Abstract
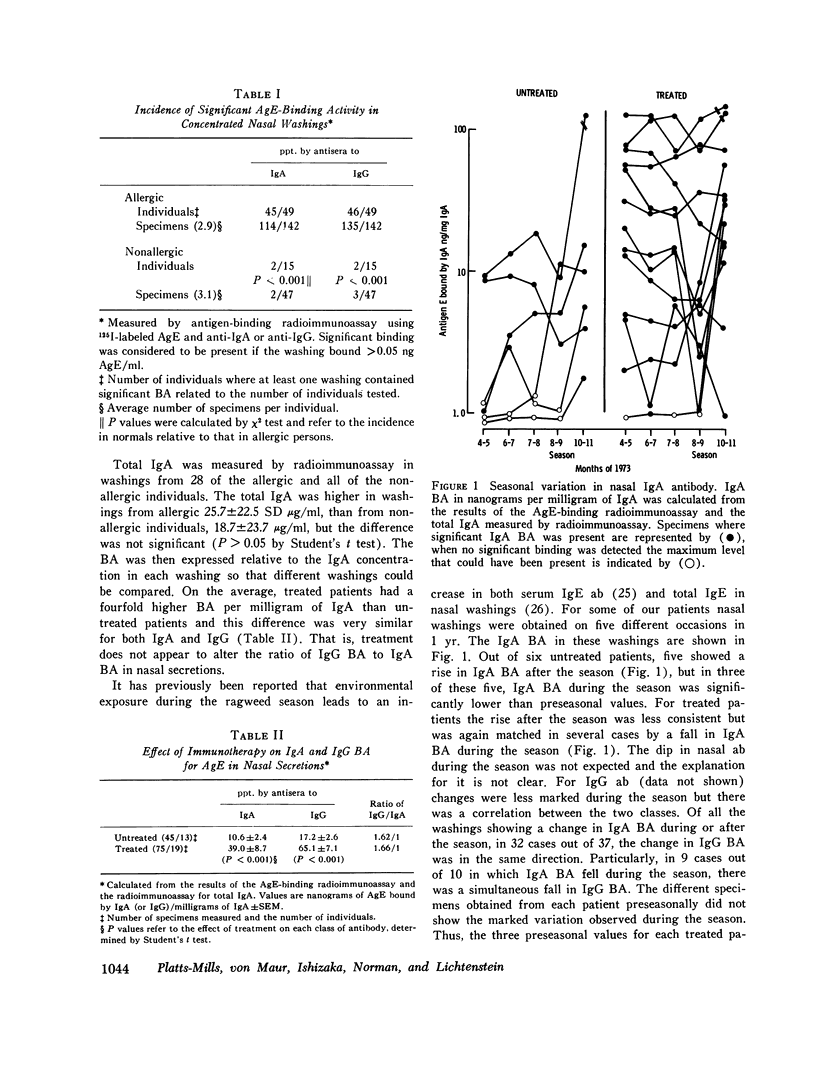
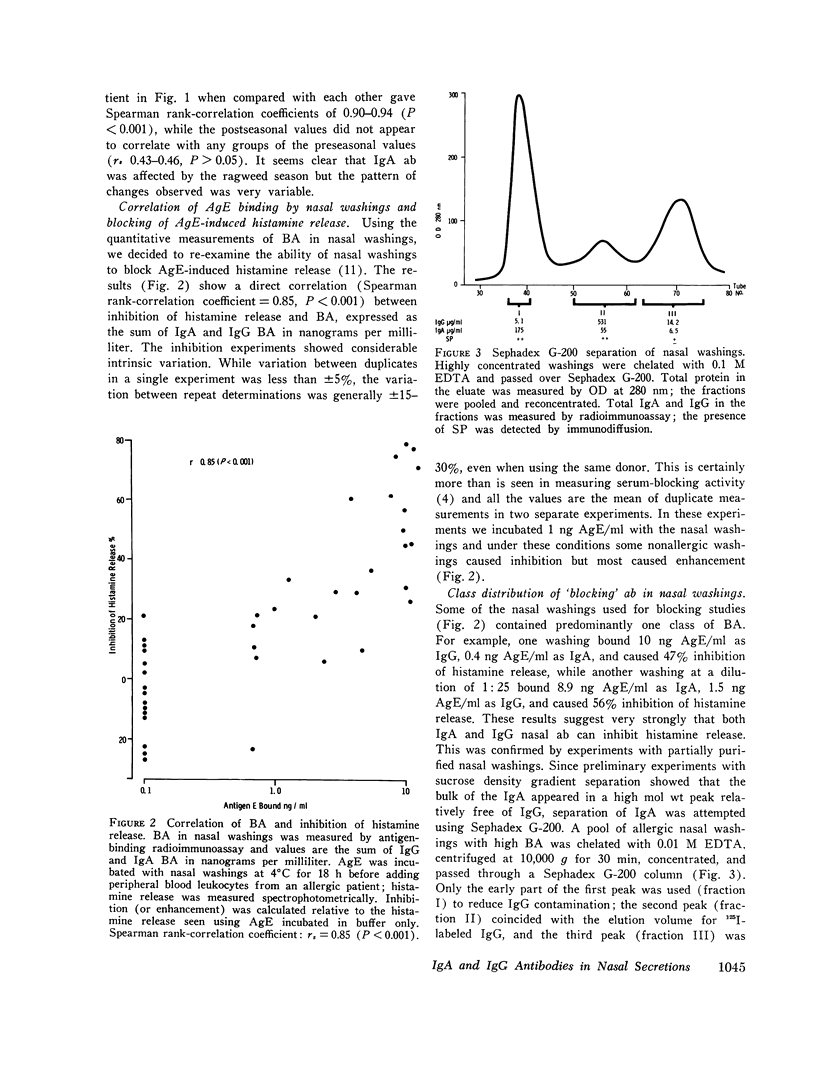
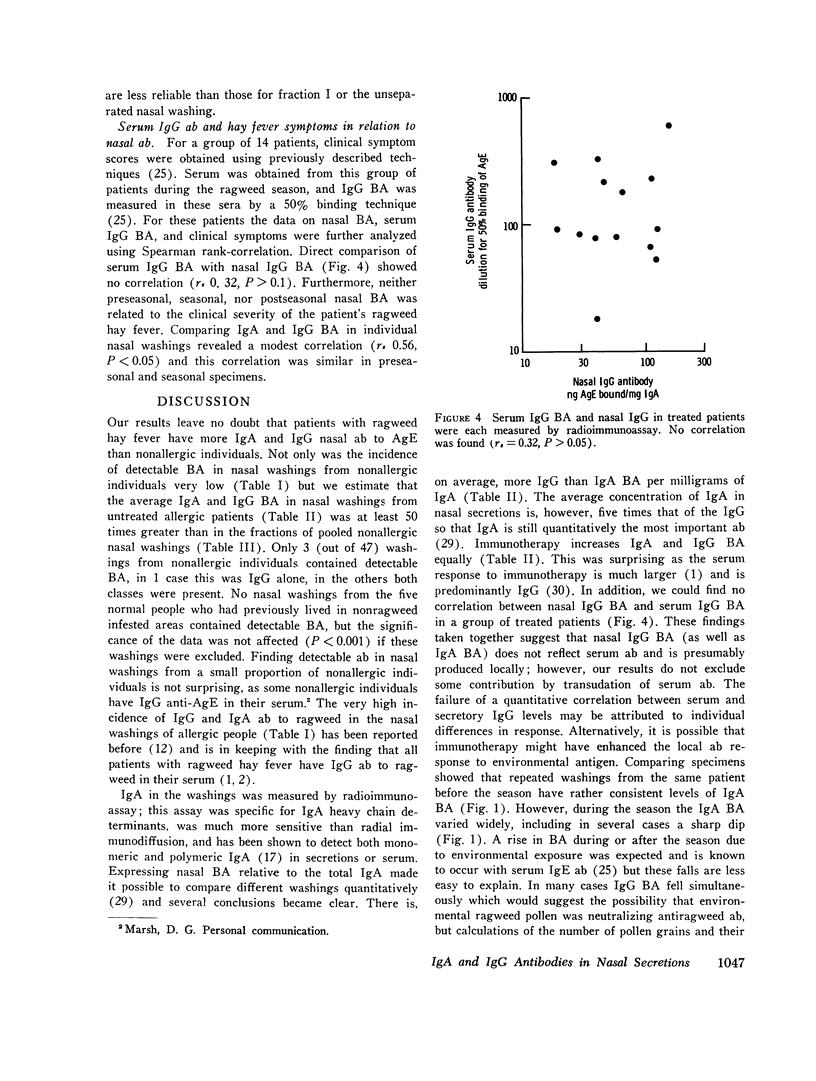
Total secretory IgA and specific anti-antigen E (AgE) antibodies (ab) in the IgA and IgG classes were measured in concentrated nasal washings from ragweed allergic and normal individuals by antigen binding or anti-alpha-radioimmunoassays. Virtually all the allergic patients had significant IgA (45/49) and IgG (46/49) ab to AgE in their nasal washings. By contrast, washings from most normal persons contained no measurable IgA (13/15) ab or IgG (13/15) ab to AgE. The total IgA levels in allergic washings were not significantly different from those in normal washings and they were used to standardize the ab measurements. Parenteral immunotherapy with ragweed extract increased specific nasal IgA ab from 10.6 +/- 2.7 (SEM) to 39.0 +/- 8.7 ng AgE bound/mg IgA and IgG ab from 17.2 +/- 2.6 to 65.1 +/- 7.4 ng AgE bound/mg IgA (P less than 0.001 for both classes). The ratio of IgA:IgG ab was not affected by therapy, and for treated patients, there was no correlation (rs + 0.32, P greater than 0.1) between nasal IgG ab and serum IgG ab. These results suggest that at least part of the nasal IgG ab is produced locally. Blocking activity in the nasal washings was measured by inhibition of histamine release and was found to correlate directly (rs + 0.85, P less than 0.001) with binding activity for AgE. Some washings from normal persons caused slight inhibition of histamine release but others caused enhancement. Nasal washings were fractionated by passage over Sephadex G-200. Inhibition of histamine release by dilutions of the IgA-rich and IgG-rich fractions correlated well with binding activity in these fractions. None of these results support the hypothesis that allergic individuals are deficient in secretory IgA or secretory ab responses. These results, however, are in keeping with the theory that hay fever occurs in a high-responder population which is genetically able to respond to low doses of inhalant antigens.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Yunginger J. W. Ragweed hay fever: treatment by local passive administration of IgG antibody. Clin Allergy. 1975 Mar;5(1):79–87. doi: 10.1111/j.1365-2222.1975.tb01838.x. [DOI] [PubMed] [Google Scholar]
- Hamaoka T., Newburger P. E., Katz D. H., Benacerraf B. Hapten-specific IgE antibody responses in mice. 3. Establishment of parameters for generation of helper T cell function regulating the primary and secondary responses of IgE and IgG B lymphocytes. J Immunol. 1974 Sep;113(3):958–973. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Human reaginic antibodies and immunoglobulin E. J Allergy. 1968 Dec;42(6):330–363. doi: 10.1016/0021-8707(68)90095-6. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Kishimoto T., Delespesse G., King T. P. Immunogenic properties of modified antigen E. I. Presence of specific determinants for T cells in denatured antigen and polypeptide chains. J Immunol. 1974 Jul;113(1):70–77. [PubMed] [Google Scholar]
- KING T. P., NORMAN P. S., CONNELL J. T. ISOLATION AND CHARACTERIZATION OF ALLERGENS FROM RAGWEED POLLEN. II. Biochemistry. 1964 Mar;3:458–468. doi: 10.1021/bi00891a026. [DOI] [PubMed] [Google Scholar]
- King T. P., Norman P. S., Lichtenstein L. M. Studies on ragweed pollen allergens. V. Ann Allergy. 1967 Oct;25(10):541–553. [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Kontou-Karakitsos K., Salvaggio J. E., Mathews K. P. Comparative nasal absorption of allergens in atopic and nonatopic subjects. J Allergy Clin Immunol. 1975 Apr;55(4):241–248. doi: 10.1016/0091-6749(75)90143-8. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskowitz S., Salvaggio J. E., Schwartz H. J. An hypothesis for the development of atopic allergy in man. Clin Allergy. 1972 Sep;2(3):237–246. doi: 10.1111/j.1365-2222.1972.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Levine B. B., Stember R. H., Fotino M. Ragweed hay fever: genetic control and linkage to HL-A haplotypes. Science. 1972 Dec 15;178(4066):1201–1203. doi: 10.1126/science.178.4066.1201. [DOI] [PubMed] [Google Scholar]
- Levine B. B., Vaz N. M. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. A potential mouse model for immune aspects of human atopic allergy. Int Arch Allergy Appl Immunol. 1970;39(2-3):156–171. doi: 10.1159/000230343. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Holtzman N. A., Burnett L. S. A quantitative in vitro study of the chromatographic distribution and immunoglobulin characteristics of human blocking antibody. J Immunol. 1968 Aug;101(2):317–324. [PubMed] [Google Scholar]
- Lichtenstein L. M., Ishizaka K., Norman P. S., Sobotka A. K., Hill B. M. IgE antibody measurements in ragweed hay fever. Relationship to clinical severity and the results of immunotherapy. J Clin Invest. 1973 Feb;52(2):472–482. doi: 10.1172/JCI107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L. Clinical and in vitro studies on the role of immunotherapy in ragweed hay fever. Am J Med. 1968 Apr;44(4):514–524. doi: 10.1016/0002-9343(68)90052-1. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L., Osler A. G. In vitro studies of human ragweed allergy: changes in cellular and humoral activity associated with specific desensitization. J Clin Invest. 1966 Jul;45(7):1126–1136. doi: 10.1172/JCI105419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Osler A. G. Studies on the mechanism of hypersensitivity phenomena. XI. The effect of normal human serum on the release of histamine from human leukocytes by ragweed pollen antigen. J Immunol. 1966 Jan;96(1):159–168. [PubMed] [Google Scholar]
- Lichtenstein L. M., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XII. An in vitro study of the reaction between ragweed pollen antigen, allergic human serum and ragweed-sensitive human leukocytes. J Immunol. 1966 Jan;96(1):169–179. [PubMed] [Google Scholar]
- Marsh D. G., Bias W. B., Hsu S. H., Goodfriend L. Association of the HL-A7 cross-reacting group with a specific reaginic antibody response in allergic man. Science. 1973 Feb 16;179(4074):691–693. doi: 10.1126/science.179.4074.691. [DOI] [PubMed] [Google Scholar]
- Marsh D. G., Bias W. B., Ishizaka K. Genetic control of basal serum immunoglobulin E level and its effect on specific reaginic sensitivity. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3588–3592. doi: 10.1073/pnas.71.9.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C. D., Lyman M., Alberto R., Cheng J. Procedures for immunochemical study of histamine release from leukocytes with small volume of blood. J Allergy. 1970 Jul;46(1):12–20. doi: 10.1016/0021-8707(70)90056-0. [DOI] [PubMed] [Google Scholar]
- Newcomb R. W., Normansell D., Stanworth D. R. A structural study of human exocrine IgA globulin. J Immunol. 1968 Nov;101(5):905–914. [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. IgA and IgA diphtheria antitoxin responses from human tonsil lymphocytes. J Immunol. 1975 Mar;114(3):1058–1064. [PubMed] [Google Scholar]
- Rossen R. D., Schade A. L., Butler W. T., Kasel J. A. The proteins in nasal secretion: a longitudinal study of the gammaA-globulin, gammaG-globulin, albumin, siderophilin, and total protein concentrations in nasal washings from adult male volunteers. J Clin Invest. 1966 May;45(5):768–776. doi: 10.1172/JCI105391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVAGGIO J. E., CAVANAUGH J. J., LOWELL F. C. A COMPARISON OF THE IMMUNOLOGIC RESPONSES OF NORMAL AND ATOPIC INDIVIDUALS TO INTRANASALLY ADMINISTERED ANTIGEN. J Allergy. 1964 Jan-Feb;35:62–69. doi: 10.1016/0021-8707(64)90050-4. [DOI] [PubMed] [Google Scholar]
- Stokes C. R., Taylor B., Turner M. W. Association of house-dust and grass-pollen allergies with specific IgA antibody deficiency. Lancet. 1974 Aug 31;2(7879):485–488. doi: 10.1016/s0140-6736(74)92014-5. [DOI] [PubMed] [Google Scholar]
- Taylor B., Norman A. P., Orgel H. A., Stokes C. R., Turner M. W., Soothill J. F. Transient IgA deficiency and pathogenesis of infantile atopy. Lancet. 1973 Jul 21;2(7821):111–113. doi: 10.1016/s0140-6736(73)93060-2. [DOI] [PubMed] [Google Scholar]
- Taylor G. The nose as a model for the study of respiratory tract allergic disease. Ann N Y Acad Sci. 1974;221:117–123. doi: 10.1111/j.1749-6632.1974.tb28207.x. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- Tse K. S., Wicher K., Arbesman C. E. Effect of immunotherapy on appearance of antibodies to ragweed in external secretions. J Allergy Clin Immunol. 1973 Apr;51(4):208–217. doi: 10.1016/0091-6749(73)90140-1. [DOI] [PubMed] [Google Scholar]
- Turk A., Lichtenstein L. M., Norman P. S. Nasal secretory antibody to inhalant allergens in allergic and non-allergic patients. Immunology. 1970 Jul;19(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- YAGI Y., MAIER P., PRESSMAN D., ARBESMAN C. E., REISMAN R. E. THE PRESENCE OF THE RAGWEED-BINDING ANTIBODIES IN THE BETA-2A, BETA-2M, AND GAMMA GLOBULINS OF THE SENSITIVE INDIVIDUALS. J Immunol. 1963 Jul;91:83–89. [PubMed] [Google Scholar]
- Yunginger J. W., Gleich G. J. Seasonal changes in serum and nassal IgE concentrations. J Allergy Clin Immunol. 1973 Mar;51(3):174–186. doi: 10.1016/0091-6749(73)90022-5. [DOI] [PubMed] [Google Scholar]



