treatment options.
A preclinical framework, with which to model key elements of opioid addiction (ie, opioid use disorder or opioid dependence), can be found in animal models of extended access to intravenous heroin self-administration. In rats, short access (1 h per day; ShA) to heroin produces stable levels of drug intake, whereas long access (6–23 h per day; LgA) to heroin self-administration can lead to escalated self-administration, which reproduces several key symptoms of heroin dependence reminiscent of the human condition (Ahmed et al, 2000a; Barbier et al, 2013; Vendruscolo et al, 2011). It has long been hypothesized that during limited access conditions, drug intake is mainly driven by the positive reinforcing properties of heroin, whereas during extended access, negative reinforcement mechanisms predominate, in which brain stress systems are recruited and the drug is taken to alleviate negative affective states associated with drug withdrawal (for review, Koob et al, 2014).
The hypocretin/orexin (HCRT) neuropeptides have recently been associated with both stress and drug-seeking behaviors (for review, see Johnson et al, 2012; Mahler et al, 2012). These neuropeptides consist of HCRT-1 and HCRT-2 (also known as orexin A and orexin B, respectively) and are synthesized solely within a restricted region of the dorsal hypothalamus, including the lateral hypothalamus proper, adjacent perifornical area, and dorsomedial hypothalamus (de Lecea et al, 1998; Sakurai et al, 1998), collectively referred to as the lateral hypothalamic area (LHA). Despite being confined within the LHA, HCRT neurons project widely throughout the brain targeting two G-protein-coupled receptors, HCRT receptors 1 and 2 (HCRT-R1 and -R2, respectively). These receptors have different affinities for HCRT peptides such that HCRT-1 binds to both receptors, whereas HCRT-2 binds selectively to HCRT-R2. Importantly, HCRT neuronal projections include reciprocal connections to the extended amygdala and other basal forebrain regions (Baldo et al, 2003; Peyron et al, 1998) implicated in negative reinforcement (for review, Koob et al, 2014). To date, HCRT-R1 has been shown to mediate the positive reinforcement of drug seeking of all major drug classes, including psychostimulants, nicotine, alcohol, and opioids. Relevant to opioids, administration of an HCRT-R1 antagonist attenuates heroin self-administration under either fixed or progressive ratio schedules of reinforcement (Smith and Aston-Jones, 2012). However, far fewer studies have examined HCRT-2 signaling in drug-seeking/taking behavior in general, and in opioid-seeking/taking in particular. Recent research has demonstrated that selective HCRT-R2 antagonism (TCS-OX2-29) blocks the expression of morphine conditioned place preference (Tabaeizadeh et al, 2013). To date, the degree to which HCRT-R2 signaling contributes to self-administration of heroin specifically, and under extended access conditions in particular, has yet to be determined.
Therefore, we tested the effect of a systemically administered HCRT-R2-selective antagonist on heroin self-administration in ShA and LgA rats. In addition, Hcrtr2 mRNA levels were measured using quantitative PCR in stress- and reward-related brain regions of ShA and LgA rats under acute heroin withdrawal conditions.
Material and Methods
Animals
Adult male Wistar rats (N=70; Charles River, Raleigh, NC, USA), weighing between 225–275 g at the beginning of the experiments, were housed in groups of 2–3 per cage in a temperature-controlled (22 °C) vivarium on a 12 h/12 h light/dark cycle (lights on at 18:00 h) with ad libitum access to food and water. The animals were allowed to acclimate to the animal facility for at least 7 days before surgery. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Surgery
Rats were anesthetized with isoflurane (1.5–2.5%) and prepared with chronic intravenous silastic catheters (Dow Corning, Midland, MI, USA) into the right jugular vein (Vendruscolo et al 2011). The catheter was secured to the vein with suture thread and passed subcutaneously to exit dorsally on the animal's back. After surgery, the catheters were flushed daily with 0.2 ml of a sterile solution containing heparinized (30 USP units/ml) saline and the antibiotic Cefazolin. Rats were allowed to recover for 7 days before behavioral testing.
Self-Administration
Intravenous self-administration sessions were conducted in standard operant conditioning chambers (Med Associates) as previously described (Barbier et al, 2013; Vendruscolo et al, 2011). In brief, rats were trained to press one of the two levers (the active lever) on a fixed-ratio 1 (FR1) schedule of reinforcement (each response resulted in fluid delivery) to obtain 0.1 ml of heroin (60 μg/kg/infusion) in 1 h sessions. Reinforced responses were followed by a 20 s timeout period, in which a cue-light (above the active lever) was turned on and lever presses did not result in additional injections. During the acquisition of heroin self-administration, food and water were not available to the rats while in the test chambers. After the acquisition of heroin self-administration, rats were split into two groups matched for lever press in the last three sessions of the acquisition phase and were given 1 h (short access or ShA) or 12 h (long access or LgA) of access to heroin self-administration. In this escalation phase, all groups were allowed to nose poke for food and water on an FR1 schedule while in the test chambers. Rats were allowed 15 days of daily escalation sessions, at the end of which ShA rats displayed stable levels of heroin self-administration, whereas LgA rats displayed an escalation of heroin intake as repeatedly reported by our laboratory (data not shown; Ahmed et al, 2000b; Barbier et al, 2013; Greenwell et al, 2009a, 2009b; Schlosburg et al, 2013; Vendruscolo et al, 2011; Walker et al, 2000).
Open Field
The apparatus, made of wood covered with impermeable Formica, had a white floor of 100 × 100 cm (divided by black lines into 25 squares of 20 × 20 cm) and 40 cm high white walls. Illumination inside the open field was 300 lux. Each rat was placed in the center of the open field and the number of squares crossed was registered for 5 min.
Pharmacological Testing
NBI-80713 (N-[(1R)-2,3-dihydro-1H-inden-1-yl]-2-{[2-(3,4-dimethoxyphenoxy)ethyl] [(4-fluorophenyl)methyl]amino}acetamide; MW=478 g/mol; Neurocrine, San Diego, CA, USA) was dissolved in 5% dimethylformamide and 5% Emulphor (Rodia) and diluted with saline. NBI-80713 is a selective HCRT-R2 antagonist (Rat HCRT-R1 Ki=87 nM; Rat HCRT-R2 Ki=2.2 nM). NBI-80713 (0, 7.5, 15 and 30 mg/kg) was intraperitoneally (IP) injected in a volume of 3 ml/kg 60 min before behavioral testing. For heroin self-administration, the animals received all doses in a within-subject Latin square design. A regular FR1 heroin self-administration session without NBI-80713 treatment was performed between testing days. For the open field test, the animals received either 0, 7.5, 15, or 30 mg/kg IP and were tested only once.
Reverse Transcription and Quantitative PCR
Brains from heroin-exposed animals were collected ∼20 and 10 h after the final ShA and LgA self-administration sessions, respectively. These brain collection time points corresponded to the typical starting time for the rats' subsequent self-administration session (n=8 per group), and thus were more likely to reflect gene expression changes related to anticipation for heroin self-administration in both groups. Brains from a separate cohort of age-matched, non-catheterized, heroin-naive animals that were handled daily (n=8) were collected and used as a control group. All brains were collected at approximately the same time of day to avoid circadian effect confounds. RNA was extracted and purified from brain region punches (2 mm diameter), including the nucleus accumbens (NAc), amygdala, and bed nucleus of the stria terminalis (BNST), using the PureLink RNA Mini Kit (Ambion, Austin, TX, USA) following the manufacturer's instructions. Anatomical substructures of the NAc, amygdala, and BNST were not assessed. cDNA was reverse transcribed from total RNA using the Superscript III First Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Gene expression levels were determined by quantitative polymerase chain reaction (qPCR) using a TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Reactions were carried out as described previously (Vendruscolo et al, 2012), and cDNA concentrations of Hcrtr2 were calculated according to the relative quantification (ddCt) method, corrected for differences in PCR efficiency, normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Primers used were as follows: TaqMan qPCR utilized commercially available Hcrtr1 (Rn00565032_m1), Hcrtr2 (Rn00565155_m1) and Gapdh (Rn99999916_s1) primer/probe sets (Applied Biosystems), with PCR conditions according to the manufacturer's protocol.
Statistical Analysis
All data are expressed as mean and standard errors of the mean (+SEM). Self-administration data were analyzed using a two-way analysis of variance (ANOVA), with group (ShA and LgA) as the between-subjects factor and treatment (0, 7.5, 15, and 30 mg/kg) or time (1, 2, 4, 6, and 12 h) as the within-subjects factor. For open field test, data were analyzed using a one-way ANOVA with treatment (0, 7.5, 15, and 30 mg/kg) as the between-subjects factor. For all behavioral tests, post hoc comparisons were performed using a Bonferroni multiple-comparison adjustment when the ANOVAs were found to be significant. For quantitative PCR analyses, data are expressed as mean percentage fold change from naive Hcrtr2 mRNA levels and were analyzed using a one-way ANOVA with group (naive, ShA, and LgA) as the between-subjects factor. When appropriate, post hoc comparisons were performed using Fisher's least significant difference test. P-values < 0.05 were considered statistically significant for all tests.
Results
Pharmacologic Properties of NBI-80173
NBI-80713 is a high-affinity HCRT-R2 antagonist with an affinity at the rat HCRT-R2 of 2.2±0.6 nM and affinity at the rat HCRT-R1 of 87±0.4 nM. Following systemic administration, NBI-80713 (30 mg/kg; IP) rapidly achieved significant plasma and brain levels with a plasma Tmax of 0.5±0.01 h and a Cmax of 983±125 ng/ml. Plasma and brain exposures were determined at 1 and 4 h. Plasma levels were determined to be 746±124 ng/ml and 661±401 ng/ml with brain levels achieving 390±4.3 ng/g and 223±98.3 ng/g at 1 and 4 h, respectively. These values correspond to an overall brain:plasma ratio of 0.53±0.1 and 0.36±0.1 at 1 and 4 h, respectively. Plasma half-life of the compound was 0.9±0.1 h with a calculated bioavailabilty of 33.9±6.3%. All values are mean±SD and confirm brain penetration and exposure of this compound over the duration of the studies.
HCRT-R2 Antagonist Reduces Heroin Self-Administration in LgA Rats
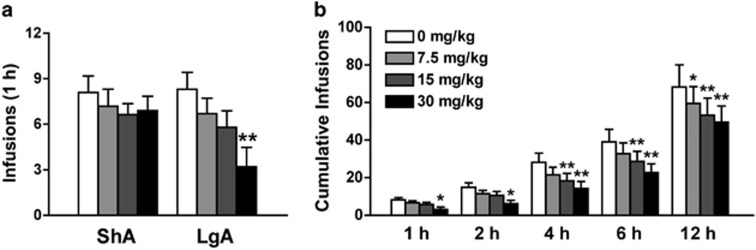
Rats were allowed either ShA (1 h; n=11) or LgA (12 h; n=10, one rat was excluded from the study due to loss of catheter patency) to heroin self-administration on an FR1 schedule of reinforcement. Systemic injection of NBI-80713 significantly decreased heroin self-administration in the first hour under an FR1 schedule of reinforcement in LgA rats at the dose of 30 mg/kg (Figure 1a; Group: F(1,19)=0.86, NS; Treatment: F(3,57)=9.66, p<0.001; Group x treatment: F(3,57)=4.15, p<0.01). The NBI-80713 treatment did not alter heroin self-administration in ShA rats. Further statistical analyses revealed a significant decrease in cumulative heroin infusions in the LgA rats at all time epochs (1, 2, 4, 6, and 12 h) for the entire 12-h session (Figure 1b; Time: F(4,36)=36.32, p<0.001; Treatment: F(3,27)=10.01, p<0.001; Time × treatment: F(12,108)=2.93, p<0.01).
Figure 1.
NBI-80713, a HCRT-receptor 2 antagonist, decreases heroin self-administration in long-access (LgA) but not in short-access (ShA) rats. The bars represent mean number (+SEM) of heroin infusions under an FR1 schedule of reinforcement. (a) NBI-80713 (0, 7.5, 15, or 30 mg/kg, i.p.) significantly decreases responding for heroin in LgA, but not in ShA, rats during the first hour of heroin self-administration at the 30 mg/kg dose. (b) NBI-80713 significantly decreases cumulative responding for heroin at all time epochs in the 12-h LgA session (1, 2, 4, 6, and 12 h). *p<0.05, **p<0.01 vs respective vehicle-treated group. n=10–11 per group.
HCRT-R2 Antagonist has no Effect on Nose Poke for Food or Water
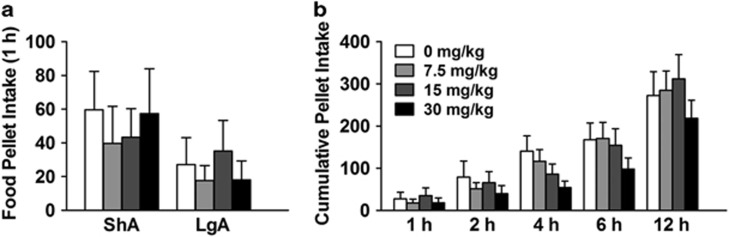
During heroin self-administration, rats were also allowed to nose poke for food and water on an FR1 schedule. There was no main effect of either NBI-80713 or group (ShA vs LgA) on responding for food during the first hour of heroin self-administration (Figure 2a; Group: F(1,19)=1.12, NS; Treatment: F(3,57)=0.78, NS; Group × treatment: F(3,57)=0.94, NS). Furthermore, there was neither a significant main effect of NBI-80713 treatment nor interaction effect between treatment and time on food intake during the 12 h LgA session (Figure 2b; Time: F(4,36)=6.61, p<0.001; Treatment: F(3,27)=0.75, NS; Time × treatment: F(12,108)=1.13, NS). Similarly, there was no significant effect of NBI-80713 administration on water intake (Supplementary Figure S1; Time: F(4,36)=5.79, p<0.01; Treatment: F(3,27)=0.89, NS; Time × treatment: F(12,108)=1.03, NS). The lack of effect on food and water responding would suggest that NBI-80713 has a specific action on heroin self-administration and does not disrupt general operant performance.
Figure 2.
NBI-80713, a HCRT-receptor 2 antagonist, has no effect on responding for food pellet self-administration in either long-access (LgA) or short-access (ShA) rats. The bars represent mean number (+SEM) of food pellet responding under an FR1 schedule of reinforcement. (a) NBI-80713 (0, 7.5, 15, or 30 mg/kg, i.p.) has no effect on responding for food pellets in either ShA or LgA rats during the first hour of heroin self-administration. (b) NBI-80713 has no significant effect on cumulative food pellet intake during the entire 12-h LgA session (1, 2, 6, and 12 h). n=10–11 per group.
HCRT-R2 Antagonist has no Effect on Locomotor Activity in an Open Field
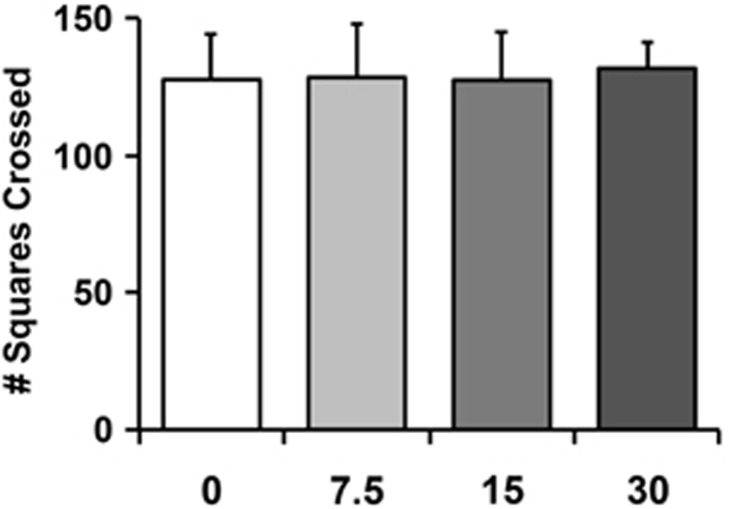
A separate group of heroin-naive rats (n=6 per group) was given systemic NBI-80713 (0, 7.5, 15, or 30 mg/kg; i.p.) before testing in an open field apparatus containing 25 squares. There was no significant effect of NBI-80713 on locomotion (number of squares crossed) at any dose tested (Figure 3; F(3,23)=0.14, NS). This indicates that there are no sedative and/or locomotor-impairing effects of NBI-80713 administration.
Figure 3.
NBI-80713, a HCRT-receptor 2 antagonist, has no effect on locomotor activity in a 5-min open field test. Bars indicate mean number (+SEM) of squares crossed following systemic injection of NBI-80713 (0, 7.5, 15, or 30 mg/kg, i.p.). n=6 per group.
Hcrtr2 mRNA in the Amygdala is Upregulated in LgA Rats
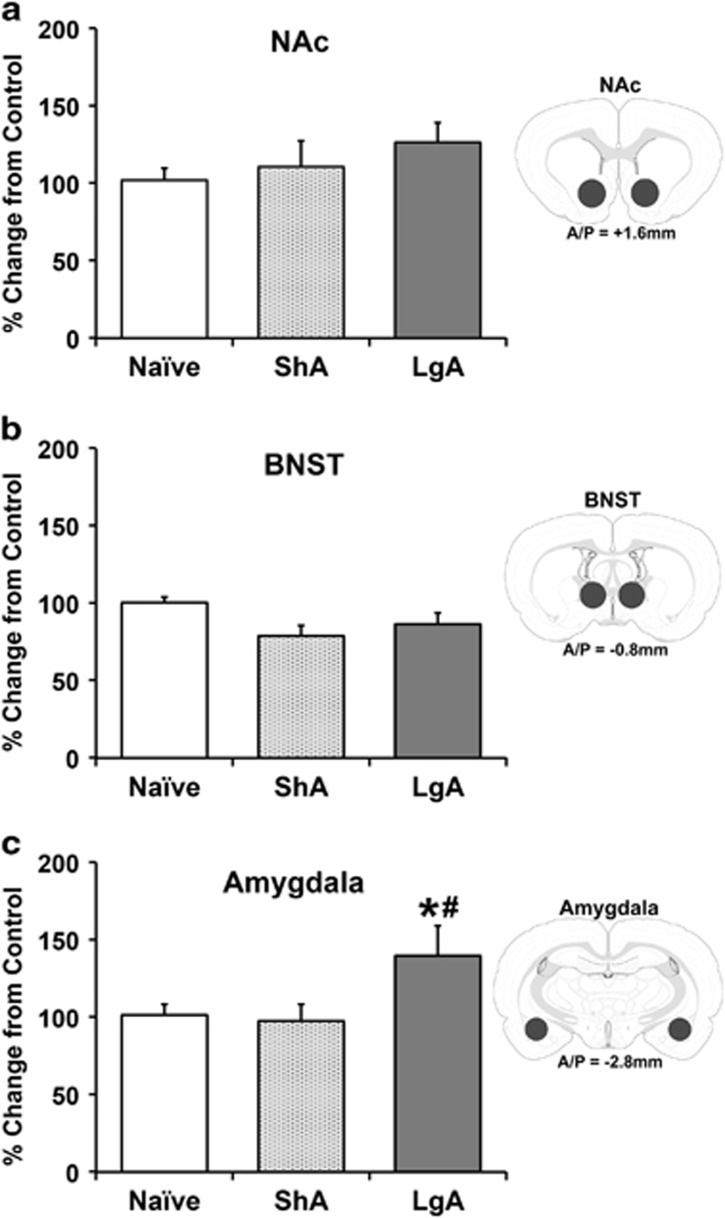
A separate group of rats (n=8 per group) was trained to self-administer heroin as described above. In addition, another separate group of age-matched heroin-naive rats (n=8) were used as a control group. Animals were killed during acute withdrawal following 15 days of escalation, and Hcrtr2 gene expression levels were determined by qPCR. Hcrtr2 mRNA levels in the NAc, BNST, and amygdala of rats exposed to ShA or LgA heroin self-administration or heroin-naive rats are shown in Figure 4. The following cases were excluded from analyses as outliers (ie, more than two SD from the mean): NAc, one case each from ShA and LgA; amygdala, one case each from naive, ShA and LgA. Compared with naive rats, ShA and LgA rats showed no change in Hcrtr2 mRNA levels in either NAc or BNST (NAc: F(2,21)=0.95, NS; BNST: F(2,23)=3.32, NS). However, LgA rats showed a significant increase in Hcrtr2 mRNA levels in the amygdala compared with both naive and ShA rats (F(2,20)=4.56, p<0.05).
Figure 4.
Effects of acute heroin withdrawal on Hcrtr2 mRNA expression levels in reward/stress-related brain regions of heroin-naive, short access (ShA) and long access (LgA) to heroin rats. Bars indicated the mean percentage change (+SEM) from control (ie, naive) values of Hcrtr2 mRNA expression. Insets illustrate the approximate location of brain punches with A/P level from Bregma. LgA rats showed no significant difference in Hcrtr2 mRNA levels in either NAc (a) or BNST (b), but did show a significant increase in the amygdala (c) compared with both naive and ShA rats. *p<0.05 vs naive; #p<0.05 vs ShA. n=7–8 per group.
Discussion
Escalation of heroin intake is thought to reflect an important aspect of the transition from initial controlled drug use to uncontrolled drug dependence or addiction, and has been associated with dysfunction of brain reward and stress systems (Ahmed et al, 2000b; Barbier et al, 2013; Edwards et al, 2012; Kenny et al, 2006; Lenoir and Ahmed, 2007; Lenoir et al, 2013; Vendruscolo et al, 2011). Here, we report that the HCRT-R2 antagonist NBI-80713 selectively decreased heroin, but not food, self-administration specifically in rats given extended access to heroin (12 h; LgA), and had no effect in rats given limited access to heroin (1 h; ShA). In addition, Hcrtr2 mRNA expression levels in LgA rats were increased specifically within the amygdala (a stress-related brain region), but not within the NAc, compared with naive rats. When combined, these results suggest that HCRT signaling at HCRT-R2s may have a role in the neuroadaptive changes that mediate negative reinforcement that are posited to drive compulsive-like intake during heroin dependence.
HCRT-R2 Antagonism Reduces Heroin Intake Specifically in Rats Allowed Extended Access
The current studies demonstrate a reduction in heroin intake in LgA rats, specifically, following systemic administration of the HCRT-R2 antagonist NBI-80713. Given HCRT neurotransmission is well-characterized as a modulator of the initiation and maintenance of arousal/waking, there is some concern about interpreting behavioral data in which HCRT-R2 antagonism could impair performance due to sedative effects (Dugovic et al, 2009; Kummangal et al, 2013). However, the current data showing a reduction in heroin self-administration do not suggest behavioral confounds of sedative or otherwise performance-impairing HCRT-R2 antagonist effects. First, NBI-80713 effects were specific to the LgA group and did not affect heroin self-administration in ShA rats. Second, NBI-80713 did not affect locomotor performance in the open field test. Third, there was no significant attenuation of general performance in operant conditions (ie, nose poke for food and water) in ShA and LgA rats. These findings strongly suggest that the decrease in heroin self-administration observed in LgA rats was specific for heroin reinforcement and not due any performance-impairing effects of the HCRT-R2 antagonist.
In addition, given the moderate selectivity of the HCRT-R2 antagonist, NBI-80713, it is possible that the attenuation of heroin self-administration in the current studies may also be at least partially mediated by HCRT-R1 antagonism. Although HCRT-R1 antagonism has not been tested in rats allowed extended access to heroin, one study did show a reduction in responding for heroin self-administration under short-access conditions (Smith and Aston-Jones, 2012), unlike what was observed in the current studies showing no effect of the HCRT-R2 antagonist on heroin self-administration in the ShA group. This behavioral observation, coupled with the nearly 40-fold affinity of the NBI-80713 compound for HCRT-R2 vs HCRT-R1, would suggest a significant role for HCRT-R2 neurotransmission in heroin self- administration under extended access conditions. However, another study showed increased Hcrt-1 gene expression and HCRT neuronal activation within the LHA under morphine withdrawal conditions, whereas HCRT-R1 antagonism attenuated somatic expression of naloxone-precipitated morphine withdrawal and reduced c-FOS expression, particularly within the extended amygdala (Laorden et al, 2012). It remains to be determined whether HCRT-R1 antagonism would have similar behavioral effects in animals that were allowed extended access to heroin.
HCRT Activation during Escalated Opioid Intake
The current studies showed a specific role for HCRT-R2 neurotransmission in LgA rats. The escalation of heroin intake observed in LgA animals contrasts with the lower, more stable intake levels observed in ShA animals (Ahmed et al, 2000b; Barbier et al, 2013; Greenwell et al, 2009a, 2009b; Schlosburg et al, 2013; Vendruscolo et al, 2011; Walker et al, 2000). It is hypothesized that escalated heroin taking is mediated, in part, by the dysregulation of brain reward and stress systems (eg, HCRT, dynorphin, substance P and corticotropin-releasing factor; CRF) particularly within subregions of the extended amygdala via negative reinforcement mechanisms (for review, see Koob et al, 2014). The extended amygdala, including the central amygdala, BNST, and a transition area in the medial and caudal portions of the NAc, as well as regions implicated in reward-related neurocircuitry such as the ventral tegmental area (VTA), have extensive reciprocal connections to HCRT neurons within the LHA (Alheid et al, 1998; Baldo et al, 2003; Peyron et al, 1998; Schmitt et al, 2012). Thus, it is relevant that direct opioid effects on HCRT neurons have been demonstrated under both reward and stress paradigms. Studies have shown that HCRT neurons exhibit enhanced FOS-activation in proportion to morphine-induced conditioned place preference (CPP; Harris et al, 2005), whereas intra-VTA injections of an HCRT-R1 antagonist attenuated morphine CPP (Narita et al, 2006). These studies suggest a potential role for HCRT modulatory actions on opioid-related reward systems. However, it is conceivable that in the case of morphine-induced CPP, activation of the HCRT system may be a downstream effect of CRF activation, as morphine is known to sensitize CRF and other stress systems (Xu et al, 2004). Consistent with this hypothesis, immunohistochemical studies indicate stress-induced activation of HCRT neurons occurs through CRF-1 receptor activation (Winsky-Sommerer et al, 2004, 2005). Additional studies demonstrate that intra-VTA infusion of HCRT-1 increases intracranial self-stimulation thresholds via activation of CRF in the central amygdala (Hata et al, 2011). Furthermore, pretreatment with a CRF-1/CRF-2-receptor antagonist was shown to attenuate HCRT-1-induced reinstatement of cocaine-seeking (Boutrel et al, 2005). Combined, these observations suggest both direct and indirect HCRT actions on ‘antireward'/brain stress systems.
Hcrt mRNA Changes in Stress/Reward-Related Brain Regions
Consistent with our pharmacological behavioral data, the current studies showed an increase in Hcrtr2 mRNA expression within a stress-related region, the amygdala, specifically in LgA animals, suggesting the amygdala might have a role in escalated heroin intake via increased HCRT transmission at HCRT-2Rs. However, Hcrtr2 mRNA levels measured in a reward-related region, the NAc, did not significantly change in ShA or LgA rats compared with naive rats, suggesting that HCRT-R2 signaling may not contribute to the positive reinforcement that is believed to motivate drug intake in a nondependent state, but rather to the negative reinforcement that drives intake during heroin dependence. We did not observe an increase in Hcrtr2 mRNA levels in a second stress-related region, the BNST. In this case, it is possible that BNST expresses higher levels of HCRT-R1. Our results do not preclude a role for HCRT neurotransmission within subregions of the BNST and NAc, especially the shell portion of NAc, under negatively reinforcing conditions such as opioid withdrawal. Indeed, enhanced HCRT neuronal activation is found during the negative affective state of opioid withdrawal. For example, Hcrt mRNA levels in the lateral hypothalamus increase during the aversive state of morphine withdrawal (Zhou et al, 2010). Although we found an increase in Hcrtr2 mRNA expression in LgA rats specifically, we cannot exclude the possibility that extended access to heroin may have modified Hcrtr2 protein expression or alternatively may reflect altered function or number of the receptors themselves.
In summary, we showed that pharmacological HCRT-R2 blockade reduced heroin self-administration specifically in LgA rats, suggesting that HCRT-R2 do not mediate the positively reinforcing properties of heroin as modeled in ShA conditions, but rather modulate negative reinforcement that drives intake during heroin dependence as modeled in LgA conditions. These observations suggest a specific functional role for HCRT-R2 signaling in compulsive-like heroin self-administration associated with extended access to the drug and suggest HCRT-R2 antagonism as a potential pharmacological strategy for the treatment of heroin dependence.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse (DA004043, DA004398), National Institute of Alcohol Abuse and Alcoholism (AA007456), and by the Pearson Center for Alcoholism and Addiction Research. A portion of this work was also supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. This is article number 28078 from the Scripps Research Institute.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, Heimer L. The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience. 1998;84:967–996. doi: 10.1016/s0306-4522(97)00560-5. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Barbier E, Vendruscolo LF, Schlosburg JE, Edwards S, Juergens N, Park PE, et al. The NK1 Receptor Antagonist L822429 Reduces Heroin Reinforcement. Neuropsychopharmacology. 2013;38:976–984. doi: 10.1038/npp.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camí J, de Torres S, San L, Solé A, Guerra D, Ugena B. Efficacy of clonidine and of methadone in the rapid detoxification of patients dependent on heroin. Clin Pharmacol Ther. 1985;38:336–341. doi: 10.1038/clpt.1985.182. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, et al. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, et al. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, ston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hata T, Chen J, Ebihara K, Date Y, Ishida Y, Nakahara D. Intra-ventral tegmental area or intracerebroventricular orexin-A increases the intra-cranial self-stimulation threshold via activation of the corticotropin-releasing factor system in rats. Eur J Neurosci. 2011;34:816–826. doi: 10.1111/j.1460-9568.2011.07808.x. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber HD, Topazian M, Gaspari J, Riordan CE, Kosten T. Clonidine and naltrexone in the outpatient treatment of heroin withdrawal. Am J Drug Alcohol Abuse. 1987;13:1–17. doi: 10.3109/00952998709001497. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76 Pt B:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummangal BA, Kumar D, Mallick HN. Intracerebroventricular injection of orexin-2 receptor antagonist promotes REM sleep. Behav Brain Res. 2013;237:59–62. doi: 10.1016/j.bbr.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Laorden ML, Ferenczi S, Pintér-Kübler B, González-Martín LL, Lasheras MC, Kovács KJ, et al. Hypothalamic orexin—a neurons are involved in the response of the brain stress system to morphine withdrawal. PloS One. 2012;7:e36871. doi: 10.1371/journal.pone.0036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD, Independent Scientific Committee on Drugs Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AAK, et al. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci USA. 2013;110:9036–9041. doi: 10.1073/pnas.1219159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, et al. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct Funct. 2012;217:233–256. doi: 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin/hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35:798–804. doi: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration, (2013
- Tabaeizadeh M, Motiei-Langroudi R, Mirbaha H, Esmaeili B, Tahsili-Fahadan P, Javadi-Paydar M, et al. The differential effects of OX1R and OX2R selective antagonists on morphine conditioned place preference in naïve versus morphine-dependent mice. Behav Brain Res. 2013;237:41–48. doi: 10.1016/j.bbr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JR, Ahmed SH, Gracy KN, Koob GF. Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res. 2000;854:85–92. doi: 10.1016/s0006-8993(99)02288-x. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de LL. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G-P, Bockstaele E, Van, Reyes B, Bethea T, Valentino RJ. Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. J Neurosci. 2004;24:8193–8197. doi: 10.1523/JNEUROSCI.1657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Proudnikov D, Yuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Res. 2010;1314:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.