Abstract
A discrete cue associated with intravenous injections of cocaine acquires greater control over motivated behavior in some rats (‘sign-trackers', STs) than others (‘goal-trackers', GTs). It is not known, however, if such variation generalizes to cues associated with other drugs. We asked, therefore, whether a discrete cue (a light) associated with the intravenous administration of an opioid drug (the short-acting mu receptor agonist, remifentanil) acquires incentive motivational properties differently in STs and GTs, as indicated by tests of Pavlovian conditioned approach and conditioned reinforcement. Consistent with studies using cocaine, STs approached a classically conditioned opioid cue more readily than GTs, and in a test of conditioned reinforcement worked more avidly to get it. Interestingly, STs and GTs did not differ in the acquisition of a conditioned orienting response. In addition, the performance of conditioned approach behavior, but not conditioned orientation, was attenuated by pretreatment with the dopamine receptor antagonist, flupenthixol, into the core of the nucleus accumbens. Lastly, food and opioid cues engaged similar amygdalo–striatal–thalamic circuitry to a much greater extent in STs than GTs, as indicated by Fos expression. Taken together, these data demonstrate that, similar to food and cocaine cues: (1) a discrete opioid cue attains greater incentive motivational value in STs than GTs; (2) the attribution of incentive motivational properties to an opioid cue is dopamine dependent; and (3) an opioid cue engages the so-called ‘motive circuit' only if it is imbued with incentive salience.
INTRODUCTION
Cues associated with natural or drug rewards can acquire such powerful control over motivated behavior that they are sometimes difficult to resist. There is, however, considerable individual variation in the ability of reward cues to motivate behavior (Mahler and de Wit, 2010; Meyer et al, 2012; Robinson and Flagel, 2009). Preclinical studies suggest this variation is due, at least in part, to intrinsic individual variation in the extent to which reward cues are attributed with incentive salience (Meyer et al, 2012; Robinson and Flagel, 2009; Yager and Robinson, 2010). For example, if a spatially discrete stimulus (a lever; the conditioned stimulus, CS) is repeatedly paired with delivery of a food reward (the unconditioned stimulus, US), in some rats ('sign-trackers', STs; Hearst and Jenkins, 1974), the CS itself becomes attractive, eliciting approach and engagement with it, and desired, in that STs will work to obtain it. In other rats ('goal-trackers', GTs; Boakes, 1977) the CS itself is less attractive—its presentation instead elicits approach to the location where food will be delivered—and GTs do not work as avidly to gain access to it. Thus, a CS acquires the properties of an incentive stimulus—the ability to attract and to act as a conditioned reinforcer—to a greater extent in some rats than others (for reviews, see Robinson et al, 2014; Saunders and Robinson, 2013a).
Importantly, the propensity to approach a food cue predicts the extent to which a discrete drug cue acquires motivational properties. For example, relative to GTs, a cocaine cue is more attractive to STs, eliciting greater approach behavior (Flagel et al, 2010; Yager and Robinson, 2013) and more desired, in that STs will work more avidly just for presentation of a cocaine cue (Saunders and Robinson, 2010; Yager and Robinson, 2013). Finally, a cocaine cue spurs greater drug-seeking behavior in STs than GTs (Saunders et al, 2013b). However, all previous studies comparing the ability of a drug cue to motivate behavior in STs and GTs have used cocaine. Therefore, it is not known if such variation generalizes to cues associated with drugs from other classes.
To begin to address this question, we asked whether the propensity to attribute incentive salience to a food cue predicts the extent to which a discrete cue associated with administration of an opioid drug (remifentanil) acquires incentive motivational properties. Remifentanil was chosen for study because not only is it a potent mu receptor agonist, but it also has a very short duration of action, which is advantageous for conditioning studies (Haidar et al, 1997). Second, to explore the neurobiology underlying individual variation in the attribution of incentive salience to an opioid cue we asked (a) whether dopamine transmission within the nucleus accumbens core is necessary for expression of conditioned approach to an opioid cue and (b) whether an opioid cue is equally effective in inducing Fos protein expression in brain regions that comprise the ‘motive circuit' in STs vs GTs.
MATERIALS AND METHODS
Pavlovian Training Using Food as the US
Adult male Sprague–Dawley rats from Harlan (Haslett, MI) and Charles River (Portage, MI) (Fitzpatrick et al, 2013) were acclimated to the colony room for 1 week before Pavlovian training using procedures similar to those described previously (Flagel et al, 2007; Meyer et al, 2012). Briefly, rats underwent five (Experiments 1–3) or seven (Experiment 4) daily sessions of Pavlovian conditioning, during which a lever (lever-CS) was inserted into the chamber 25 times for 8 s, and immediately upon retraction a single 45-mg banana-flavored pellet (the US) was delivered into the food cup. Following completion of training, animals were classed into three groups: (1) those that preferentially interacted with the lever-CS (STs), (2) those that preferentially interacted with the food cup during the lever-CS presentation (GTs), and (3) those that had no strong preference for either the lever-CS or food cup. See Supplementary Methods.
Experiment 1: Individual Variation in Pavlovian Conditioned Approach using Remifentanil as the US
Following Pavlovian training using food as the US, STs and GTs were outfitted with an intravenous jugular catheter. Behavioral testing was conducted in chambers identical to those used to screen animals for ST and GT, except the food cup and lever were removed from the chamber and two stimulus lights were placed on the left and right sides of the wall opposite the house light, 13.5 cm above the floor. Before training, rats were assigned to either Paired (CS and US presented together) or unpaired (UP) groups (US explicitly not paired with presentation of the CS). Each session consisted of 22 trials occurring on a variable time (VT) schedule with a mean of 360 s (300–420 s). For rats in the Paired groups, each trial consisted of illumination of the stimulus light (light-CS) for 10 s, which coincided with an intravenous infusion of 1.6 or 3.2 μg/kg remifentanil hydrochloride (weight of the salt, dissolved in 0.9% saline in 50 μl). Rats in the UP group received noncontingent infusions of 3.2 μg/kg remifentanil that were explicitly not paired with illumination of the light-CS (remifentanil was delivered on a VT schedule with a mean of 150 s after the CS was extinguished). We only tested rats in the UP group with 3.2 μg/kg of remifentanil as this dose produced the most approach in the paired rats.
Video analysis
All Pavlovian conditioning sessions using remifentanil as the US were video-recorded. Video was scored offline by an observer blind to treatment condition for two different conditioned responses (CRs), as described previously (Yager and Robinson, 2013). (1) Conditioned Orientation: an orienting response was scored if the rat made a head and/or body movement in the direction of the CS during the CS period, regardless of whether the rat approached the CS. (2) Conditioned Approach: an approach response was scored if the rat moved towards the CS during the CS period, bringing its nose to within 1 cm of the light, which required it to rear (Supplementary Methods).
Experiment 2: Individual Variation in the Conditioned Reinforcing Properties of a Pavlovian Conditioned Remifentanil Cue
One week following the last Pavlovian training session with remifentanil as the US, rats from Experiment 1 underwent a single 40-min test for conditioned reinforcement, in which they had the opportunity to nose poke for presentation of the remifentanil cue (Supplementary Methods).
Experiment 3: The Role of Nucleus Accumbens Core Dopamine in the Expression of Pavlovian Conditioned Approach to a Remifentanil Cue
An independent cohort of rats was trained on the Pavlovian task using food as the US to identify STs, and only STs were used in this experiment. Rats were then prepared with catheters and guide cannulas placed 2 mm above the target site in the nucleus accumbens core. Rats then underwent Pavlovian training with remifentanil as the US for 8 days exactly as in Experiment 1. Before the 9th training session, rats were given a vehicle microinjection. Subsequently, rats received microinjections of the dopamine antagonist, flupenthixol (5, 10, and 20 μg), in a counterbalanced order, 35 min before being moved to the testing chambers for the start of the session (Supplementary Methods).
Experiment 4: Individual Variation in Fos Expression Elicited by Pavlovian Conditioned Food and Remifentanil Cues
Ten days following Pavlovian training using either food or remifentanil as the US, as described above and in the Supplementary Methods, rats were re-exposed to either the food (lever-CS) or remifentanil (light-CS) cue, under extinction conditions, for 4 s a total of 10 times (once per minute). After the last CS presentation, rats were returned to their home cages, and then 60 min later their brains were obtained and processed for Fos immunohistochemistry (Supplementary Methods).
RESULTS
STs and GTs both Orient to a Remifentanil Cue, but only STs Avidly Approach it
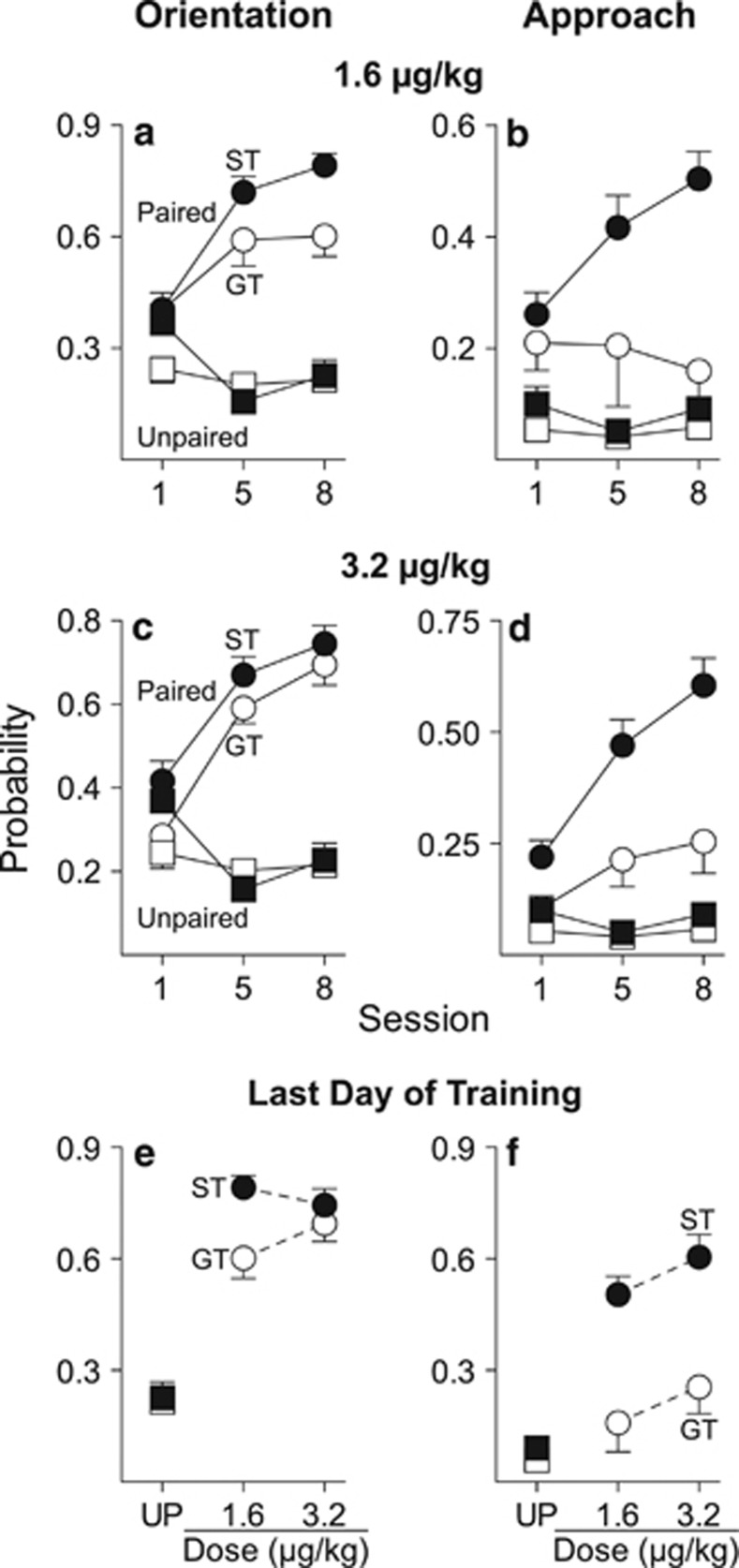
As reported previously (Flagel et al, 2007; Meyer et al, 2012), two distinct phenotypes emerged as a result of Pavlovian training using food as the US (Supplementary Figure S1; Supplementary Results). STs and GTs were then used to test the attractiveness of a remifentanil cue. Figures 1a and c show that with both doses of remifentanil, paired STs and GTs acquired a conditioned orienting response, as indicated by a significant increase in the probability of orienting behavior across sessions (1.6 μg/kg: F(2, 39.25)=23.59, p<0.001; 3.2 μg/kg: F(2, 18)=99.62, p<0.001), and they did so at a similar rate, as indicated by nonsignificant group effects and nonsignificant group by session interactions. However, Figures 1b and d show that with both doses of remifentanil paired STs more readily approached the remifentanil cue than did GTs (effect of group, 1.6 μg/kg: F(1, 45.04)=15.17, p<0.001; 3.2 μg/kg: F(1, 45.59)=20.18, p<0.001; group × session interaction, 1.6 μg/kg: F(2, 41.38)=3.84, p=0.03; 3.2 μg/kg: n.s.). Importantly, neither STs nor GTs in the unpaired group acquired an orienting or approach CR. Figures 1e and f summarize the dose–response functions for the probability of conditioned orientation and approach on the final day of training (Supplementary Results).
Figure 1.
CS-directed orientation and approach to a cue associated with a noncontingent intravenous injection of remifentanil. All Unpaired rats were trained with 3.2 μg/kg remifentanil (STs n=10, GTs n=11). Data represent means±SEM. Probability of orientation (a) and approach (b) to the remifentanil cue in rats that received 1.6 μg/kg remifentanil as the US (Paired STs n=11, GTs n=8). Probability of orientation (c) and approach (d) to the remifentanil cue in rats that received 3.2 μg/kg remifentanil as the US (Paired STs n=12, GTs n=10). Dose–response functions for the probability of conditioned orientation (e) and approach (f) on the final day of training where each data point represents an independent group of rats. CS, conditioned stimulus; GT, goal-trackers; ST, sign-trackers; UP, unpaired.
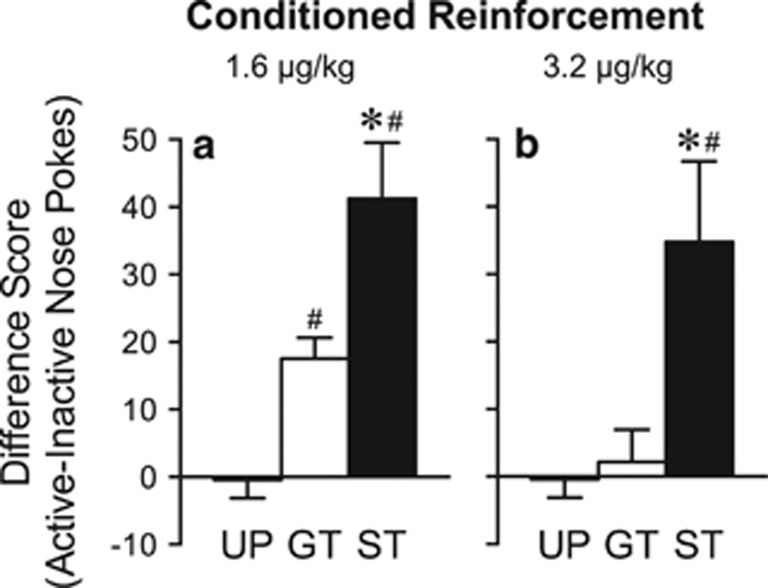
A Remifentanil Cue is a more Effective Conditioned Reinforcer in STs than GTs
Figure 2 shows the mean difference in responses into the Active minus the Inactive port during the conditioned reinforcement test. A one-way ANOVA resulted in a significant main effect of group for both doses (1.6 μg/kg: F(2, 37)=20.09, p<0.001; 3.2 μg/kg: F(2, 40)=8.11, p=0.001). Follow-up tests indicated that, with both training doses, STs made more responses than either GTs or the UP group (p's<0.01), whereas GTs and the UP group only differed from one another when 1.6 μg/kg remifentanil was used during conditioning (p=0.02).
Figure 2.
Performance during the conditioned reinforcement test. During this 40-min test, a nose poke into one port (Active) resulted in 2-s presentation of the cue either previously paired or unpaired with noncontingent remifentanil delivery. Nose pokes into the other port (Inactive) had no consequence. All UP rats were trained with 3.2 μg/kg remifentanil (n=21). Data represent the means±SEM difference in nose pokes into the Active minus Inactive port for rats that were trained with (a) 1.6 μg/kg remifentanil (Paired STs n=11, GTs n=8) or (b) 3.2 μg/kg remifentanil (Paired STs n=12, GTs n=10). *, indicates a significant group difference between STs and GTs. #, indicates a significant difference from UP. p<0.05.GT, goal-trackers; ST, sign-trackers; UP, unpaired.
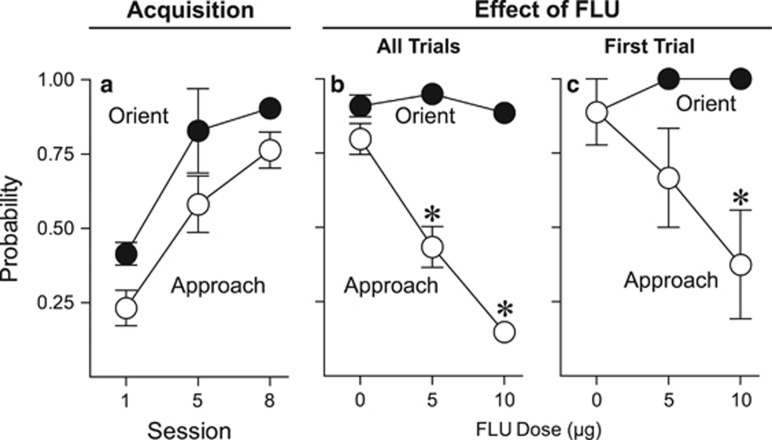
Dopamine Receptor Blockade in the Nucleus Accumbens Core Suppresses Conditioned Approach to a Remifentanil Cue, but not Conditioned Orientation
Pavlovian training with food as the US was very similar to Experiment 1; therefore, these data are not shown. It is important to point out that this experiment only utilized rats identified as STs. As in Experiment 1, STs acquired orienting and approach CRs (main effect of session, orientation: F(2, 18.03)=54.29, p<0.001; approach: F(2, 17.06)=26.99, p<0.001; Figure 3a). Upon review of video from the test sessions, we found that the 20-μg dose of flupenthixol produced nonspecific motor effects (Supplementary Figure S2; Supplementary Results). Thus, data using this dose were not included in any further analyses. Figure 3b shows that flupenthixol dose-dependently decreased approach to the remifentanil cue (F(2, 15.22)=47.409, p<0.001) without affecting conditioned orientation (F(2, 14)=3.565, p=0.17), and did so on the very first trial (that is, in the absence of any new learning; Figure 3c; F(2, 16.973)=4.98, p=0.02). See Supplementary Results for details and Supplementary Figure S3 for locations of microinjection tips.
Figure 3.
Effect of flupenthixol in STs (n=9) on performance of conditioned orientation and approach to a remifentanil cue. Data are presented as the mean±SEM. (a) Acquisition of CS-directed orientation and approach to a cue associated with a noncontingent intravenous injection of 3.2 μg/kg remifentanil in rats that were classified as STs. (b) Effect of flupenthixol on conditioned orientation and approach to the remifentanil cue across the entire session. (c) Effect of flupenthixol on conditioned orientation and approach to the remifentanil cue on the very first trial. CS, conditioned stimulus; FLU, flupenthixol; GT, goal-trackers; ST, sign-trackers; UP, unpaired. *, indicates significant difference relative to vehicle. p<0.05.
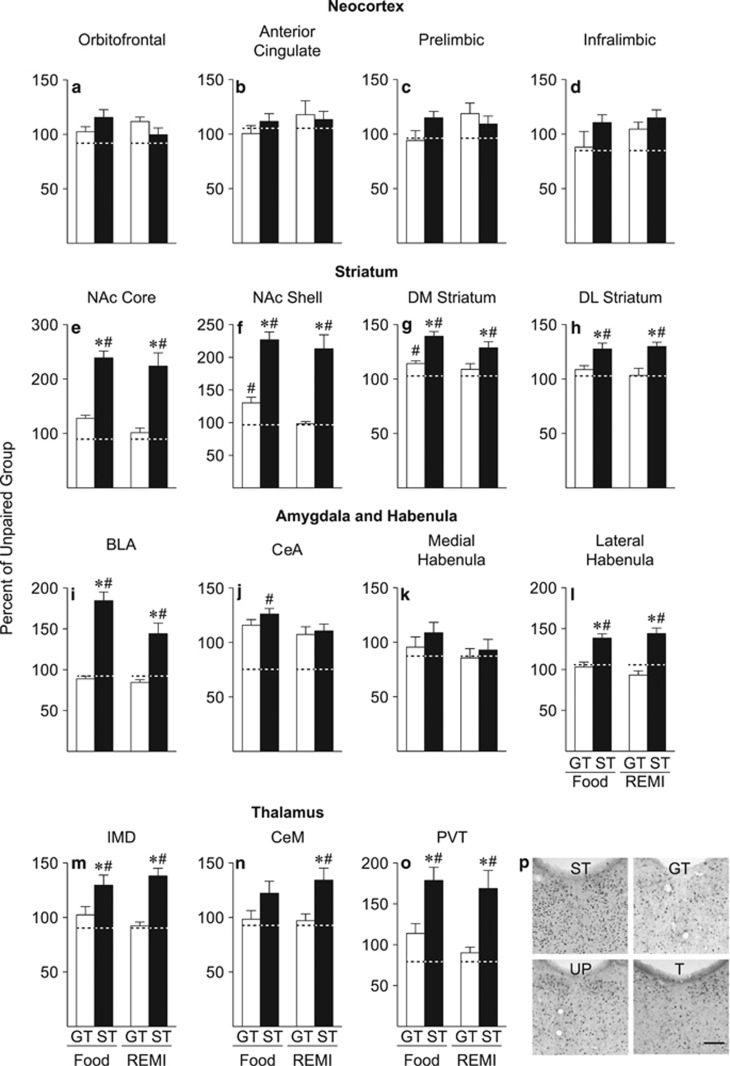
Both Food and Remifentanil Cues Elicit much Greater Fos Expression throughout the ‘Motive Circuit' in STs than GTs
Pavlovian training with food and remifentanil as the US were the same as in Experiment 1 and produced similar effects (Supplementary Figures S4 and S5; Supplementary Results). Figure 4 shows the mean (±SEM) number of Fos-positive cells in STs and GTs, exposed to either the food or the remifentanil cue, expressed as a percent of Fos-positive cells in the relevant UP control group (food or remifentanil used as the US). The actual cell counts for each group are shown in Supplementary Table S1, and one-way ANOVAs were conducted on the number of Fos cells as a function of group, and not the percent data. The graphs depict the data as a percent of the respective UP group to decrease the number of bars used in each graph, which facilitates visually making group comparisons.
Figure 4.
Mean±SEM percent of Fos cells relative to the respective unpaired (UP) groups (UP food cue n=6, UP remifentanil cue n=6) in the (a) orbitofrontal cortex, (b) anterior cingulate cortex, (c) prelimbic cortex, (d) infralimbic cortex, (e) NAc core, (f) NAc shell, (g) DM striatum, (h) DL striatum, (i) BLA, (j) CeA, (k) medial habenula, (l) lateral habenula, (m) IMD, (n) CeM, and (o) PVT of rats presented with either the food cue (STs n=6, GTs n=5) or the REMI cue (STs n=6, GTs n=6) on the test day. Dashed lines indicate the percent of Fos cells in transport control rats relative to unpaired rats. (p) Representative images of PVT sections immunostained for Fos in each experimental group. BLA, basolateral amygdala; CeA, central nucleus of the amygdala; CeM, central medial nucleus of the thalamus; DM, dorsomedial; DL, dorsolateral; IMD, intermedidorsal nucleus of the thalamus; NAc, nucleus accumbens; PVT, paraventricular nucleus of the thalamus; REMI, remifentanil; T, transport control; UP, unpaired. *, indicates a significant difference from GTs. #, indicates a significant difference from UP. p<0.05. Scale bar, 100 μm.
Fos Immunoreactivity
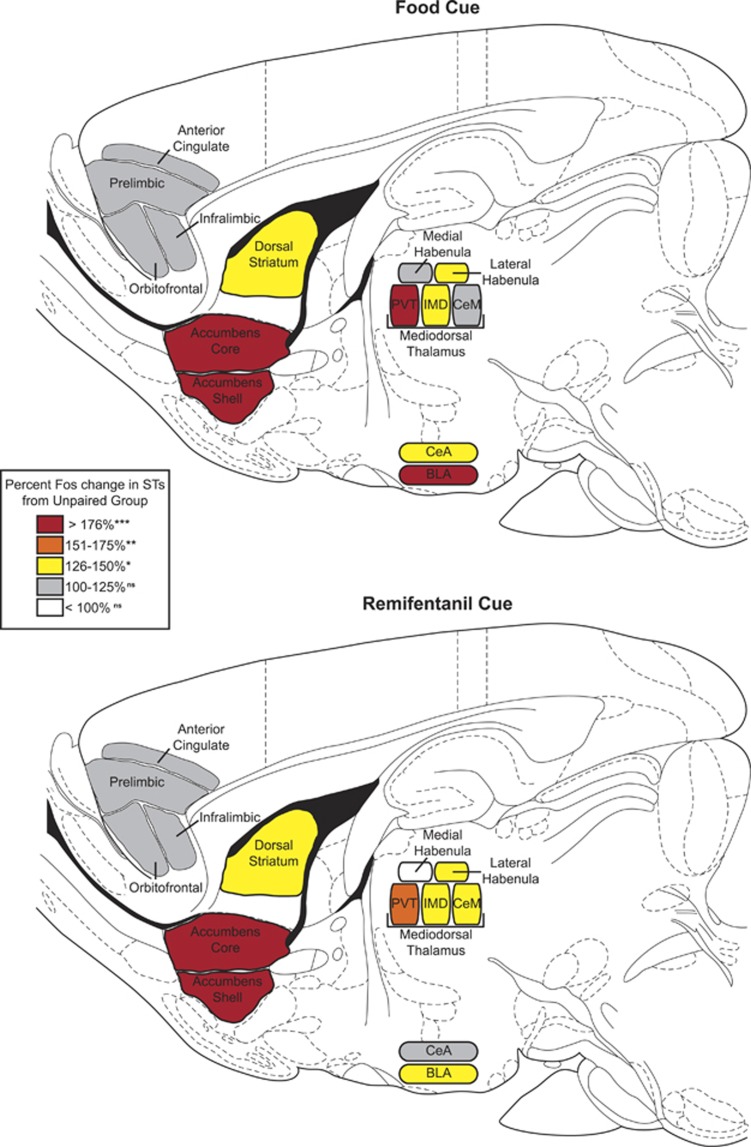
In the nucleus accumbens core and shell, dorsomedial and dorsolateral striatum, basolateral amygdala, lateral habenula, and paraventricular and intermediodorsal nuclei of the thalamus, presentation of both the food and the remifentanil cue elicited greater Fos expression in STs than in GTs or the respective UP group, which did not differ from one another (Figure 4; all p's<0.05; Supplementary Results). There were no significant group differences in Fos expression elicited by either the food or the remifentanil cue in any region of the prefrontal cortex we analyzed or in the medial habenula. In the central nucleus of the amygdala, presentation of the food cue elicited greater Fos expression in STs than the UP food group, whereas there were no significant group differences in Fos expression after presentation of the remifentanil cue (food: F(2, 14)=6.055, p=0.013; remifentanil: F(2, 15)=0.565, p=0.58). However, in the central medial nucleus of the thalamus, there were significant group differences in Fos expression elicited by the remifentanil cue, but not by the food cue (food: F(2, 14)=2.851, p=0.091; remifentanil: F(2, 15)=5.971, p=0.012). Figure 5 provides a visual summary of these results.
Figure 5.
Summary of Fos changes after presentation of either the food or remifentanil cue. Colors represent the percent change in Fos activation in STs compared with the Unpaired control groups. BLA, basolateral amygdala; CeA, central nucleus of the amygdala; CeM, central medial nucleus of the thalamus; IMD, intermedidorsal nucleus of the thalamus; PVT, paraventricular nucleus of the thalamus. ns, nonsignificant, p>0.05; *p<0.05; **p<0.01; ***p<0.001.
DISCUSSION
It is clear that cues associated with opioid drugs can be attributed with incentive salience. Opioid cues are attractive (Madsen and Ahmed, 2014; Peters and De Vries, 2013) and act as conditioned reinforcers (Bertz et al, 2014; Bertz and Woods, 2013). Of course, studies on opioid cue-induced reinstatement of drug-seeking behavior are consistent with this notion (Davis and Smith, 1976; Shalev et al, 2002). Here we were specifically interested in whether the propensity to attribute incentive salience to a food cue predicts variation in the extent to which an opioid (remifentanil) cue acquires motivational properties, as previously shown for a cocaine cue (Flagel et al, 2010; Saunders and Robinson, 2010; Saunders et al, 2013b; Yager and Robinson, 2013). It did.
Individual Variation in the Motivational Properties of an Opioid Cue
First, STs more readily approached the remifentanil cue than did GTs. Second, the remifentanil cue was a more effective conditioned reinforcer in STs than GTs. Interestingly, there was no difference between STs and GTs in the acquisition of a conditioned orienting response to the remifentanil cue. This is important because with drug as the US there is no ‘goal' to approach. It is also consistent with previous findings for both food and cocaine cues (Yager and Robinson, 2013). We conclude that GTs did not approach the remifentanil cue because it was not attributed with sufficient incentive salience to attract animals into close proximity with it, even though they did learn the CS–US association (they acquire a conditioned orienting response). Thus, variation in the propensity to attribute incentive salience to reward cues is seen using food cues and cues associated with drugs from at least two different classes, suggesting that this represents a fundamental trait (for example, Meyer et al, 2012).
Dopamine and Pavlovian Conditioned Approach
It is well established that the primary rewarding effects of psychomotor stimulant drugs are mediated by dopamine neurotransmission within the nucleus accumbens (NAc; Di Chiara and Imperato, 1988; Lyness et al, 1979; Roberts et al, 1980; Wise and Bozarth, 1987), but this might not be the case for opioids (for review see Badiani et al (2011). For example, systemic blockade of dopamine receptors and either selective lesions of dopamine terminals or blockade of dopamine D1 receptors within the NAc decreases cocaine self-administration but has little to no effect on heroin self-administration (Ettenberg et al, 1982; Gerrits et al, 1994; Maldonado et al, 1993; Pettit et al, 1984).
Although the primary reinforcing effects of opioids may not be dopamine-dependent, dopamine does appear to be required for cues associated with opioids to acquire secondary (conditioned) reinforcing effects. For example, systemic injection of dopamine receptor antagonists or injection of a dopamine D1 receptor antagonist into the NAc core attenuated the reinstatement of heroin seeking by heroin-associated cues (Bossert et al, 2007; Lai et al, 2013), indicating that the ability of an opioid cue to serve as a conditioned reinforcer requires dopamine. Here we show that dopamine in the NAc core is also required for a remifentanil cue to elicit a sign-tracking CR, which is thought to reflect the extent to which the cue is attributed with incentive salience (Flagel et al, 2011b; Saunders and Robinson, 2012). Importantly, although flupenthixol dose-dependently reduced conditioned approach behavior, it had no effect on conditioned orienting, as reported previously when food was used as the US (Saunders and Robinson, 2012). This suggests that the decrement in approach behavior was not because dopamine blockade degraded the CS–US association, but specifically attenuated the incentive value of the cue, necessary for it to remain attractive. Consistent with this interpretation, flupenthixol suppressed approach behavior on the very first trial, indicating that the decrement in performance occurred in the absence of new learning. These findings, together with our previous reports (Flagel et al, 2011b; Saunders and Robinson, 2012; Saunders et al, 2013b), indicate that dopamine transmission within the NAc core is necessary for maintaining the motivational properties of multiple classes of reward cues, including opioid cues.
Engagement of ‘Motive Circuitry' by Reward Cues
There is now a wealth of evidence in both humans and non-human animals that cues associated with different classes of rewards (for example, food, drugs, and sex) engage overlapping neural systems, including the mesocorticolimbic dopamine system and other cortico–striatal–thalamic loops that comprise a so-called ‘motive circuit' (Childress et al, 1999; Frohmader et al, 2010; Kelley et al, 2005; Tang et al, 2012; Tomasi et al, 2014). However, in most studies the predictive and incentive values of cues are confounded, and it is not possible to know which property of a cue is sufficient to engage these neural circuits. It is important, therefore, that Flagel et al (2011a) reported that the predictive value of a food cue is not sufficient to engage motivational circuitry—it must be imbued with incentive salience (that is, it did so in STs but not GTs). Here we asked whether this would also be the case for an opioid cue and whether food and opioid cues engaged similar circuitry.
In almost every region we examined, both the food and remifentanil cues elicited greater Fos expression in STs relative to GTs, or rats that received UP CS–US presentations. Furthermore, there were a number of regions (for example, NAc core, dorsolateral striatum, midline thalamic nuclei, basolateral amygdala, and lateral habenula) where presentation of either the food or remifentanil cue had no effect on Fos expression in GTs (that is, they did not differ from the UP groups) while presentation of either cue produced robust Fos expression in STs. However, one limitation of the study is that Fos was only quantified from a portion of each structure and may not be representative of the entire region. Interestingly, these data parallel some recent human imaging work that has shown individual variation in the ability of both food and drug cues to elicit brain activity throughout the ‘motive circuit' (Beaver et al, 2006; Janes et al, 2010; Kilts et al, 2014). It was also interesting that the food and opioid cue engaged essentially the same brain regions in STs. However, there were a few brain areas where we found a dissociation between subregions in the extent to which both the food and the remifentanil cue elicited Fos expression. For example, presentation of the food and remifentanil cue elicited robust Fos expression in STs within the basolateral amygdala (BLA) but not in the central nucleus of the amygdala (CeA). This finding is consistent with a series of studies showing that, whereas lesions of the BLA attenuate ST behavior, lesions of the CeA do not affect acquisition or expression of sign-tracking behavior (Chang et al, 2012a, 2012b). In addition, presentation of the food and remifentanil cue elicited robust Fos expression in the lateral habenula of STs, but not the medial habenula, which is consistent with the ability of an opioid cue to reinstate drug-seeking behavior and increase Fos expression in the lateral habenula (Madsen et al, 2012). Interestingly, Danna et al (2013) recently reported that modulation of lateral habenula outputs strongly influences sign-tracking, but not goal-tracking behavior, perhaps because of its influence on dopamine neurotransmission.
We should point out that the food cup may also have incentive value, as both STs and GTs eventually approach the location of food delivery (DiFeliceantonio and Berridge, 2012; Mahler and Berridge, 2009). However, in Flagel et al (2011a), the food cup was removed from the chamber on test day to specifically isolate the ability of the food cue to elicit c-fos mRNA expression. Thus, they could not assess c-fos mRNA expression when a GT CR was made. It is possible that approach to the food cup might be sufficient to activate some of the same brain regions in GTs as in STs. For this reason, we decided to leave the food cup in the chamber on the test day. Nevertheless, we did not find any region where Fos expression was greater in GTs than in STs. One possible explanation for this is that the 3 days before the cue exposure test day, rats were placed into the chambers (with the food cup present) to minimize the influence of any contextual cues. These habituation sessions may have decreased the amount of goal-tracking observed on the test day (Supplementary Figure S4), which may have resulted in less overall Fos expression in GTs.
CONCLUSIONS
The propensity of an individual to attribute incentive salience to a food cue predicted the extent to which an opioid (remifentanil) cue became attractive and desired, consistent with previous studies using cocaine (Robinson et al, 2014). In addition, the ability of a remifentanil cue to motivate approach behavior required dopamine transmission within the NAc core, and a distributed network of brain regions that comprises a so-called ‘motive circuit', including the dopamine-rich ventral and dorsal striatum, were engaged by food and opioid cues only if they were attributed with incentive salience. It is important to emphasize that in GTs both the food and remifentanil cues functioned as fully predictive CSs, evoking CRs, but this property was not sufficient to engage this circuitry. This dissociation suggests that these brain regions may be especially important in mediating motivational processes. The dopamine system has been the primary focus of research on incentive motivation and reward, but the diversity of brain regions selectively engaged in STs suggests that a number of other brain regions deserve attention. For example, the paraventricular nucleus of the thalamus (for review see Haight and Flagel (2014), the BLA (Chang et al, 2012a, 2012b), and the lateral habenula (Danna et al, 2013) all appear to exert different effects on sign-tracking than on goal-tracking behavior. It is also of note that the food and opioid cues engaged essentially the same brain reward circuitry, suggesting that similar psychological and neurobiological mechanisms may underlie the attribution of incentive salience to cues associated with very different types of rewards.
FUNDING AND DISCLOSURE
This research was supported by grants from the National Institute on Drug Abuse to LMY (F31 DA030799) and TER (P01 DA031656). The authors declare no conflicts of interest.
Acknowledgments
We thank Anusari Dewasurendra, Kyle Eaton, and Elizabeth O'Donnell for assistance with behavioral testing.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse of the National Institutes of Health.
Supplementary Material
References
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertz JW, Chen J, Woods JH.2014Effects of pramipexole on the acquisition of responding with opioid-conditioned reinforcement in the rat Psychopharmacology (Berl)e-pub ahead of print 3 July 2014doi: 10.1007/s00213-014-3659-2 [DOI] [PMC free article] [PubMed]
- Bertz JW, Woods JH. Acquisition of responding with a remifentanil-associated conditioned reinforcer in the rat. Psychopharmacology (Berl) 2013;229:235–243. doi: 10.1007/s00213-013-3102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes R.1977Performance on learning to associate a stimulus with positive reinforcementIn: Davis H, Hurwits H, (eds)Operant-Pavlovian Interactions Lawrence Erlbaum Associates: Hillsdale; 67–97. [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Effects of lesions of the amygdala central nucleus on autoshaped lever pressing. Brain Res. 2012;1450:49–56. doi: 10.1016/j.brainres.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiol Learn Mem. 2012;97:441–451. doi: 10.1016/j.nlm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna CL, Shepard PD, Elmer GI. The habenula governs the attribution of incentive salience to reward predictive cues. Front Hum Neurosci. 2013;7:781. doi: 10.3389/fnhum.2013.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlovian J Biol Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFeliceantonio AG, Berridge KC. Which cue to 'want'? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behav Brain Res. 2012;230:399–408. doi: 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl) 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, et al. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS ONE. 2013;8:e75042. doi: 10.1371/journal.pone.0075042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Frohmader KS, Wiskerke J, Wise RA, Lehman MN, Coolen LM. Methamphetamine acts on subpopulations of neurons regulating sexual behavior in male rats. Neuroscience. 2010;166:771–784. doi: 10.1016/j.neuroscience.2009.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MA, Ramsey NF, Wolterink G, van Ree JM. Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration in the rat. Psychopharmacology (Berl) 1994;114:486–494. doi: 10.1007/BF02249340. [DOI] [PubMed] [Google Scholar]
- Haidar SH, Moreton JE, Liang Z, Hoke JF, Muir KT, Eddington ND. Evaluating a possible pharmacokinetic interaction between remifentanil and esmolol in the rat. J Pharm Sci. 1997;86:1278–1282. doi: 10.1021/js970079e. [DOI] [PubMed] [Google Scholar]
- Haight JL, Flagel SB. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci. 2014;8:79. doi: 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Sign Tracking: the Stimulus-Reinforcer Relation and Directed Action. Monograph of the Psychonomic Society: Austin; 1974. [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick Bde B, Holmes AJ, Sousa J, et al. Neural substrates of attentional bias for smoking-related cues: an FMRI study. Neuropsychopharmacology. 2010;35:2339–2345. doi: 10.1038/npp.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: Studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–14. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Kennedy A, Elton AL, Tripathi SP, Young J, Cisler JM, et al. Individual differences in attentional bias associated with cocaine dependence are related to varying engagement of neural processing networks. Neuropsychopharmacology. 2014;39:1135–1147. doi: 10.1038/npp.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Chen W, Zhu H, Zhou X, Liu H, Zhang F, et al. Low dose risperidone attenuates cue-induced but not heroin-induced reinstatement of heroin seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2013;16:1569–1575. doi: 10.1017/S1461145712001563. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Friedle NM, Moore KE. Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav. 1979;11:553–556. doi: 10.1016/0091-3057(79)90040-6. [DOI] [PubMed] [Google Scholar]
- Madsen HB, Ahmed SH.2014Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues Addict Biole-pub ahead of print 7 March 2014doi: 10.1111/adb.12134 [DOI] [PubMed]
- Madsen HB, Brown RM, Short JL, Lawrence AJ. Investigation of the neuroanatomical substrates of reward seeking following protracted abstinence in mice. J Physiol. 2012;590:2427–2442. doi: 10.1113/jphysiol.2011.225219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. Which cue to ‘want?' Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: Individual differences in cue-induced craving after food or smoking abstinence. PLoS ONE. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Robledo P, Chover AJ, Caine SB, Koob GF. D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav. 1993;45:239–242. doi: 10.1016/0091-3057(93)90112-7. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, et al. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, De Vries TJ. Pavlovian conditioned approach, extinction, and spontaneous recovery to an audiovisual cue paired with an intravenous heroin infusion. Psychopharmacology (Berl) 2013;231:447–453. doi: 10.1007/s00213-013-3258-7. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: implications for addiction. Neurosci Biobehav R. 2013;37:1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine ‘craving': role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND.2014Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors Hum Brain Mappe-pub ahead of print 21 August 2014doi: 10.1002/hbm.22617 [DOI] [PMC free article] [PubMed]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–34. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2013;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.