Abstract
Polycystic Ovarian Syndrome (PCOS) has been associated with numerous reproductive and metabolic abnormalities. Despite tremendous advances in the management of reproductive dysfunction, insight into the metabolic implications of PCOS is limited by the lack of uniform diagnostic criteria, the heterogeneity of the condition and the presence of confounders including obesity. Obesity clearly has a role in long term health and may best predict both reproductive and metabolic dysfunction as well as negatively affect the response to treatment in women with PCOS. Diabetes, cardiovascular disease and cancer are also at the forefront of any risk assessment or comprehensive treatment strategy for these women. Lifestyle modifications including dietary changes, increased exercise and weight loss are appropriate first line interventions for many women with PCOS. Pharmaceuticals including metformin, lipid lowering agents and oral contraceptives should be tailored to the individual’s risk profile and treatment goals.
Introduction
Polycystic Ovarian Syndrome (PCOS) likely has long-term effects on health and longevity. It is a disorder that typically has its onset early in life, (how early remains a matter of speculation). Thus, the metabolic abnormalities associated with the syndrome, such as insulin resistance, have a long duration over the lifespan to compound their effects. Further in other populations, insulin resistance is associated with a progressive increase in cardiovascular risk factors and events, though its natural history in PCOS is still cloudy. Understanding the true health implications of PCOS is problematic due to the heterogenous nature of this condition, disagreement over the diagnostic criteria, the lack of long term cohort studies, and confounding factors (such as obesity and long term treatments with OCPs or metformin). More attention is being paid to the potential chronic health risks associated with PCOS, and often the worst is assumed.
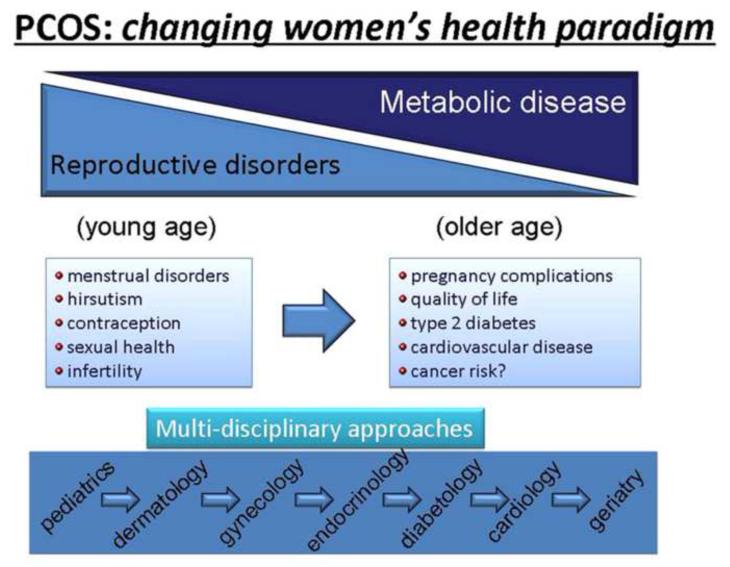
The recent Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group addressed the full range of PCOS’ health implications. Their goal was to summarize the current data and identify knowledge gaps in the understanding of PCOS sequelae. Pregnancy complications, quality of life, type 2 diabetes mellitus, cardiovascular disease and cancer risk were identified as relevant long term metabolic complications of PCOS (Figure 1) In addition, it was stressed that obesity appears to play an independent role in all of the long term complications and may best predict both reproductive and metabolic dysfunction, as well as negatively affect the response to treatment.(Fauser, 2012)
Figure 1.
While there is considerable overlap between PCOS and the metabolic syndrome, it is important to note that they are not identical or interchangeable. Although a large proportion of women with PCOS also have the metabolic syndrome (~30-40%), most do not.(Ehrmann, 2006) The prevalence of PCOS may not be increased in women with the metabolic syndrome when compared to appropriate controls.(Korhonen, 2001) Likewise, the incidence of metabolic syndrome may vary based on age in women with PCOS.(Glintborg, 2012) Despite the limitation of our current knowledge, recognition of the long term health implication of PCOS is crucial. Our review will address the potential associations and possible interventions for obesity, cancer, type II diabetes, and cardiovascular disease in the setting of PCOS.
Obesity
Obesity has reached epidemic proportions in the United States and clearly contributes to the morbidity seen with PCOS. In the United States, upper body fat distribution is highly predictive of poor health, particularly diabetes and cardiovascular disease.(Lord, 2006) However, the association between obesity and PCOS is not universal and may in part be a cultural and ethnic phenomenon. Large case series of women who meet the criteria for PCOS from the United Kingdom and the Netherlands have reported BMIs of < 25 in 80% and 56% of these women, respectively. (Ehrmann, 1999, Knochenhauer, 1998, Legro, 1999) Similarly, women with PCOS from China also tend to have normal BMIs.(Chen, 2011) Although women with BMI<30 have metabolic complications, the greatest health implications of PCOS are clearly associated with excess weight and abdominal circumference, especially in the setting of androgen excess.(Glueck, 2005) Likewise, increased high upper body fat distribution or visceral adiposity further compounds the insulin resistance and type 2 diabetes. PCOS more than doubles the likelihood of diabetes and this relative risk increases to almost 4 fold in the setting of obesity.(Norman, 2001) The ESHRE/ASRM 3rd Consensus on PCOS also pointed out that diabetes is likely to increase as the prevalence of obesity continues to rise.(Fauser, 2012) Persistence of PCOS into the late reproductive life may convey a risk of DM as high as 7 fold.(Pasquali, 2011) Obesity increases one’s lifetime risk of cancer and cardiovascular disease.(Berrington de Gonzalez, 2010, Boggs, 2011) Any intervention that reduces excess weight has potential to improve the health and wellness of women with PCOS Lifestyle modifications that include weight loss and exercise should form the foundation for treating obese women with PCOS. Although definitive data are lacking, Lifestyle modifications has been shown to decrease adipose tissue and improve insulin sensitivity associated with PCOS. In the adult population the benefits of exercise in Lifestyle Modifications on glucose levels and other cardiovascular risk factors are well documented. The addition of exercise to dietary caloric restriction also results in more rapid, though only modest incremental decreases in weight.(Hall, 2011) A recent randomized trial of 130 morbidly obese adult patients found that the addition of exercise resulted in greater reductions in waist circumference and hepatic fat mass.(Moran, 2011)
Many studies have suggested an improvement in cardiovascular parameters and in the underlying hormone abnormalities seen with obese anovulatory women after lifestyle modifications. However, there are few well controlled randomized studies to date that have evaluated the effects of lifestyle modifications specifically on women with PCOS. A recent meta-analysis of lifestyle modification in PCOS suggested that despite the limited and heterogeneous studies there are benefits on body composition, hyperandrogenism and insulin resistance in women with PCOS, although many parameters remain unaffected.(Moran, Hutchison, 2011) In addition, most of the available studies have only focused on reproductive outcomes or restoration of menstrual cyclicity and ovulation. In small cohort studies of women with PCOS, modest weight loss has been shown to improve reproductive and metabolic function. Moderate calorie restriction with 2% to 5% weight loss resulted in a 21% decline in free testosterone; 9 of 18 women with irregular cycles resumed regular ovulation; and 2 of the 18 women became pregnant.(Guzick, 2004) Another group of 24 obese patients with PCOS was placed on a 1000kcal, low fat diet for 6-7 months. 13 subjects lost more than 5% of their starting body weight with resulting increases in SHBG and a reduction in fasting serum insulin levels (11.2 (5.2-32) vs. 2.3 (0.1-13.8) p = 0.018) More than half of the women who lost >5% of their body mass had normalization of ovarian function.(Kiddy, 1992)
Reduction in centripetal adipose fat may be the key to normalization of reproductive and metabolic function. An observational cohort study evaluated 40 obese infertile patients with PCOS enrolled in a 24 week structured exercise program (SET) or a dietary intervention group. The SET group consisted of 3 times weekly exercise sessions on a bicycle ergometer for 30 minutes to reach a target maximal oxygen consumption of 60-70%. The diet group utilized a high protein (35% protein, 45% carbohydrate, 20% fat) and an 800 kcal total deficit per day. Weekly interactive educational sessions were offered to all subjects in the dietary program. Both groups had improvement in menstrual cyclicity defined by luteal progesterone >10. However, the SET group had a higher ovulation rate than the diet group. (24.8% vs. 15.1% p= 0.032) While stratifying by intervention group, and then analyzing case vs. control (ovulatory vs. nonovulatory), the cases (ovulatory) had more change in waist circumference than the control group. (SET: −9.6cm vs. −2.5cm p <0.05; diet group: −9.4cm vs. −2.8cm p <0.05) The study may have been limited by the low number of subjects and 25% drop out rate. None the less, it appears that loss of central adipose tissue contributes to resumption of menstrual cyclicity.(Palomba, 2008) Normalization of the menstrual cycle is often associated with improvements in ovulation and parallels the increase in SHBG and reduction in insulin resistance seen with loss of the more metabolically active visceral fat. (Park, 2005)
Hollman et al demonstrated a reduction in impaired glucose tolerance from 50% to 4% after a 32 week lifestyle modifications program in a cohort of obese, anovulatory patients after only modest weight loss. The improvement in glucose tolerance was associated with increased pregnancy rates.(Hollmann, 1996) Other researchers have suggested that lifestyle modifications also improves spontaneous pregnancy rates (Crosignani, 2003, Clark, 1995, Clark, 1998), response to fertility medication(Palomba, 2010), and success following the use of Assisted Reproductive Technologies.(Luke, 2011, Shah, 2011, Moran, 2011)
While the optimum life style modification has not been identified, a majority of these studies have utilized structured exercise programs with protein rich hypocaloric diets and on occasion, psychological support. The carbohydrate versus low-fat diet debate was addressed in a 2 year trial of 307 obese adults (mean BMI 36 kg/m2). Study subjects were randomized to a low-carbohydrate diet that allowed unrestricted consumption of fat or to a low-fat diet with approximately 30% of calories from fat. Behavioral interventions, including self-monitoring and physical activity sessions were included in the protocol for both arms of the study. Weight loss was comparable between the two groups. The majority of the lipid profile did not differ between the two. Although the mean HDL cholesterol levels were significantly higher in the low-carbohydrate group, the absolute difference was only 2 mg/dL. The generalizability to PCOS was limited by the inclusion of men and the exclusion of those with diabetes and hyperlipidemia. The 50% drop out rate at 2 years is indicative of the difficulty with diet and lifestyle modifications. (Foster, 2010)
In the absence of definitive data, clinicians must rely on expert opinion and guidance. A 500 calorie per day restriction is recommended by the CDC for weight loss in the general population. Patients are often counseled that the weekly caloric deficit of 3500 calories should lead to a 1-2 pound weight loss. However this counseling likely overestimate the amount of weight that will be lost by almost 100%, based on careful modeling incorporating counter-regulatory mechanisms.(Hall, Sacks, 2011) The Androgen Excess and Polycystic Ovarian Syndrome Society has provided specific recommendations for addressing obesity in women with PCOS. (Table 1) (Wild, Carmina,2010)
Table 1.
Lifestyle modification principles suggested for obesity management in polycystic ovary syndrome (PCOS).
| Guidelines for dietary and lifestyle intervention in PCOS |
|---|
| 1. Lifestyle modification is the first form of therapy, combining behavioral (reduction of psychosocial stressors), dietary, and exercise management. |
| 2. Reduced-energy diets (500–1000 kcal/day reduction) are effective options for weight loss and can reduce body weight by 7% to 10% over a period of 6 to 12 months. |
| 3. Dietary pattern should be nutritionally complete and appropriate for life stage and should aim for <30% of calories from fat, <10% of calories from saturated fat, with increased consumption of fiber, fibre, whole-grain breads and cereals, and fruit and vegetables. |
| 4. Alternative dietary options (increasing dietary protein, reducing glycemic index, reducing carbohydrate) may be successful for achieving and sustaining a reduced weight but more research is needed in PCOS. |
| 5. The structure and support within a weight-management program is crucial and may be more important than the dietary composition. Individualization of the program, intensive follow-up and monitoring by a physician, and support from the physician, family, spouse, and peers will improve retention. |
| 6. Structured exercise is an important component of a weight-loss regime; aim for >30 minutes per /day. |
Reprinted from Fertility and Sterility. Vol 92, No 6. Lisa Moran, PhD, Renato Pasquali, M.D., Helena J Teedle, PhD., Kathleen M. Hoeger, M.D., and Robert J. Norman, M.D. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society, 1966-1982, Copyright 2009, with permission from Elsevier
Numerous pharmaceuticals have been utilized in the treatment of obesity, but the ideal diet pill has also remained elusive. Side effects and unacceptable risk profile has negated any benefit of weight loss for numerous medical therapies for obesity. The only therapy currently FDA approved for the long term management of obesity, Orlistat, has been shown to reduce BMI and the progression to Type II diabetes mellitus. However, its use is limited by gastrointestinal side effects.(Derosa, Cicero,2012) The recently completed phase 3 SEQUEL trial (Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults) demostrated weight reduction with reduced cardiovascular risk factors and improved metabolic function following the use of phentermine / tobiramate in conjunction with lifestyle modification.(Garvey, Ryan,2012) Many patients also seek weight loss in herbal weight loss pills. A recent systematic review of the current literature by the Johns Hopkins’ Weight Management Center concluded that nonprescription dietary supplements as an adjunct to weight loss currently cannot be strongly recommended.(Poddar, Kolge,2011) Furthermore, these medications have not been adequately assessed in women with PCOS.
Weight loss and a reduction of centripetal fat has been demonstrated with the use of insulin sensitizing agent metformin in the Diabetes Prevention Program. Women with PCOS may also experience weight loss on this class of drugs.(Legro, 2007) Metformin, a biguanide antihyperglycemic, together with a low-calorie diet, is associated in some studies with more weight loss than a low-calorie diet alone. (Barbieri, 2003), though larger multi-center studies have not found this to be the case.(Ladson, 2011, Tang, 2006) Women taking metformin in the PPCOSI trial experienced a significant reduction in weight of almost 1 body mass index (BMI) unit.(Legro, Barnhart, 2007) A 2009 meta-analysis of treatment with metformin in women with PCOS showed a statistically significant decrease in BMI compared with placebo (weighted mean difference −0.68; 95% CI −1.13 to −0.24).(24)
There was some indication of greater effect with high-dose metformin (> 1500 mg per day) and longer duration of therapy (> 8 weeks). Women in need of profound weight loss may also benefit from surgical intervention. Bariatric surgery has been shown to result in significant weight loss and improvement in endocrine function in those with obesity and stigmata of PCOS.(Escobar-Morreale, 2005)
Cancer
An increased risk for endometrial, ovarian and breast cancer in women with PCOS has been suggested. Clearly it has been associated with an increased risk for breast and endometrial cancer in population based studies.(Legro, Barnhart, 2007) It is important to note that many confounders including obesity, hyperglycemia and anovulation (unopposed estrogen) with resultant infertility make it difficult to define the absolute risk of these neoplasms attributed to PCOS alone. This is especially true in the case of ovarian and breast cancer where a paucity of data limit conclusions. However, the link between PCOS and endometrial cancer appears to be supported by both biologic plausibility and the preponderance of evidence suggesting a 2 -3 fold increase risk of endometrial cancer in the setting of anovulation, menstrual irregularity and PCOS.(Chittenden, 2009, Coulam, 1983) (28, 29) Proper endometrial surveillance (ultrasound and/or sampling) and periodic induction of uterine bleeding with progesterone withdrawal may lessen the risk of endometrial cancer. Oral contraceptives also result in a significant reduction in the risk of endometrial and ovarian cancer (Cramer, 2012) with no increase in long term metabolic risk.(Costello, 2007) In those desiring fertility, pregnancy decreases one’s risk of all three cancer types.(Adami, 2008) Recent evidence supports the use of a progesterone releasing IUD in the prevention and treatment of uterine hyperplasia and early endometrial cancer but its use specifically in women with PCOS has not been investigated.(Gallos, 2010)
Agents that induce ovulation and improve the chance of pregnancy would also combat the unopposed estrogen and possibly lower the risk endometrial hyperplasia and cancer. Insulin sensitizing agents role in endometrial cancer prevention have been addressed in a limited manner. Endometrial samples were obtained before and after treatment with metformin or rosiglitazone alone or in combination in a prospective cohort study. Those with abnormal histology (simple hyperplasia) on endometrial biopsy at baseline tended to normalise after treatment. However, the small sample size, lack of long term follow up and absence of endometrial cancer in the study population preclude any meaningful recommendation for all women with PCOS.(Legro, 2007) Life style modifications that normalize ovulation and restore fertility would in theory reduce one’s lifetime risk of cancer in reproductive tissues. Recent evidence supports a reduction in numerous biomarkers of endometrial cancer with weight loss.(Linkov, 2011) In addition, weight loss with improvements in glucose dynamics might reverse the increased risk of breast(Hemminki, 2010, Rosato, 2011) and endometrial cancer(Rosato, Zucchetto, 2011) seen with glucose intolerance and type 2 diabetes. Despite the concern for endometrial cancer in the setting of PCOS, the current data do not support increased surveillance solely on the basis of a diagnosis of PCOS. Screening with an endometrial biopsy or ultrasound assessment of the endometrial lining should be based on the standard dogma of abnormal uterine bleeding in a women 35 years or older or with significant risk factors for endometrial cancer.
Diabetes
PCOS is commonly associated with glucose metabolism abnormalities and is an independent risk factor for the development of diabetes. Impaired glucose tolerance, measured by oral glucose tolerance test, may approach 30–40% in obese populations with PCOS.(Legro, Kunselman, 1999, Ehrmann, 1999) Progression of insulin resistance without hyperglycemia to glucose intolerance and ultimately diabetes is variable but may occur in a third of those affected within 2 – 3 years(Legro, 2005) and exceed 50% within 10 years.(Norman, Masters, 2001) Overall, numerous studies have suggested a 2 -3 fold increase in the incidence of diabetes with PCOS, even after controlling for BMI.(Chang, 2011) Likewise, the familial link seen with PCOS is noted in glucose metabolism abnormalities as the parents of women with PCOS have a significant higher prevalence of diabetes than controls.(Legro, Kunselman, 1999)
The negative association between glucose dynamics and PCOS is compounded by presence of obesity, especially in the setting of androgen excess and oligo-ovulation. The second iteration of the Nurses Health Study demonstrated that women who reported long or irregular cycles between 18 and 22 years of age were twice as likely to subsequently develop diabetes even when controlling for obesity.(Solomon, 2001) Likewise, the Coronary Artery Risk Development in Young Adults (CARDIA) study, a biracial longitudinal study of over 5000 men and women from four US communities, found that women who reported PCOS between 20 and 32 years of age were 2 -3 times more likely to develop diabetes over the next 18 years. Obese women with PCOS were 3 – 4 times more likely to develop diabetes than women without PCOS or obesity, but the association between PCOS and an increased rate of incident diabetes remained even in women with a BMI of less than 25.(Wang, 2011)
Once again, diet, exercise and weight loss are the clinicians most valuable allies in the fight against the insulin resistance, glucose intolerance and diabetes associated with PCOS, especially those with concomitant obesity. As noted above, successful lifestyle modifications may have far reaching beneficial effects for glucose metabolism. Medical therapy with insulin sensitizers may also play a role with metformin’s niche in this fight being its ability to reduce progression to diabetes in those at greatest risk. Although not specifically focused on women with PCOS, the Diabetes Prevention Program showed a 31% (95% CI 17–43%) reduction in the conversion to type 2 diabetes with metformin compared with placebo in individuals with impaired glucose tolerance.(Orchard, 2005) This program also demonstrated that the thiazolidinediones, including troglitazone and rosiglitazone are equivalent if not superior to metformin in delaying the development of diabetes in at risk populations. However the risk, including hepatoxicity which forced the removal of troglitazone from the market, increased CVD events with rosiglitazone which severely restricted its use, and the rising concern about bladder cancer with pioglitazone, as well as the lack of proven benefit in women with PCOS argue against the use of this class of medications. (Buchanan, 2002, Knowler, 2005) In a longitudinal cohort followed for 20 years, a history of at least 6 months of metformin (or oral contraceptive) use did not have a beneficial effect on the long term parameters including BMI, waist circumference, testosterone levels or fasting insulin.(Carmina, 2012)
Cardiovascular risk
Cardiovascular disease (CVD) is a major concern for women with PCOS, but a clear cause and effect relationship has not been established, nor can anyone state if events are even elevated in this group of women compared to others. Several CVD risk factors have been associated with PCOS including insulin resistance, type 2 diabetes mellitus, and possibly hypertension.(Yusuf, 2004) Women with PCOS also appear to have a higher incidence of obstructive sleep apnea and associated cardiovascular risk factors compared to weight matched controls.(Nandalike, Agarwal,2012) Dyslipidemia are found in up to 70% of women with PCOS in the United States and are most commonly characterized by high triglycerides and increased low density lipoprotein (LDL) cholesterol with low levels of high-density lipoprotein (HDL)-cholesterol.(Legro, 2001) In addition, obesity is an independent risk factor for heart disease and a common component of the PCOS clinic picture.
The mood disorders associated with PCOS may also contribute to vascular disease indirectly through obesity and insulin resistance as well as through alterations in the stress response(Benson, 2009), inflammatory markers(Sverrisdottir, 2008), and sympathetic tone(Wild, 2010). CVD risk also clusters in fathers of women with PCOS. Fathers of women with PCOS self reported a higher prevalence of heart attacks and stroke compared to a reference population. In addition, an increased 10 year coronary artery disease risk was noted in these men. Of note, no association between PCOS and self report of CV disease was seen in the women with PCOS or their mothers.(Taylor, 2011)
Age is one key risk factor for CVD that may not correlate with the severity of PCOS. The temporal association between CVD risk and PCOS is tenuous as PCOS stigmata may wane in the 5th decade(Brown, 2011), well before the peak incidence of CVD. Likewise, our understanding of the link between PCOS and CVD is limited as much of the available literature focuses on women with PCOS during their reproductive years, a time largely free of CVD for most women. A recent 20 year longitudinal study of young women with PCOS demonstrated a reduction in potential risk factors for CV disease including androgemia, BMI and insulin resistance over time. Only abdominal circumferences had worsened by the 5th decade.(Carmina, Campagna, 2012) Similarly a longitudinal case control study of cardiovascular risk factors over time in women with and without PCOS suggested a plateauing of levels at an earlier age in the cases with PCOS compared to controls without PCOS, who tended to progressively worsen.(Talbott, 2000)
Conversely, the Women’s Ischemia Evaluation Study (WISE) suggested that postmenopausal women with a history of menstrual irregularity and evidence of hyperandrogenemia are at increased risk for cardiovascular disease and also CV events. Women with a semblance of PCOS had more multivessel cardiovascular disease (32% vs. 25%, OR 1.7; 95% CI, 1.1–2.8, adjusted for age and BMI), and lower event-free survival (hazard ratio 1.6; 95% CI, 1.2–2.1) when compared to women without evidence of PCOS. The CVD risk correlated with diabetes, hypertriglyceridemia, and increased free T.(Shufelt, 2011)
An adverse cardiovascular risk profile does not equate to a cause and effect relationship between PCOS and CV disease. Although many of the common features associated with PCOS are clearly defined risk factors for CVD, the high prevalence of CVD in the population may lead to considerable overlap by default.(Legro, 2003) The vast majority of the evidence linking PCOS to cardiovascular events including myocardial infarction and stroke rely on retrospective assessment of PCOS. Long term prospective studies of CVD endpoints in women with well characterized PCOS are lacking and may not be possible as the PCOS diagnostic criteria, menstrual or ovulatory dysfunction, are by definition universal in the postmenopausal period.(Legro, 2003) Thus, there are inherent shortcomings in any attempt to establish a direct correlation between PCOS and the largely postmenopausal CVD. However, the tremendous impact of CVD on the health and well being of women begs for further efforts to define the risk and development of effective intervention strategies.
The Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society recommends an assessment of CVD risk factors in women with PCOS and assignment of PCOS related CVD risk categories. The “at risk category women with PCOS who also display includes obesity (especially abdominal adiposity), cigarette smoking, hypertension, dyslipidemia (increased LDL-C and/or non-HDL-C), subclinical vascular disease, IGT and a family history of premature CVD (<55 yr of age in male relative, <65 yr of age in female relative). Women with PCOS who also meet criteria for the metabolic syndrome, type 2 diabetes mellitus and overt vascular or renal disease are considered to be high risk for CVD.(Wild, Carmina, 2010) This group recommended that BP, waist circumference and BMI be determined at every visit, a lipid profile (total cholesterol, LDL-C, non-HDL-C, HDL-C, and triglycerides) be obtained every 2 years and a 2-h post 75-g oral glucose challenge be performed with a BMI greater than 30 kg/m2, or alternatively in lean PCOS women with advanced age (>40 yr), personal history of gestational diabetes, or family history of T2DM. Screening for depression, anxiety and quality of life was also suggested. (Dokras, 2011) The recent 3rd PCOS Consensus Workshop Group recommended CVD risk assessment at any age is for psychosocial stress, blood pressure, glucose, lipid profile (cholesterol, triglycerides, HDL, LDL, and non-HDL cholesterol), waist circumference, physical activity, nutrition, and smoking.(Fauser, 2012)
The optimum multifaceted approach to women with PCOS that reduces and prevents CVD has yet to be determined. However, life style modification lifestyle modifications should form the foundation of any effort to reduce CVD risk. Short term weight loss improves numerous CVD risk factors including waist circumference, androgen levels, insulin resistance and dysplipidemias in women with PCOS.(Andersen, 1995) Dietary recommendation for overweight women with PCOS includea hypocaloric, low saturated fat, increased mono- and polyunsaturated fat diet (500–1000 kcal/d reduction; <30% calories from fat, <10% calories from saturated fat; increased consumption of fiber, whole-grain breads, cereals, fruits, and vegetables). At least 30 min of moderate-intensity physical activity daily with an initial weight loss of 5 – 10% and a long term goal of 10 – 20% weight loss to reach an abdominal circumference of less than 88 cm has been suggested.(Andersen, Seljeflot, 1995)
Several pharmacologic interventions have been proposed for the reduction of CVD risk in women with PCOS. Insulin sensitizers, including metformin, may contribute to improvements in the CVD risk profile by enhancing glucose metabolism, reducing triglycerides, elevating HDL and contributing to modest weight loss in the short term. Metformin also decreases the inflammatory marker C-reactive protein(Morin-Papunen, 2003) and may improve atherosclerosis(Agarwal, 2010, Diamanti-Kandarakis, 2005) In the United Kingdom Prospective Diabetes Study (UKPDS), metformin was the only diabetes medication that reduced all cause mortality.(1998) However, the benefit of metformin above and beyond lifestyle modifications is questionable and no reduction in cardiovascular events has been demonstrated in women with PCOS. Dyslipidemias also warrant lifestyle modifications and appropriate cholesterol lowering agents in some cases. Antihypertensive medications are of clear benefit when indicated but weight loss medication cannot be recommended for women with PCOS based on the available literature.(Moran, 2009, Wild, Carmina, 2010)
Summary
PCOS is associated with several reproductive and metabolic abnormalities. Obesity is one of the most common findings in PCOS but is itself an independent risk factor for many of the disease states that have been attributed to PCOS. Likewise, altered glucose metabolism including impaired glucose tolerance, insulin resistance and type 2 diabetes mellitus are well known co-morbidities to PCOS. Both of these pathologic conditions must be mentioned as significant confounders in any discussion of the link between PCOS and cancer or cardiovascular disease. Standardization of diagnostic criteria and prospective longitudinal data is required to improve our understanding of long term health risk associated with PCOS. Likewise, the management of PCOS must include lifestyle modifications and pharmacologic intervention targeted to the individual disease process.
Longterm management of PCOS Highlights.
Longterm complications of PCOS include type 2 diabetes mellitus, cardiovascular disease and cancer risk.
Obesity and glucose intolerance are common but not universal features of PCOS that compound an accurate assessment of the risk inherent in this syndrome.
Lifestyle modifications form the foundation for treating obese women with PCOS and may reduce the incidence of diabetes, cardiovascular disease and cancer.
The management of PCOS also includes pharmacologic interventions, including metformin and oral contraceptives, that are targeted to the individual’s disease process and treatment goals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65. [PubMed] [Google Scholar]
- Adami HO, Hunter D, Trichopoulos D. Textbook of Cancer Epidemiology. Oxford University Press; 2008. 2009 PtOSOS. [Google Scholar]
- Agarwal N, Rice SP, Bolusani H, Luzio SD, Dunseath G, Ludgate M, et al. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95(2):722–30. doi: 10.1210/jc.2009-1985. [DOI] [PubMed] [Google Scholar]
- Andersen P, Seljeflot I, Abdelnoor M, Arnesen H, Dale PO, Lovik A, et al. Increased insulin sensitivity and fibrinolytic capacity after dietary intervention in obese women with polycystic ovary syndrome. Metabolism. 1995;44(5):611–6. doi: 10.1016/0026-0495(95)90118-3. [DOI] [PubMed] [Google Scholar]
- Benson S, Arck PC, Tan S, Hahn S, Mann K, Rifaie N, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology. 2009;34(5):727–35. doi: 10.1016/j.psyneuen.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DA, Rosenberg L, Cozier YC, Wise LA, Coogan PF, Ruiz-Narvaez EA, et al. General and abdominal obesity and risk of death among black women. N Engl J Med. 2011;365(10):901–8. doi: 10.1056/NEJMoa1104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZA, Louwers YV, Fong SL, Valkenburg O, Birnie E, de Jong FH, et al. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril. 2011;96(5):1259–65. doi: 10.1016/j.fertnstert.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51(9):2796–803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- Carmina E, Campagna AM, Lobo RA. A 20-year follow-up of young women with polycystic ovary syndrome. Obstet Gynecol. 2012;119(2 Pt 1):263–9. doi: 10.1097/AOG.0b013e31823f7135. [DOI] [PubMed] [Google Scholar]
- Chang CM, Wang SS, Dave BJ, Jain S, Vasef MA, Weisenburger DD, et al. Risk factors for non-Hodgkin lymphoma subtypes defined by histology and t(14;18) in a population-based case-control study. Int J Cancer. 2011;129(4):938–47. doi: 10.1002/ijc.25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Li Z, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55–9. doi: 10.1038/ng.732. [DOI] [PubMed] [Google Scholar]
- Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19(3):398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995;10(10):2705–12. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcomes for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502–5. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- Costello MF, Shrestha B, Eden J, Johnson NP, Sjoblom P. Metformin versus oral contraceptive pill in polycystic ovary syndrome: a Cochrane review. Hum Reprod. 2007;22(5):1200–9. doi: 10.1093/humrep/dem005. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol. 1983;61(4):403–7. [PubMed] [Google Scholar]
- Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26(1):1–12. doi: 10.1016/j.hoc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18(9):1928–32. doi: 10.1093/humrep/deg367. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Alexandraki K, Protogerou A, Piperi C, Papamichael C, Aessopos A, et al. Metformin administration improves endothelial function in women with polycystic ovary syndrome. Eur J Endocrinol. 2005;152(5):749–56. doi: 10.1530/eje.1.01910. [DOI] [PubMed] [Google Scholar]
- Derosa G, Cicero AF, D’Angelo A, et al. Effects of 1-year orlistat treatment compared to placebo on insulin resistance parameters in patients with type 2 diabetes. J Clin Pharm Ther. 2012;37(2):187–95. doi: 10.1111/j.1365-2710.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(1):145–52. doi: 10.1097/AOG.0b013e318202b0a4. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22(1):141–6. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(1):48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millan JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005;90(12):6364–9. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38. e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147–57. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203(6):547, e1–10. doi: 10.1016/j.ajog.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss an dmetabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glintborg D, Mumm H, Ravn P, Andersen M. Age Associated Differences in Prevalence of Individual Rotterdam Criteria and Metabolic Risk Factors during Reproductive Age in 446 Caucasian Women with Polycystic Ovary Syndrome. Horm Metab Res. 2012 doi: 10.1055/s-0032-1304608. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Dharashivkar S, Wang P, Zhu B, Gartside PS, Tracy T, et al. Obesity and extreme obesity, manifest by ages 20-24 years, continuing through 32-41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol. 2005;122(2):206–12. doi: 10.1016/j.ejogrb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Guzick DS. Polycystic ovary syndrome. Obstet Gynecol. 2004;103(1):181–93. doi: 10.1097/01.AOG.0000104485.44999.C6. [DOI] [PubMed] [Google Scholar]
- Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–37. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. Oncologist. 2010;15(6):548–55. doi: 10.1634/theoncologist.2009-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Runnebaum B, Gerhard I. Effects of weight loss on the hormonal profile in obese, infertile women. Hum Reprod. 1996;11(9):1884–91. doi: 10.1093/oxfordjournals.humrep.a019512. [DOI] [PubMed] [Google Scholar]
- Kiddy DS. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;36(1):7. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54(4):1150–6. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen S, Hippelainen M, Niskanen L, Vanhala M, Saarikoski S. Relationship of the metabolic syndrome and obesity to polycystic ovary syndrome: a controlled, population-based study. Am J Obstet Gynecol. 2001;184(3):289–96. doi: 10.1067/mob.2001.109596. [DOI] [PubMed] [Google Scholar]
- Ladson G, Dodson WC, Sweet SD, Archibong AE, Kunselman AR, Demers LM, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95(3):1059–66. e1–7. doi: 10.1016/j.fertnstert.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84(1):165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111(8):607–13. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24(3):302–12. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90(6):3236–42. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–66. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- Legro RS, Zaino RJ, Demers LM, Kunselman AR, Gnatuk CL, Williams NI, et al. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196(4):402, e1–10. doi: 10.1016/j.ajog.2006.12.025. discussion e10-1. [DOI] [PubMed] [Google Scholar]
- Linkov F, Maxwell GL, Felix AS, Lin Y, Lenzner D, Bovbjerg DH, et al. Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: Implications for cancer risk reduction. Gynecol Oncol. 2011 doi: 10.1016/j.ygyno.2011.12.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J, Thomas R, Fox B, Acharya U, Wilkin T. The central issue? Visceral fat mass is a good marker of insulin resistance and metabolic disturbance in women with polycystic ovary syndrome. BJOG. 2006;113(10):1203–9. doi: 10.1111/j.1471-0528.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil Steril. 2011;96(4):820–5. doi: 10.1016/j.fertnstert.2011.07.1100. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril. 2009;92(6):1966–82. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;7:CD007506. doi: 10.1002/14651858.CD007506.pub3. [DOI] [PubMed] [Google Scholar]
- Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(10):4649–54. doi: 10.1210/jc.2002-021688. [DOI] [PubMed] [Google Scholar]
- Nandalike K, Agarwal C, Strauss T, Coupey SM, Isasi CR, Sin S, et al. Sleep and cardiometabolic function in obese adolescent girls with polycystic ovary syndrome. Sleep Med. 2012 Aug 22; doi: 10.1016/j.sleep.2012.07.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod. 2001;16(9):1995–8. doi: 10.1093/humrep/16.9.1995. [DOI] [PubMed] [Google Scholar]
- Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142(8):611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23(3):642–50. doi: 10.1093/humrep/dem391. [DOI] [PubMed] [Google Scholar]
- Palomba S, Falbo A, Giallauria F, Russo T, Rocca M, Tolino A, et al. Six weeks of structured exercise training and hypocaloric diet increases the probability of ovulation after clomiphene citrate in overweight and obese patients with polycystic ovary syndrome: a randomized controlled trial. Hum Reprod. 2010;25(11):2783–91. doi: 10.1093/humrep/deq254. [DOI] [PubMed] [Google Scholar]
- Park HS, Lee K. Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: a study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet Med. 2005;22(3):266–72. doi: 10.1111/j.1464-5491.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Stener-Victorin E, Yildiz BO, Duleba AJ, Hoeger K, Mason H, et al. PCOS Forum: research in polycystic ovary syndrome today and tomorrow. Clin Endocrinol (Oxf) 2011;74(4):424–33. doi: 10.1111/j.1365-2265.2010.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar K, Kolge S, Bezman L, Mullin GE, Cheskin LJ. Nutraceutical Supplements for Weight Loss A Systematic Review. Nutr Clin Pract. 2011 Oct;26(5):539–52. doi: 10.1177/0884533611419859. [DOI] [PubMed] [Google Scholar]
- Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011;22(4):884–9. doi: 10.1093/annonc/mdq464. [DOI] [PubMed] [Google Scholar]
- Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES, et al. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(10):3671–89. doi: 10.1210/jc.2008-0222. [DOI] [PubMed] [Google Scholar]
- Shufelt CL, Johnson BD, Berga SL, Braunstein GD, Reis SE, Bittner V, et al. Timing of hormone therapy, type of menopause, and coronary disease in women: data from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Menopause. 2011;18(9):943–50. doi: 10.1097/gme.0b013e3182113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA. 2001;286(19):2421–6. doi: 10.1001/jama.286.19.2421. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab. 2008;294(3):E576–81. doi: 10.1152/ajpendo.00725.2007. [DOI] [PubMed] [Google Scholar]
- Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20(11):2414–21. doi: 10.1161/01.atv.20.11.2414. [DOI] [PubMed] [Google Scholar]
- Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod. 2006;21(1):80–9. doi: 10.1093/humrep/dei311. [DOI] [PubMed] [Google Scholar]
- Taylor MC, Reema Kar A, Kunselman AR, Stetter CM, Dunaif A, Legro RS. Evidence for increased cardiovascular events in the fathers but not mothers of women with polycystic ovary syndrome. Hum Reprod. 2011;26(8):2226–31. doi: 10.1093/humrep/der101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117(1):6–13. doi: 10.1097/AOG.0b013e31820209bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95(5):2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]