Abstract
BACKGROUND AND PURPOSE:
Cerebrovascular collaterals have been increasingly recognized as predictive of clinical outcomes in Moyamoya disease in Asia. The aim of this study was to characterize collaterals in North American adult patients with Moyamoya disease and to assess whether similar correlations are valid.
MATERIALS AND METHODS:
Patients with Moyamoya disease (n = 39; mean age, 43.5 ±10.6 years) and age- and sex-matched control subjects (n = 33; mean age, 44.3 ± 12.0 years) were graded via angiography. Clinical symptoms of stroke or hemorrhage were graded separately by imaging. Correlations between collateralization and disease severity, measured by the modified Suzuki score, were evaluated in patients with Moyamoya disease by fitting a regression model with clustered ordinal multinomial responses.
RESULTS:
The presence of leptomeningeal collaterals (P = .008), dilation of the anterior choroidal artery (P = .01), and the posterior communicating artery/ICA ratio (P = .004) all correlated significantly with disease severity. The presence of infarct or hemorrhage and posterior steno-occlusive disease did not correlate significantly with the modified Suzuki score (P = .1). Anterior choroidal artery changes were not specific for hemorrhage. Patients with Moyamoya disease were statistically more likely than controls to have higher posterior communicating artery/ICA ratios and a greater incidence of leptomeningeal collaterals.
CONCLUSIONS:
As with Moyamoya disease in Asian patients, the presence of cerebrovascular collaterals correlated with the modified Suzuki score for disease severity in North American patients with Moyamoya disease. However, anterior choroidal artery changes, which correlated with increased rates of hemorrhage in Asian studies, were not specific to hemorrhage in North Americans.
Moyamoya disease (MMD) has been largely described by the Asian experience, yet varying epidemiologic and clinical features in North Americans with MMD suggest that pathophysiologic differences exist. MMD in North Americans and Europeans most commonly affects young women in the third-to-fourth decades of life, whereas MMD in Asians typically begins in childhood.1–3 North American and European patients present with ischemic stroke or TIA as the most frequent manifestation,1,4–9 whereas hemorrhage is more frequent in Asian cohorts.3 Additionally, gene associations found in Asian cohorts have not been replicated by European investigators.10
Shared features for all MMD include predominantly anterior circulation involvement with progressive arteriopathy involving the ICA and proliferation of distinctive basal vessels—changes that have been well-correlated with disease severity.11–13 Other collaterals, including dilated anterior choroidal arteries (AchoAs) and posterior communicating arteries (PcomAs), are less well-studied, particularly in North Americans. Histologic analysis of these collaterals has demonstrated evidence of stress related to increased flow, which may predispose patients to hemorrhage.14 Dilated anterior choroidal and posterior communicating arteries have been shown to be strongly predictive of hemorrhage in Asian MMD.15 The purpose of this study was to assess correlations between disease severity with less well-characterized collaterals, including the PcomA/ICA ratio, leptomeningeal collaterals (LMC), and AchoA changes, in a cohort of adult North Americans with MMD. Our primary hypothesis is that these collaterals correlate variably with disease severity, as measured by the modified Suzuki score (mSS) and clinical findings of stroke or hemorrhage. Our secondary hypothesis is that these collaterals correlate with disease compared with control subjects.
Materials and Methods
This retrospective review was approved by the local institutional review board. Subjects were identified by an electronic medical record search for all adult patients with MMD who underwent DSA from 2002 to 2012 at our institution. Idiopathic MMD was defined as mSS I–IV in at least 1 cerebral hemisphere without associated predisposing disease. Control subjects were selected sequentially from angiograms obtained for any non-Moyamoya indication. Two certified neuroradiologists, blinded to clinical and imaging findings, graded disease severity separately and resolved disagreement by consensus. Modifications have been made to the Suzuki classification so that the score can be applied to individual cases, rather than longitudinally.16,17 The mSS used for this study (Table 1) includes 5 stages of disease severity,18 examples of which are shown in Fig 1. In separate sessions divided by >2 weeks, the following were graded from DSA:
1) LMC circulation to the anterior circulation was classified into 2 stages: Stage 1, leptomeningeal cortical branches were found coursing from the posterior cerebral artery (PCA) to the frontal, temporal, or parietal Lobes; Stage 2, there was no leptomeningeal collateral circulation.19,20
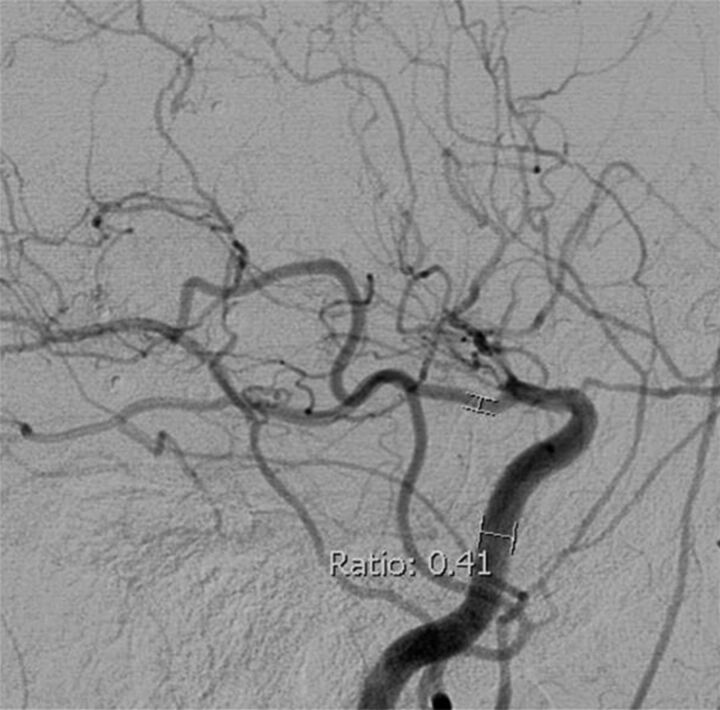
2) Ratio of the PcomA lumen diameter to the ipsilateral precavernous ICA lumen diameter21 is shown in Fig 2. Because the distal ICA is stenosed in MMD, the ICA measurement was performed at the precavernous portion with the widest, parallel lumen. If a PcomA infundibulum was present, the diameter was measured distal to infundibulum.
3) Proximal segment of the posterior cerebral artery (P1) steno-occlusive changes were noted (1, no stenosis or occlusion; 2, stenosis or occlusion).2,22
Table 1:
Modified Suzuki scoring
| Score | Description of classification |
|---|---|
| 0 | No evidence of disease |
| I | Mild-to-moderate stenosis around ICA bifurcation with absent or slightly developed ICA MMDa |
| II | Severe stenosis around the ICA bifurcation or occlusion of either proximal anterior or MCA branches with well-developed ICA MMD |
| III | Occlusion of both anterior and MCA branches with well-developed ICA MMD (only a few of anterior or MCA branches or both are faintly opacified in antegrade fashion through meshwork of ICA MMD) |
| IV | Complete occlusion of both anterior and MCA branches with absent or small amount of ICA MMD (without opacification of either anterior or MCA branches in antegrade fashion) |
ICA Moyamoya disease indicates perforating collateral vessels at or around the terminal ICA.
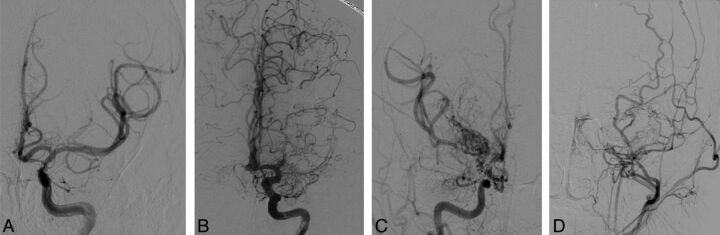
Fig 1.
Modified Suzuki scoring. Anteroposterior projections from DSA demonstrate a moderately stenosed left ICA without anterior cerebral artery or MCA involvement or Moyamoya perforators (mSS I) (A); an occluded left M1 with well-developed ICA Moyamoya perforators (mSS II) (B); an occluded right ICA, A1 and M1 with extensive Moyamoya perforators (mSS III) (C); and an occluded left ICA, M1 and A1 with absent Moyamoya perforators (mSS IV)—external carotid collaterals are visualized from a common carotid injection (D).
Fig 2.
PcomA/ICA ratio method. Lateral projection from DSA with the PcomA/ICA ratio in a patient with MMD with distal ICA occlusion beyond the PcomA origin.
An endovascular neurosurgeon (with 21 years of experience), blinded to clinical and imaging findings, separately graded the AchoA (zero, normal; 1, dilated with distal branching; and 2, dilated with abnormal branches serving as collaterals to other regions).15 The site of ICA occlusion was identified in subjects when the AchoA was not visualized. Hemorrhage or infarct or both were graded on CT or MR imaging performed within 30 days of DSA, and the location of the infarct was classified by territory. Exclusion criteria were the following: 1) For PcomA/ICA ratios, the hemisphere was excluded if the PcomA was not visualized on DSA; 2) both hemispheres were excluded for surgical revascularization before DSA; and 3) for AchoA grading, the hemisphere was excluded if the AchoA was not visualized.
Statistical Analysis
For univariate analysis for the association of the disease severity (mSS) with other collateral and clinical characteristics, the Kruskal-Wallis or Fisher exact test (2-sided) was used. For the comparison of the PcomA/ICA ratio between subjects with MMD and controls, the Wilcoxon rank sum test was used. Because hemispheres were considered separately, a multivariate regression model based on generalized estimating equations23 for clustered ordinal responses with uniform local odds ratio structure was fit to evaluate the correlations between collateralization and disease severity among subjects with MMD. Hemispheres with an mSS of zero were included in the analysis because the typical progression of MMD is bilateral involvement.24 Due to the small number of observations for mSS stages zero and I (6 and 7, respectively), we combined them as stage 1 in the regression model. Asian and African American races were also combined as nonwhite. For multivariate analysis, the CT/MR imaging findings of infarct and hemorrhage were combined. The Fisher exact test was used to correlate the incidence of hemorrhage in patients of Asian descent with non-Asian patients. All MMD statistics were adjusted by age, sex, race (white or nonwhite), LMC, the presence of infarct or hemorrhage, P1 and AchoA classification, and PcomA/ICA ratio.
Results
We examined angiograms from 39 subjects with MMD (78 cerebral hemispheres). The mean age was 43.5 ± 10.6 years (range, 26–64 years). The median age was 44 years. Twenty-eight patients were women. Age- and sex-matched controls (n = 33; mean age, 44.3 ± 12.0 years) were included. Subjects with MMD were white (n = 28), African American (n = 7), and Asian (n = 4); control subjects were white (n = 27), African American (n = 5), and Hispanic (n = 1).
Fourteen hemispheres (9 left, 5 right) were excluded from measurement of the PcomA/ICA ratio secondary to lack of ipsilateral PcomA (n = 2), lack of DSA lateral projection (n = 1), prior aneurysm coiling (n = 1), and ipsilateral ICA occlusion (n = 10). All hemispheres excluded due to ICA occlusion had mSS grades of IV. Six subjects with MMD (15%) had unilateral involvement. Collateral characteristics by mSS are summarized in Table 2. Interobserver agreement for mSS rating meets acceptable statistical criteria with a Fleiss-Cohen κ statistic of 0.845 (95% CI, 0.785–0.904).
Table 2:
Collateral and clinical characteristics by modified Suzuki score in subjects with Moyamoya diseasea
| Metric (P Value) | N | 0 (n = 6) | I (n = 7) | II (n = 30) | III (n = 20) | IV (n = 15) |
|---|---|---|---|---|---|---|
| PcomA/ICAb (.024) | 64 | 0.125 | 0.205 | 0.295 | 0.330 | 0.520 |
| LMC (.001) | 78 | |||||
| 1 | 17% (1) | 29% (2) | 63% (19) | 80% (16) | 93% (14) | |
| 2 | 83% (5) | 71% (5) | 37% (11) | 20% (4) | 7% (1) | |
| CT/MR (.06)c | 78 | |||||
| 1 | 83% (5) | 57% (4) | 37% (11) | 15% (3) | 33% (5) | |
| 2 | 17% (1) | 42% (3) | 60% (18) | 70% (14) | 67% (10) | |
| 3 | 0% (0) | 0% (0) | 3% (1) | 15% (3) | 0% (0) | |
| AchoA (<.001) | 78 | |||||
| 0 | 83% (5) | 43% (3) | 13% (4) | 5% (1) | 7% (1) | |
| 1 | 17% (1) | 43% (3) | 37% (11) | 10% (2) | 0% (0) | |
| 2 | 0% (0) | 14% (1) | 50% (15) | 85% (17) | 33% (5) | |
| NV | 0% (0) | 0% (0) | 0% (0) | 0% (0) | 60% (9) | |
| P1 (.14) | 78 | |||||
| 1 | 100% (6) | 86% (6) | 90% (27) | 95% (19) | 67% (10) | |
| 2 | 0% (0) | 14% (1) | 10% (3) | 5% (1) | 33% (5) |
Note:—N indicates the number of nonmissing values; NV, not visualized.
Numbers after percentages are frequencies.
PcomA/ICA ratio provided is median.
CT/MR 1—no infarct or hemorrhage, 2—infarct, 3—hemorrhage.
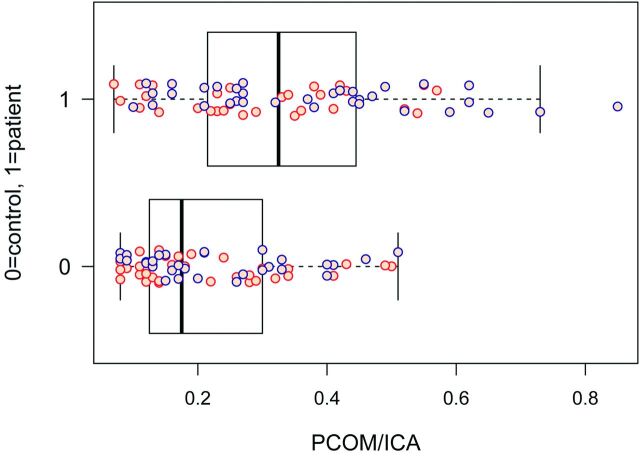
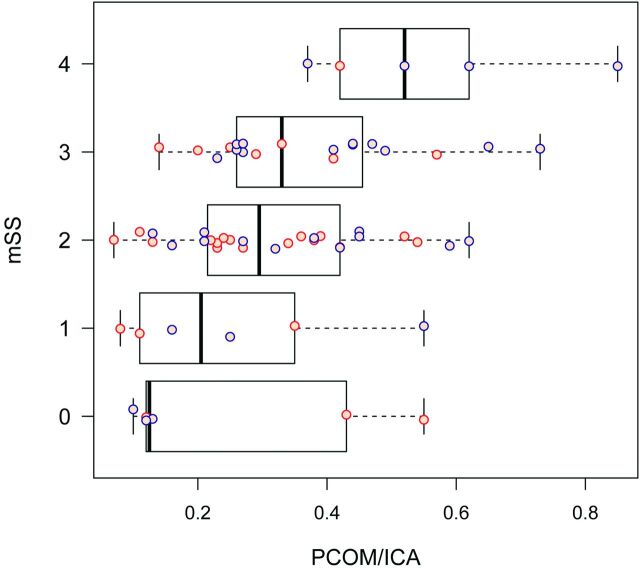
Figure 3 demonstrates PcomA/ICA ratios for subjects compared with controls. The mean PcomA/ICA ratio for subjects was 0.34, compared with 0.22 for controls. After we adjusted for age, sex, race, and LMC, a linear mixed-effects model estimate mean PcomA/ICA ratio difference between subjects and controls was significant at .115 (P = .0002, 95% CI, 0.058–0.172). PcomA/ICA ratios for subjects increased with increasing mSS (Fig 4). The multivariate regression model for correlated ordinal responses showed that for every 0.1-U increase in the PcomA/ICA ratio, the OR of having a more severe mSS classification (eg, mSS of II increases to mSS of III) was 1.61 (P = .004; 95% CI, 1.17–2.21).
Fig 3.
PcomA/ICA ratio in subjects with MMD versus control subjects by hemisphere. The PcomA/ICA ratio in a patient with MMD (n = 1) was significantly higher (P < .001) compared with control subjects (n = 0). Orange dots are observations for the left cerebral hemisphere, and blue dots are for the right cerebral hemisphere. The dark band represents the median in this boxplot.
Fig 4.
PcomA/ICA ratio in patients with MMD by mSS and hemisphere. The PcomA/ICA ratio increased with increasing mSS (P = .024). Orange dots are observations for the left cerebral hemisphere, and blue dots are for the right cerebral hemisphere.
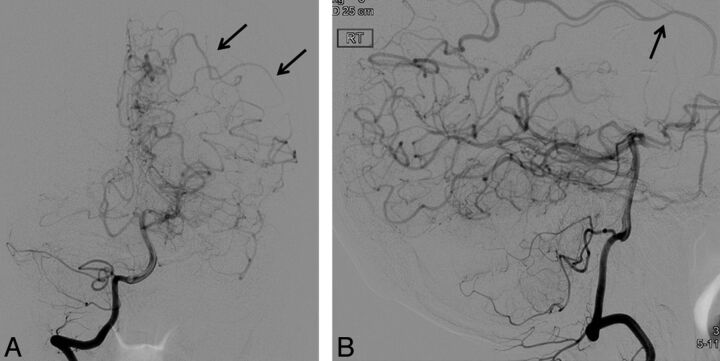
The regression model also demonstrated a significant association between mSS and the presence of LMC (P = .008) for subjects. The OR of having a more severe mSS classification was 4.79 times higher (95% CI, 1.51–15.21) for MMD hemispheres with LMC, compared with those without LMC. Only 2 of 66 hemispheres in control subjects had LMC (1 with a history of seizures and 1 with previously coiled aneurysms, but neither with vascular stenosis). Figure 5 demonstrates the appearance of LMC in 1 subject with MMD. All hemispheres with P1 steno-occlusive involvement had LMC. However, P1 steno-occlusive change was not significantly associated with mSS (P = .485).
Fig 5.
Anteroposterior (A) and lateral (B) projections from DSA with right vertebral injection in patient with MMD demonstrate leptomeningeal cortical branches (arrows) from the PCA to the left parietal and temporal lobes.
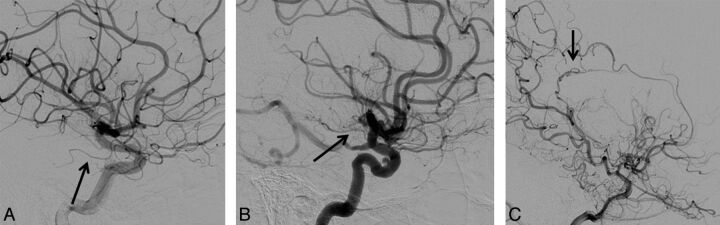
AchoA grades are shown in Fig 6. Table 3 shows the distribution of AchoA grades by imaging findings. There was a significant association between the AchoA classification and mSS (P = .02). The OR of having a more severe mSS classification was 2.76 times higher (95% CI, 0.57–13.24) for hemispheres with grade I AchoA versus control subjects (P = .21), and the OR increased to 17.2 (95% CI, 2.26–131.1) when comparing grade II AchoA with control subjects (P = .01). In 9 hemispheres, the AchoA was occluded due to ICA occlusion proximal to the AchoA origin and lack of collateral AchoA filling via posterior collaterals. All such hemispheres were mSS IV; none had hemorrhage and 5 of 9 had infarcts. All hemispheres with hemorrhage (4 of 78) had AchoA grade 2, and none had P1 steno-occlusive findings.
Fig 6.
Lateral projections from DSA in 3 patients with Moyamoya disease, with the AchoA identified by the arrow. A, The AchoA appears normal without proliferative vessels (stage zero). B, The AchoA is thickened with distal branching (stage I). C, The AchoA is dilated, and abnormal branches serve as collaterals (stage II).
Table 3:
Distribution of anterior choroidal artery grades by imaging findingsa
| Symptom of Hemisphere | N | AchoA Grade |
||
|---|---|---|---|---|
| 0 (No.) | 1 (No.) | 2 (No.) | ||
| No symptom | 24 | 29% (7) | 33% (8) | 38% (9) |
| Infarct | 41 | 17% (7) | 22% (9) | 61% (25) |
| Hemorrhage | 4 | 0% (0) | 0% (0) | 100% (4) |
Note:—N indicates the number of nonmissing values.
P = .18.
There was no statistically significant association between mSS and imaging findings of infarct or hemorrhage (P = .11). Forty-six of 78 MMD hemispheres (59%) had infarcts. Of 15 mSS hemispheres, 5—including the only mSS IV hemisphere without LMC—had no infarcts, 2 had infarcts involving the ipsilateral basal ganglia, and all remaining mSS hemispheres had a watershed pattern of infarcts. No patient with mSS IV had posterior circulation or cortical MCA territory infarcts.
Two of 4 subjects with MMD with hemorrhage were of Asian descent. The Fisher exact test gave a 2-sided P = .045 for the correlation between the incidence of hemorrhage in patients of Asian descent with non-Asian patients, though findings were limited by the low number of hemispheres with hemorrhage. Only 1/78 MMD hemispheres had both hemorrhage and infarct on imaging. This hemisphere had no P1 steno-occlusive changes or LMC and had grade 2 AchoA changes.
Median follow-up time for subjects with angiography (19 of 39 subjects with MMD) was 463 days (minimum, 105 days; maximum, 1740 days).
Discussion
The primary findings of this work are the following: 1) As with prior studies on adult MMD, we observed a strong correlation between vascular collaterals, including the presence of LMC, dilated AchoAs, and larger PcomA/ICA ratios, and mSS in adult MMD. Despite these collateral networks, most subjects with MMD in our study had infarcts, and the presence of infarct and/or hemorrhage did not correlate with disease severity, as reflected by the mSS, due to the high frequency of infarcts in subjects with MMD with less severe mSSs (eg, 60% of mSSs II subjects). 2). In contrast to Asian studies, AchoA changes were sensitive but not specific for intracranial hemorrhage, and P1 steno-occlusive involvement did not appear to correlate with disease severity. Taken together, these findings suggest that mSS may not provide a comprehensive predictive model for the complex hemodynamic stress, which causes infarcts and hemorrhages in subjects with MMD. Additionally, as more nuanced predictive models arise, such as AchoA changes, our findings suggest the models may not generalize across ethnic cohorts.
The ischemic stress in MMD is imparted by progressive anterior circulation stenosis, which may include vascular constrictive changes, which narrows the terminal ICA and, in patients with mSS III–IV, occludes both the anterior and middle cerebral arteries.21 When ICA stenosis occurs distal to the AchoA, both the AchoA and PcomA may be subjected to increased collateral flow and stress.14 The correlation of the PcomA:ICA ratio in our study with disease severity may reflect a compensation mechanism for the hemodynamic stress imposed by progressive ICA arteriopathy.25–27 PcomA collateral flow has been shown to be protective against watershed infarcts in patients with atherosclerotic ICA occlusion.28 Although ICA stenosis in MMD can occlude the PcomA, stenosis typically occurred distal to the PcomA origin in our cohort (87%). The high rate of mSS IV in subjects with MMD in our study with a watershed pattern of infarcts (85% of mSS IV hemispheres with infarcts) suggests inadequate flow despite increased collaterals.
As in other North American studies, our cohort was much more likely to present with ischemia (59%) than hemorrhage (5%).1,6–8,29,30 In contrast, Asian studies reported higher rates of hemorrhage, ranging from 25% to 62% (Japan, China, and Korea) compared with 10%–29% (Iowa and Hawaii).3,15,29,31,32 Hemorrhage has been shown to be the most significant factor affecting poor outcome in Asian cohorts, and ruptured thin-walled basal perforators have been cited as the source of high rates of hemorrhage.3,33 However, studies have not shown a correlation between the reduction in perforators following surgery and rates of hemorrhage.34,35 More recently, Asian studies have implicated abnormal branching of the AchoA and dilation of the PcomA as a hemorrhagic source, particularly in subjects with intraventricular hemorrhage.15,31,36,37
Grade 1 or 2 AchoA changes were found in 89% of hemispheres with hemorrhage compared with only 44% of ischemic hemispheres in Asian MMD.15 However, grade 1 or 2 AchoA changes were present in most (80%) subjects with MMD in our study and did not differentiate hemorrhage and infarct. Despite this high rate of AchoA changes, only 4 hemispheres presented with hemorrhage, compared with 27% in the Japanese cohort.15 These findings suggest that AchoA changes in North Americans may not correlate with increased risk of hemorrhage to the extent that has been found in Asian cohorts. The impact of AchoA occlusion on future hemorrhage and ischemia risk is not clear from our study due to the small sample size and relatively short median follow-up. Only 1 MMD hemisphere had both hemorrhage and infarct, which is in line with published studies, suggesting the etiology for infarct and hemorrhage may be distinct.3,22 The incidence of P1 steno-occlusive changes in our cohort (12%) was lower than that reported in Asian studies (20%–43%).20,38
It is unclear whether findings from intracranial atherosclerotic disease studies translate to MMD.27,39 In the Warfarin Aspirin Symptomatic Intracranial Disease trial, very few cases of severe stenosis with good collaterals resulted in stroke, suggesting a protective role for LMC.26 However, in moderately stenosed patients (50%–69% stenosis), the presence of LMC was associated with an increased risk of stroke.26 LMC have been shown to be independent predictors of an increased oxygen extraction fraction in patients with atherosclerotic ICA occlusion.40
In our study, LMC correlated significantly with disease severity. The hemodynamic importance of LMC to the anterior circulation in MMD has recently been suggested in a Japanese cohort with P1 disease, in which P1 lesions led to MCA territory infarcts much more frequently than posterior infarcts.22 P1 stenosis in patients with MMD following revascularization was associated with decreased LMC, which may increase ischemic symptoms.28,38,41 P1 disease in our cohort did not correlate with disease severity, though it was most frequent in mSS IV hemispheres. Postrevascularization studies showing decreased steal phenomenon following revascularization suggest a dynamic component to MMD.9,13,42
Certainly, we could have used other published grading scales. Togao et al20 described a 4-stage system in their study, building a multivariate model for the angiographic findings in subjects with MMD, ranging from no PCA occlusive change in stage 1 to PCA occlusion with almost no visualization of distal branches in stage 4. Using this system, Togao et al found 32 stage 1 PCA arteries, 1 stage 2 artery, 5 stage 3 arteries, and 2 stage 4 arteries. Our study had an even larger percentage of subjects with MMD (87%) without PCA steno-occlusive disease. Because only 10 hemispheres had PCA steno-occlusive disease, subcategorization of PCA by stenosis degree was not thought to be statistically robust.
The findings of this work should be considered in the context of 4 limitations. First, as with most MMD studies, this study was limited by sample size (n = 39), owing to the relatively small prevalence of MMD in the general population (3 cases per 100,000).43 This led to a relatively small number of hemispheres with hemorrhage. However, this is one of the larger studies of North American MMD; therefore, we believe the results presented should be used as an exemplar for motivating larger studies. Second, constraints are imposed by the retrospective design, which precluded direct PcomA luminal diameter measurement due to differences in magnification on DSA. The PcomA/ICA ratio was, therefore, used as a surrogate. The distal ICA diameter has been shown to decrease in patients with MMD, either due to vascular constrictive changes or as a flow-related phenomenon due to more distal disease; thus, the precavernous ICA was measured.21 Third, our controls included subjects with intracranial pathology, including aneurysms and vascular stenosis, a limitation imposed by the standard for use of DSA at our institution, where DSA is reserved for subjects with suspected intracranial pathology, due to its invasive nature. Finally, our study represents a static view of what is known to be a dynamic disease. The 20 subjects with MMD without follow-up data, and the relatively short follow-up for several other subjects with MMD preclude drawing conclusions on whether the variables measured have prognostic implications. Longitudinal studies of these collateral pathways are necessary to understand the interplay between ischemic and hemorrhage risks.9,13,42
Conclusions
MMD in North Americans remains poorly characterized: The etiology is unknown; the natural history, unclear; and optimal treatment, untested. The varied clinical course for MMD may reflect the effectiveness of collaterals in compensating for the hemodynamic stress imposed by progressive ICA arteriopathy without imparting increased risk of hemorrhage. In our analysis, the presence of LMC, dilation of the AchoA, and the PcomA/ICA ratio were independent predictors of mSS severity for North Americans with MMD. However, AchoA changes did not correlate with increased risk of hemorrhage to the extent that has been found in Asian cohorts.
ABBREVIATIONS:
- AchoA
anterior choroidal artery
- LMC
leptomeningeal collaterals
- MMD
Moyamoya disease
- mSS
modified Suzuki score
- P1
proximal segment of the posterior cerebral artery
- PCA
posterior cerebral artery
- PcomA
posterior communicating artery
Footnotes
Disclosures: Megan K. Strother—RELATED: Grant: National Institute of Neurological Disorders and Stroke (5R01NS078828–02).* Morgan D. Anderson—RELATED: Grant: National Institute of Neurological Disorders and Stroke (5R01NS078828–02).* Yu Shyr—UNRELATED: Consultancy: GlaxoSmithKline, Aduro BioTech, Immunogen, Grants/Grants Pending: National Institutes of Health.* Travis R. Ladner—UNRELATED: Grants/Grants Pending: American Association of Neurological Surgeons Medical Student Summer Research Fellowship Program, Comments: The American Association of Neurological Surgeons through the Neurosurgery Research and Education Foundation is offering the American Association of Neurological Surgeons Medical Student Summer Research Fellowship program. Twenty Fellowships in the amount of $2500 will be awarded to medical students in the United States or Canada who have completed 1 or 2 years of medical school and wish to spend a summer working in a neurosurgical laboratory, mentored by a neurosurgical investigator who is a member of the American Association of Neurological Surgeons and will sponsor the student. Manus J. Donahue—RELATED: Grant: National Institutes of Health/National Institute of Neurological Disorders and Stroke (5 R01 NS078828 02),* UNRELATED: Consultancy: McLean Hospital (Boston, Massachusetts), Comments: I am a paid consultant on 2 National Institutes of Health–funded grants to McLean Hospital. *Money paid to the institution.
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (5R01NS078828–02) and the American Association of Neurological Surgeons Medical Student Summer Research Fellowship Program.
References
- 1. Khan N, Achrol AS, Guzman R, et al. Sex differences in clinical presentation and treatment outcomes in moyamoya disease. Neurosurgery 2012;71:587–93, discussion 593 [DOI] [PubMed] [Google Scholar]
- 2. Bao XY, Duan L, Li DS, et al. Clinical features, surgical treatment and long-term outcome in adult patients with moyamoya disease in China. Cerebrovasc Dis 2012;34:305–13 [DOI] [PubMed] [Google Scholar]
- 3. Han DH, Kwon OK, Byun BJ, et al. , for the Korean Society for Cerebrovascular Disease. A co-operative study: clinical characteristics of 334 Korean patients with moyamoya disease treated at neurosurgical institutes (1976–1994): the Korean Society for Cerebrovascular Disease. Acta Neurochir (Wien) 2000;142:1263–74, discussion 1273–74 [DOI] [PubMed] [Google Scholar]
- 4. Duan L, Bao XY, Yang WZ, et al. Moyamoya disease in China: its clinical features and outcomes. Stroke 2012;43:56–60 [DOI] [PubMed] [Google Scholar]
- 5. Zipfel GJ, Sagar J, Miller JP, et al. Cerebral hemodynamics as a predictor of stroke in adult patients with moyamoya disease: a prospective observational study. Neurosurg Focus 2009;26:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiu D, Shedden P, Bratina P, et al. Clinical features of moyamoya disease in the U.S. Stroke 1998;29:1347–51 [DOI] [PubMed] [Google Scholar]
- 7. Lee DJ, Liebeskind DS. Characterization of inpatient moyamoya in the U.S.: 1988–2004. Front Neurol 2011;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kainth D, Chaudhry SA, Kainth H, et al. Epidemiological and clinical features of moyamoya disease in the USA. Neuroepidemiology 2013;40:282–87 [DOI] [PubMed] [Google Scholar]
- 9. Hallemeier CL, Rich KM, Grubb RL, et al. Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke 2006;37:1490–96 [DOI] [PubMed] [Google Scholar]
- 10. Krischek B, Kasuya H, Khan N, et al. Genetic and clinical characteristics of moyamoya disease in Europeans. Acta Neurochir Suppl 2011;112:31–34 [DOI] [PubMed] [Google Scholar]
- 11. Suzuki J, Kodama N. Moyamoya disease: a review. Stroke 1983;14:104–09 [DOI] [PubMed] [Google Scholar]
- 12. Kuroda S, Hashimoto N, Yoshimoto T, et al. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke 2007;38:1430–35 [DOI] [PubMed] [Google Scholar]
- 13. Nariai T, Matsushima Y, Imae S, et al. Severe haemodynamic stress in selected subtypes of patients with moyamoya disease: a positron emission tomography study. J Neurol Neurosurg Psychiatry 2005;76:663–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009;360:1226–37 [DOI] [PubMed] [Google Scholar]
- 15. Morioka M, Hamada JI, Kawano T, et al. Angiographic dilatation and branch extension of the anterior choroidal and posterior communicating arteries are predictors of hemorrhage in adult moyamoya patients. Stroke 2003;34:90–95 [DOI] [PubMed] [Google Scholar]
- 16. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288–99 [DOI] [PubMed] [Google Scholar]
- 17. Mugikura S, Takahashi S, Higano S, et al. Predominant involvement of ipsilateral anterior and posterior circulations in moyamoya disease. Stroke 2002;33:1497–500 [DOI] [PubMed] [Google Scholar]
- 18. Donahue MJ, Ayad M, Moore R, et al. Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease. J Magn Reson Imaging 2013;38:1129–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bokkers RP, van Laar PJ, van de Ven KC, et al. Arterial spin-labeling MR imaging measurements of timing parameters in patients with a carotid artery occlusion. AJNR Am J Neuroradiol 2008;29:1698–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Togao O, Mihara F, Yoshiura T, et al. Cerebral hemodynamics in moyamoya disease: correlation between perfusion-weighted MRI and cerebral angiography. AJNR Am J Neuroradiol 2006;27:391–97 [PMC free article] [PubMed] [Google Scholar]
- 21. Kaku Y, Morioka M, Ohmori Y, et al. Outer-diameter narrowing of the ICA and MCA in moyamoya disease detected on 3D constructive interference in steady-state MR image: is arterial constrictive remodeling a major pathogenesis? Acta Neurochir (Wien) 2012;154:2151–57 [DOI] [PubMed] [Google Scholar]
- 22. Hishikawa T, Tokunaga K, Sugiu K, et al. Clinical and radiographic features of moyamoya disease in patients with both cerebral ischemia and hemorrhage. Br J Neurosurg 2013;27:198–201 [DOI] [PubMed] [Google Scholar]
- 23. Touloumis A. multgee: GEE solver for Correlated Nomiinal or Ordianal Multinomial Responses, Version 1.3. http://cran.r-project.org/package=multgee
- 24. Czabanka M, Pena-Tapia P, Schubert GA, et al. Proposal for a new grading of moyamoya disease in adult patients. Cerebrovasc Dis 2011;32(1):41–50 [DOI] [PubMed] [Google Scholar]
- 25. Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011;69:963–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liebeskind DS, Cotsonis GA, Saver JL, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab 2011;31:1293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang AP, Liu HM, Lai DM, et al. Clinical significance of posterior circulation changes after revascularization in patients with moyamoya disease. Cerebrovasc Dis 2009;28:247–57 [DOI] [PubMed] [Google Scholar]
- 28. Hendrikse J, Hartkamp MJ, Hillen B, et al. Collateral ability of the circle of Willis in patients with unilateral ICA occlusion. Stroke 2001;32:2768–73 [DOI] [PubMed] [Google Scholar]
- 29. Kleinloog R, Regli L, Rinkel GJ, et al. Regional differences in incidence and patient characteristics of moyamoya disease: a systematic review. J Neurol Neurosurg Psychiatry 2012;83:531–36 [DOI] [PubMed] [Google Scholar]
- 30. Mesiwala AH, Sviri G, Fatemi N, et al. Long-term outcome of superficial temporal artery-MCA bypass for patients with moyamoya disease in the US. Neurosurg Focus 2008;24:E15. [DOI] [PubMed] [Google Scholar]
- 31. Liu W, Zhu S, Wang X, et al. Evaluation of angiographic changes of the anterior choroidal and posterior communicating arteries for predicting cerebrovascular lesions in adult moyamoya disease. J Clin Neurosci 2011;18:374–78 [DOI] [PubMed] [Google Scholar]
- 32. Wetjen NM, Garell PC, Stence NV, et al. Moyamoya disease in the Midwestern United States. Neurosurg Focus 1998;5:e1. [DOI] [PubMed] [Google Scholar]
- 33. Fukui M, Kono S, Sueishi K, et al. Moyamoya disease. Neuropathology 2000;20(suppl):S61–S64 [DOI] [PubMed] [Google Scholar]
- 34. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg 1997;99(suppl 2):S238–S240 [PubMed] [Google Scholar]
- 35. Okada Y, Shima T, Nishida M, et al. Effectiveness of superficial temporal artery-MCA in adult moyamoya disease: cerebral hemodynamics and clinical course in ischemic and hemorrhagic varieties. Stroke 1998;29:625–30 [DOI] [PubMed] [Google Scholar]
- 36. Nah HW, Kwon SU, Kang DW, et al. Moyamoya disease-related versus primary intracerebral hemorrhage. Stroke 2012;7:1947–50 [DOI] [PubMed] [Google Scholar]
- 37. Dolati P, Sutherland G, Wong J, et al. Distal anterior choroidal artery aneurysm following iatrogenic posterior cerebral artery occlusion: a case report and review of literature. Acta Neurochir (Wien) 2012;154:53–57 [DOI] [PubMed] [Google Scholar]
- 38. Yamada I, Himeno Y, Suzuki S, et al. Posterior circulation in moyamoya disease: angiographic study. Radiology 1995;197:239–46 [DOI] [PubMed] [Google Scholar]
- 39. Bang OY, Saver JL, Kim SJ, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 2011;42:2235–39 [DOI] [PubMed] [Google Scholar]
- 40. Yamauchi H, Kudoh T, Sugimoto K, et al. Patterns of collaterals, type of infarcts, and hemodynamic impairment in carotid artery occlusion. J Neurol Neurosurg Psychiatry 2004;75:1697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vajkoczy P. Moyamoya disease: collateralization is everything. Cerebrovasc Dis 2009;28:258. [DOI] [PubMed] [Google Scholar]
- 42. Lim SM, Chae EJ, Kim MY, et al. Steal phenomenon through the anterior communicating artery in moyamoya disease. Eur Radiol 2007;17:61–66 [DOI] [PubMed] [Google Scholar]
- 43. Wakai K, Tamakoshi A, Ikezaki K, et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neuro Neurosurg 1997;99(suppl 2):S1–S5 [DOI] [PubMed] [Google Scholar]