Abstract
Adenosine is a well-known endogenous modulator of neuronal excitability with anticonvulsant properties. Thus, the modulation exerted by adenosine might be an effective tool to control seizures. In this study, we investigated the effects of drugs that are able to modulate adenosinergic signaling on pentylenetetrazole (PTZ)-induced seizures in adult zebrafish. The adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) decreased the latency to the onset of the tonic-clonic seizure stage. The adenosine A1 receptor agonist cyclopentyladenosine (CPA) increased the latency to reach the tonic-clonic seizure stage. Both the adenosine A2A receptor agonist and antagonist, CGS 21680 and ZM 241385, respectively, did not promote changes in seizure parameters. Pretreatment with the ecto-5′nucleotidase inhibitor adenosine 5′-(α,β-methylene) diphosphate (AMPCP) decreased the latency to the onset of the tonic-clonic seizure stage. However, when pretreated with the adenosine deaminase (ADA) inhibitor, erythro-9-(2-hydroxy-3-nonyl)-adenine (EHNA), or with the nucleoside transporter (NT) inhibitors, dipyridamole and S-(4-Nitrobenzyl)-6-thioinosine (NBTI), animals showed longer latency to reach the tonic-clonic seizure status. Finally, our molecular analysis of the c-fos gene expression corroborates these behavioral results. Our findings indicate that the activation of adenosine A1 receptors is an important mechanism to control the development of seizures in zebrafish. Furthermore, the actions of ecto-5′-nucleotidase, ADA, and NTs are directly involved in the control of extracellular adenosine levels and have an important role in the development of seizure episodes in zebrafish.
Introduction
Epilepsy is a common neurological disorder characterized by the occurrence of recurrent and unpredictable seizures.1 In many cases, conventional antiepileptic drugs (AEDs) are not able to provide satisfying control of seizures.2 Adenosine is a well-known endogenous modulator of neuronal excitability and provides anticonvulsant effects.3 Therefore, adenosine modulation might be an effective tool to control epileptic seizures in patients resistant to conventional AEDs.4–6
Adenosine is a purine nucleoside that can be produced by extracellular nucleotide hydrolysis or be cell released through NTs.3 Once released in the extracellular space, ATP is rapidly dephosphorylated into adenosine by ectonucleotidases, an enzyme cascade system that catalyzes the successive hydrolysis of purine and pyrimidine nucleoside tri-, di-, and monophosphates to their respective nucleosides. Nucleoside triphosphates and diphosphates may be hydrolyzed by the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family members, whereas nucleoside monophosphates are hydrolyzed by ecto-5′-nucleotidase, generating adenosine.7 Adenosine is metabolized by two possible pathways: deamination to inosine through adenosine deaminase (ADA) and phosphorylation to AMP through adenosine kinase.8 Finally, adenosine can be cell released through nucleoside transporters (NTs) in bidirectional equilibrative processes driven by chemical gradients and unidirectional concentrative processes driven by sodium electrochemical gradients.9,10
Adenosine exerts its effects through the activation of the specific G-protein-coupled receptors: A1, A2A, A2B, and A3 receptors. The A1 and A2A receptors are the most sensitive to adenosine and the most abundant in the central nervous system.11 During a seizure, extracellular adenosine levels rapidly rise and are believed to play an important role in the arrest and termination of the seizure through binding to the inhibitory adenosine A1 receptor.6,12 Nevertheless, adenosine levels rise significantly during epileptic seizures, which could activate all adenosine receptors, including the A2A receptors, which facilitate neuronal transmission.13,14
Zebrafish is a small freshwater teleost that has been used as a model organism to study epilepsy. Pentylenetetrazole (PTZ)-induced seizures in zebrafish larvae15,16 and adults17,18 cause the behavioral and electrographic alterations that would be expected from a seizure episode. Furthermore, previous studies have demonstrated the adenosine receptor subtypes, A1, A2A.1, A2A.2, and A2B, in zebrafish.19,20
Taking into account that adenosine is able to control neuronal excitability and that its signaling is regulated by nucleotide and nucleoside-metabolizing enzymes and adenosine transporters, the modulation of these mechanisms may represent an important target in epilepsy therapies. Considering that zebrafish is an effective model widely used in epilepsy studies, the investigation of the adenosine signaling in zebrafish may improve our knowledge on the role of adenosine in epilepsy. Therefore, the aim of this study was to verify the role of adenosine on seizure episodes induced by PTZ in zebrafish.
Materials and Methods
Animals
Adult (6–8 months old) wild-type zebrafish (Danio rerio) with Tübingen background were obtained from a local commercial supplier (Red Fish) and acclimated for 2 weeks before the experiments. The animals were housed in a 50-L thermostated aquarium filled with unchlorinated water constantly aerated at a targeted temperature of 26°C±2°C. Fish were kept under a 14-h light/10-h dark cycle photoperiod and fed twice a day with commercial flake fish food supplemented with live brine shrimp. The use and maintenance of zebrafish were done in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health, and the experiments were designed to minimize discomfort or suffering to the animals, as well as the number of animals used in the experiments. The protocol was approved by the Ethics Committee of the Pontificia Universidade Católica do Rio Grande do Sul (PUCRS) under the number 11/00255-CEUA.
Materials
The drugs erythro-9-(2-hydroxy-3-nonyl)-adenine (EHNA), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), cyclopentyladenosine (CPA), dipyridamole, S-(4-Nitrobenzyl)-6-thioinosine (NBTI), CGS 21680 hydrochloride hydrate, α, β-methylene adenosine 5′-diphosphate (AMPCP), pentylenetetrazole (PTZ), and tricaine were purchased from Sigma. ZM 241385 was purchased from Tocris Bioscience.
Drug pretreatments
This study investigated the effects of different drugs that are able to modulate adenosine signaling on PTZ-induced seizures in zebrafish. All drugs were injected intraperitoneally (i.p.) 30 min before the PTZ exposure. Each drug was tested in three different doses. The doses for each drug tested are indicated in Table 1 and were chosen based on previous studies conducted in rodents.21–23 The AMPCP (diluted in saline) and NBTI (diluted in 3% DMSO) doses were selected based on preliminary studies conducted in our laboratory. Intraperitoneal injections were conducted using a 3/10-mL U-100 BD Ultra-Fine™ Short Insulin Syringe 8 mm (5/16 inch)×31G Short Needle (Becton Dickinson and Company) as previously described.24 Anesthesia of the animals before the injection was obtained by its immersion in a 100 mg/L tricaine solution until the animal showed lack of motor coordination and reduced respiration rate. After the injection, the animals were placed in a separate tank with highly aerated unchlorinated tap water (26°C±2°C) to facilitate their recovery from anesthesia.
Table 1.
Pretreatments with Adenosinergic Drugs on Pentylenetetrazole-Induced Seizures in Zebrafish
Locomotor activity
After recovery from anesthesia, the animals were individually placed in glass tanks (12×8×13.5 cm, length×width×height) to analyze locomotor activity. The animals' behavior was registered by a video camera for 30 min and further analyzed using the ANY-Maze recording software (Stoelting Co.) to track the distance traveled by the animals.
PTZ-induced seizures
To induce seizures, zebrafish were individually exposed to 7.5 mM PTZ in a 250-mL beaker. The PTZ treatment was selected based on preliminary studies conducted in our laboratory. All PTZ treatments were videotaped and evaluated later by trained observers. The seizure-like behavior was classified according to each stage: stage I—dramatically increased swimming activity, stage II—whirlpool swimming behavior, and stage III—clonus-like seizures, followed by loss of posture when the animal falls to one side and remains immobile for 1–3 s, as previously reported for zebrafish.15,17 The animals were submitted to the PTZ treatment until they reached stage III. The latencies to the first episode of seizure activity in stages I, II, and III were analyzed for each animal during PTZ exposure.
Gene expression analysis by quantitative real time reverse transcription-polymerase chain reaction
The immediate early gene c-fos is rapidly and transiently upregulated in response to physiological stimuli and has been used as a marker for neuronal activity and seizure occurrence.25 To obtain detailed results about seizure manifestation in zebrafish, we analyzed the regulation of c-fos expression in the following experimental groups: vehicle (animals that were treated with vehicle and were not exposed to PTZ), vehicle+PTZ (animals that were exposed to PTZ without receiving pretreatment), and drugs+PTZ (animals that were pretreated with the different compounds studied and exposed to PTZ). All animals exposed to PTZ were tested at seizure stage III. Animals in the vehicle groups were individually placed in 250-mL beakers filled with unchlorinated water for 5 min following the saline or DMSO 3% injections.
The gene expression of c-fos was analyzed by quantitative real-time reverse transcription-polymerase chain reaction (RT-qPCR). Total RNA was isolated with Trizol® reagent (Invitrogen) in accordance with the manufacturer's instructions. The total RNA was quantified by spectrophotometry and then treated with Deoxyribonuclease I (Invitrogen) to eliminate genomic DNA contamination, in accordance with the manufacturer's instructions. The cDNA was synthesized with the ImProm-II™ Reverse Transcription System (Promega) from 1 μg of total RNA, following the manufacturer's instructions. Quantitative PCR was performed using SYBR® Green I (Invitrogen) to detect double-strand cDNA synthesis. Reactions were done in a volume of 25 μL using 12.5 μL of diluted cDNA, containing a final concentration of 0.2×SYBR Green I (Invitrogen), 100 μM dNTP, 1×PCR Buffer, 3 mM MgCl2, 0.25 U Platinum® Taq DNA Polymerase (Invitrogen), and 200 nM each of Rpl13α and c-fos reverse and forward primers (Table 2).26 The PCR cycling conditions were an initial polymerase activation step for 5 min at 95°C, 40 cycles of 15 s at 95°C for denaturation, 35 s at 60°C for annealing, and 15 s at 72°C for elongation. At the end of the cycling protocol, a melting curve analysis was included and fluorescence measured from 60°C to 99°C and it showed a single peak in all cases. Rpl13α was used as a reference gene for normalization. Relative expression levels were determined with 7500 and 7500 Fast Real-Time PCR Systems Software v.2.0.6 (Applied Biosystems). The efficiency per sample was calculated using LinRegPCR version 2012.3 Software (http://LinRegPCR.nl). Relative mRNA expression levels were determined using the 2−ΔΔCT method.
Table 2.
Primer Sequences for Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction Experiments
| Gene | Primer sequences (5′-3′) | Accession number (mRNA) | Amplicon size (bp) |
|---|---|---|---|
| Rpl13αa | F-TCTGGAGGACTGTAAGAGGTATGC | NM_212784 | 147 |
| R-AGACGCACAATCTTGAGAGCAG | |||
| c-Fosb | F-GCTCCTGGCTAAAGCGGAGCTG | NM_205569.1 | 171 |
| R-GACGTGTAGGTGGTGCAGGCTGG |
According to aTang et al.,26bdesign by authors.
Statistical analysis
The results are expressed as mean±SD. The number of animals (n) is provided in the figures. Data on locomotor activity and seizure latency were analyzed by one-way ANOVA followed by Dunnett's post hoc test. The relative mRNA expression data were analyzed by one-way ANOVA followed by Duncan's post hoc test. A p-value <0.05 was considered as a significant difference.
Results
Locomotor activity
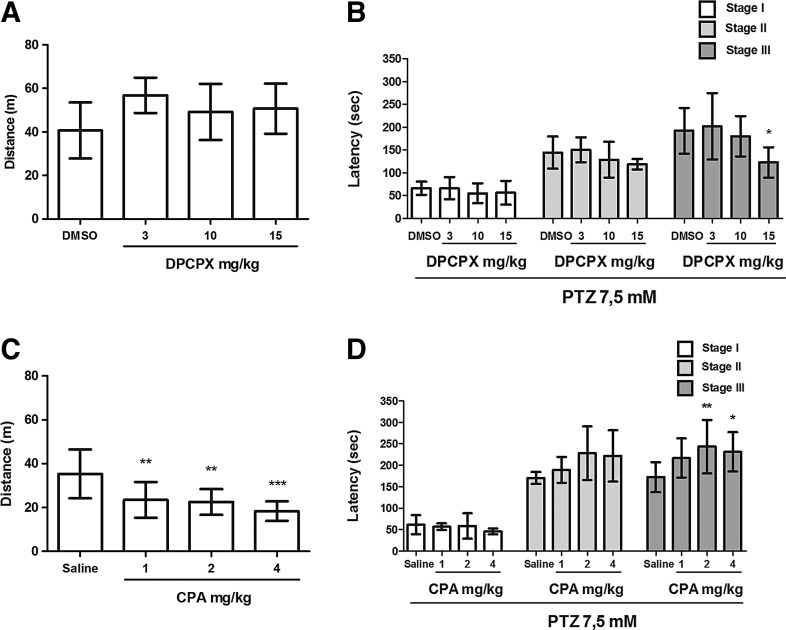
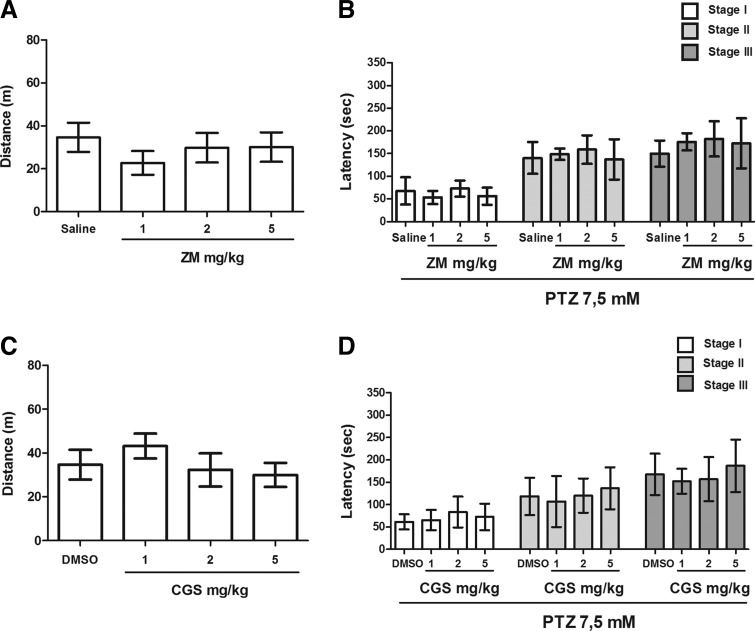
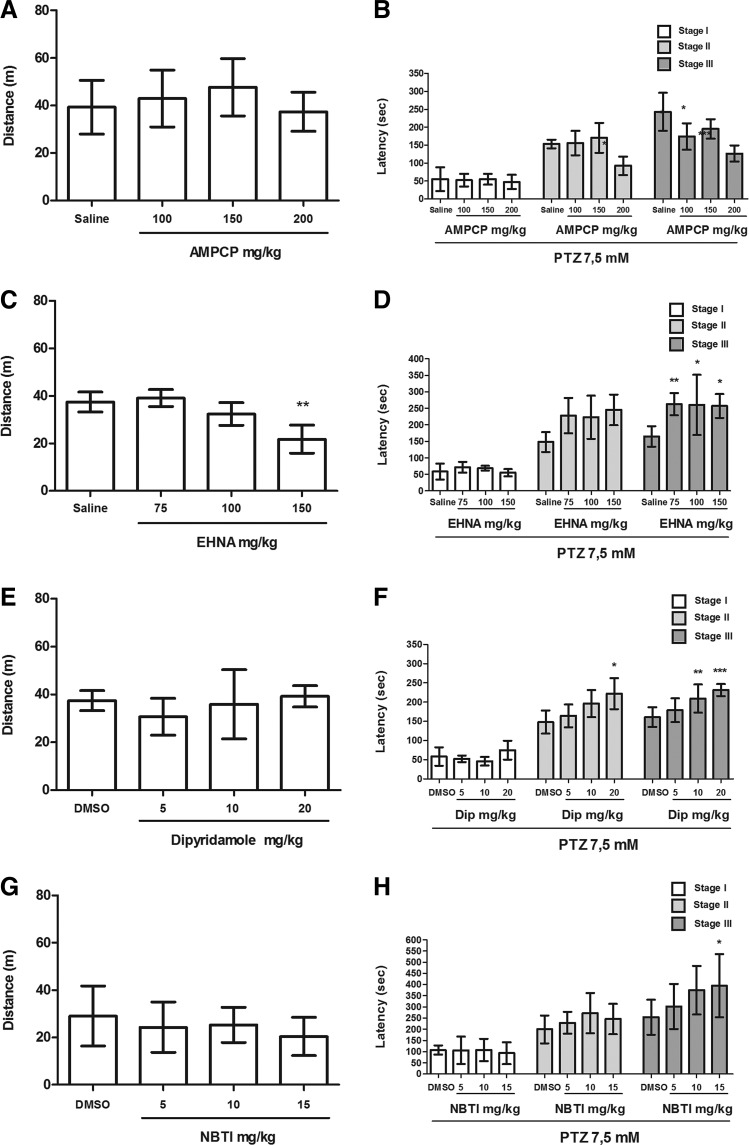
DPCPX, CGS 21680, ZM 241385, AMPCP, dipyridamole, and NBTI did not alter locomotor activity in zebrafish (Figs. 1–3). However, the results have shown that CPA was able to reduce locomotor activity of zebrafish in all tested doses (1, 2, 4 mg/kg) [Fig. 1C; F(3,47)=8.021]. In addition, the highest dose of EHNA (150 mg/kg) significantly reduced the distance traveled by animals during the 30-min drug pretreatment [Fig. 3C; F(4,27)=13.99].
FIG. 1.
Effect of DPCPX (3, 10, 15 mg/kg) and CPA (1, 2, 4 mg/kg) on locomotor activity and progression of the pentylenetetrazole (PTZ)-induced seizures in zebrafish. (A, C) Locomotor activity was measured as distance traveled during the 30 min following drug pretreatments. (B, D) Latency to the first behavioral manifestation of each seizure stage (I, II, and III) during PTZ exposure. The data are expressed as the mean±SD from 8 and 12 animals for DPCPX and CPA groups, respectively, and were analyzed by one-way ANOVA followed by Dunnett's post hoc test. The symbols represent statistical difference when compared with the respective vehicle control group (DMSO 3%). *p<0.05, **p<0.005, ***p<0.0005.
FIG. 2.
Effect of ZM 241385 (1, 2, 5 mg/kg) and CGS 21680 (1, 2, 5 mg/kg) on locomotor activity and progression of the PTZ-induced seizures in zebrafish. (A, C) Locomotor activity was measured as distance traveled during the 30 min following drug pretreatments. (B, D) Latency to the first behavioral manifestation of each seizure stage (I, II, and III) during PTZ exposure. The data are expressed as the mean±SD from 8 and 12 animals for ZM 241385 and CGS 21680 groups, respectively, and were analyzed by one-way ANOVA followed by Dunnett's post hoc test.
FIG. 3.
Effect of AMPCP (100, 150, 200 mg/kg), EHNA (75, 100, 150 mg/kg), dipyridamole (5, 10, 20 mg/kg), and NBTI (5, 10, 15 mg/kg) on locomotor activity and progression of the PTZ-induced seizures in zebrafish. (A, C, E, G) Locomotor activity was measured as distance traveled during the 30 min following drug pretreatments. (B, D, F, H) Latency to the first behavioral manifestation of each seizure stage (I, II, and III) during PTZ exposure. The data are expressed as the mean±SD from 12, 8, 8, and 12 animals for AMPCP, EHNA, dipyridamole, and NBTI, respectively, and were analyzed by one-way ANOVA followed by Dunnett's post hoc test. The symbols represent statistical difference when compared with the respective vehicle control group (DMSO 3% and Saline). *p<0.05, **p<0.005, ***p<0.0005.
Behavioral seizure parameters
Behavioral seizure parameters were evaluated in zebrafish exposed to PTZ. To investigate seizure development, we analyzed the latencies to the first behavior signal characterizing each seizure stage (I, II, and III). All animals showed progressive behavioral alterations until they reached the most severe seizure stage, stage III, which corresponds to tonic-clonic seizure in zebrafish.15
Effects of adenosine A1 and A2A agonist and antagonist receptors on PTZ-induced seizures
The selective adenosine A1 receptor antagonist, DPCPX, aggravated the behavioral response to seizures. The highest DPCPX pretreatment dose (15 mg/kg) induced faster stage III onset [Fig. 1B, F(3,31)=4.13, p<0.05]. The animals exposed to PTZ without DPCPX pretreatment reached stage III at 191.7±50.26 s, whereas animals pretreated with 3, 10, and 15 mg/kg DPCPX reached stage III at 201.8±72.86, 180.0±44.09, and 122.2±33.53 s, respectively.
The selective adenosine A1 receptor agonist, CPA, provided significant protection against PTZ-induced seizures. The latency to the tonic-clonic seizure stage was prolonged in animals pretreated with CPA [Fig. 1D, F(3,48)=4.37, p<0.05]. The animals exposed to PTZ without CPA pretreatment reached stage III at 172.1±34.86 s, whereas animals pretreated with 1, 2, and 4 mg/kg CPA reached stage III at 216.9±45.56, 243.3±62.66, and 231.1±45.78 s, respectively.
Both the adenosine A2A receptor antagonist (ZM 241385) and agonist (CGS 21680) were not able to change behavioral seizure responses (Fig. 2). Stage III was observed at 149.8±28.78, 175.7±19.01, 182.3±36.68, and 172.6±55.43 s in saline, 1, 2, and 5 mg/kg ZM 241385, respectively (Fig. 2B). Similarly, the A2A selective agonist, CGS21680, was not able to change the behavioral seizure response (Fig. 2D). Stage III was observed at 167.3±46.28 (saline), 152.0±28.0 (1 mg/kg CGS 21680), 156.9±49.7 (2 mg/kg CGS 21680), and 186.7±58.64 s (5 mg/kg CGS 21680).
Effect of modulation of extracellular adenosine levels on PTZ-induced seizures
The inhibition of nucleotide hydrolysis by the ecto-5′-nucleotidase inhibitor, AMPCP, exacerbated the PTZ-induced seizure response in zebrafish. The latency to the tonic-clonic seizure stage was faster in animals pretreated with all doses of AMPCP [Fig. 3B, F(3,46)=12.26, p<0.05]. The animals pretreated with AMPCP reached stage III at 174.3±36.99 (100 mg/kg), 195.5±27.01 (150 mg/kg), and 126.8±22.56 s (200 mg/kg), whereas the saline group reached stage III at 242.8±53.25 s. The highest AMPCP dose (200 mg/kg) also reduced the latency to stage II [Fig. 3B, F(3,28)=7.25, p<0.05]. Stage II was observed at 153.6±11.93 (saline), 156.0±33.87 (100 mg/kg), 170.4±41.71 (150 mg/kg), and 92.5±25.88 (200 mg/kg).
The inhibition of ADA by the ADA inhibitor, EHNA, provided significantly increased tonic-clonic seizure onset [Fig. 3D, F(3,42)=6.48, p<0.05]. The animals pretreated with EHNA reached stage III at 263.0±33.94 (75 mg/kg), 260.5±91.20 (100 mg/kg), and 257.33±36.07 s (150 mg/kg), whereas the animals without pretreatment reached stage III at 164.9±30.81 s.
Finally, the adenosine reuptake inhibition by the nonspecific NT inhibitor, dipyridamole, and by the inhibitor of equilibrative nucleoside transporters (ENTs), NBTI, provided significant protection against PTZ-induced seizures since animals pretreated with the highest doses of these drugs took longer to reach the tonic-clonic seizure stage. Animals pretreated with higher dipyridamole doses (10 mg/kg and 20 mg/kg) took longer to reach the tonic-clonic seizure stage [Fig. 3F, F(3,32)=10.03, p<0.05]. The animals pretreated with dipyridamole reached stage III at 178.8±30.8 (5 mg/kg), 209.2±36.72 (10 mg/kg), and 231.3±15.74 s (20 mg/kg), whereas the saline group reached stage III at 160.9±25.81 s. Animals pretreated with 20 mg/kg dipyridamole also took longer to reach stage II [Fig. 3F, F(3,27)=5.20, p<0.05]. Stage II was observed at 148.0±30.05, 164.3±29.52, 196.1±35.33, and 222.0±40.44 s in saline, 5, 10, and 20 mg/kg groups, respectively. In a similar manner, animals pretreated with the highest dose of NBTI (15 mg/kg) reached stage III at 395.167±141.3 (15 mg/kg), 375.3±108.5 (10 mg/kg), and 302.2±100.6 (5 mg/kg), whereas the control group reached stage III at 254.5±78.06 [Fig. 3H, F (3, 46)=3.07, p<0.05].
Effects of adenosine signaling modulation on seizure-induced changes in c-fos gene expression
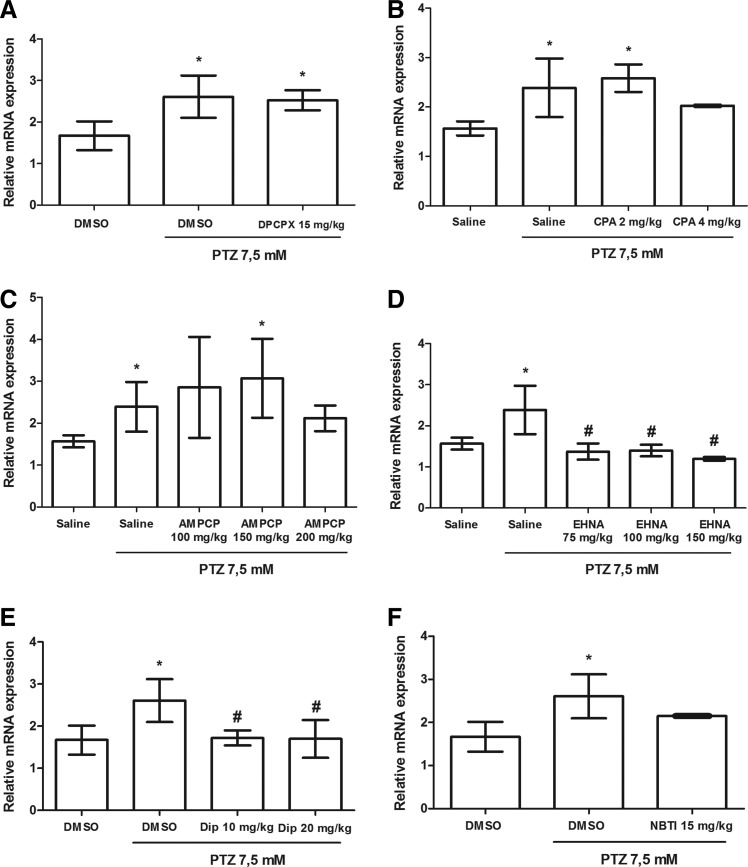
To investigate whether PTZ-induced seizures alter the c-fos gene expression, we analyzed its relative mRNA expression levels. The results have shown that c-fos expression was upregulated at stage III of seizure (Fig. 4A–F). Animals that received only vehicle treatment and were not exposed to PTZ presented relative mRNA expression of 1.53 (saline) and 1.61 (DMSO 3%), whereas animals that were exposed to PTZ following vehicle treatment presented relative mRNA expression of 2.37 (Saline+PTZ) and 2.65 (DMSO 3%+PTZ). In addition, the results have shown that EHNA [Fig. 4D; (F 4,18)=8.77] and dipyridamole [Fig. 4E; (F 3,12)=3.98] pretreatments were able to prevent the seizure-induced increase of c-fos expression. Animals pretreated with EHNA in all tested doses presented relative mRNA expression of 1.37 (75 mg/kg), 1.39 (100 mg/kg), and 1.19 (150 mg/kg). Animals pretreated with the highest doses of dipyridamole presented relative mRNA expression of 1.72 (10 mg/kg) and 1.69 (20 mg/kg). Despite having no significant effect, pretreatment with CPA and NBTI presents an apparent tendency to establish normal levels of the c-fos gene expression in response to PTZ-induced seizures. Nevertheless, DPCPX and AMPCP pretreatments were not able to modulate the c-fos alterations induced by seizures.
FIG. 4.
Effects of the studied compounds on gene expression of c-fos in zebrafish submitted to PTZ-induced seizures. (A–F) Relative mRNA expression at seizure stage III. The data are expressed as the mean±SD from four samples and were analyzed by one-way ANOVA followed by Duncan's post hoc test. The symbol *represents statistical difference when compared with the vehicle control group (animals that were treated with respective vehicle and were not exposed to PTZ). The symbol #represents statistical difference when compared with the vehicle+PTZ group (animals that were exposed to PTZ without receive pretreatment). *,#p<0.05.
Discussion
Adenosine signaling is an endogenous system involved in seizure control.3 Adenosine has been described as able to suppress seizure spread and to induce the termination of ongoing seizures.3 Our results reinforce the role of adenosine receptors in the development and control of seizures. The anticonvulsant effect induced by the activation of adenosine A1 receptors in rodents21,27 is well known. Adenosine augmentation therapies suppress and prevent seizures.6 Studies have shown that the adenosine A1 agonist promoted increased latency of seizure onset, decreased seizure occurrence, shorter seizure duration, and reduced mortality rate in chemical-induced seizure models.28 In our study, the A1 receptor agonist, CPA, increased the latency to reach the tonic-clonic seizure stage, demonstrating anticonvulsant properties in zebrafish. On the other hand, the pretreatment with adenosine A1 antagonist, DPCPX, induced a lower latency to reach the tonic-clonic seizure stage, suggesting proconvulsant effects in zebrafish. These findings confirm the anticonvulsant effect induced by the activation of adenosine A1 receptors since CPA increased the latency to seizures in all doses tested. We have also observed that CPA promoted a decrease on locomotor activity in zebrafish. A previous study supports that adenosine receptor agonists, including the selective A1 receptor agonist, CPA, produced dose-dependent suppression of spontaneous locomotor activity.29
The extracellular adenosine levels increase during seizures, and this has been proposed as a mechanism for seizure control.3,12 Nevertheless, during a seizure, extracellular adenosine levels rise, which could activate all types of adenosine receptors, including A2A, which facilitates neuronal transmission.14 In contrast to the well-known inhibitory effects of the A1 receptor, there are controversial results about the role of A2A receptors in seizures. A previous study showed that the pretreatment with the selective A2A antagonist, ZM 241385, had no effect on seizure parameters.27 However, the pretreatment with the selective A2A receptor agonist, CGS 21680, increased the after-discharge duration.27 These results oppose a previous report, which considers an anticonvulsant effect for the selective A2A receptor agonist.30 Interestingly, in our study, both the A2A receptor agonist and antagonist, CGS 21680 and ZM 241385, respectively, did not promote changes in seizure parameters. These results indicate that anticonvulsant effects of adenosine are promoted mainly through the activation of the adenosine A1 receptor.
Adenosine can be released from the cytoplasm through NTs or be formed by the extracellular breakdown of ATP by ectonucleotidases.3 Cellular release of adenosine is a physiological consequence of seizures and induces seizure arrest and termination.12 Besides cellular adenosine release, ATP release and its degradation into adenosine through ectonucleotidases represent an important source of extracellular adenosine.12 Despite its importance, the role of adenosine production and degradation for the control of seizure episodes still remains unclear. A previous study has shown that neither genetic deletion nor pharmacological inhibition of ecto-5′-nucleotidase plays a role in A1 receptor activation. This finding suggests that adenosine is not generated from ATP and is released directly by neurons.31 In active spiking neurons, A1 receptors were activated in an NT-dependent manner.32 However, another investigation has demonstrated the key role of ATP release from astrocytes on regulating adenosine signaling. By releasing ATP, which accumulates as adenosine, astrocytes tonically suppress synaptic transmission.33 Studies have proposed the equal ability of adenosine and AMP to activate A1 receptors and restrain excitatory neurotransmission, suggesting that the inhibition of extracellular AMP conversion into adenosine aiming to avoid A1 receptor activation might be nonsensical.34,35 AMP acts as an endogenous anticonvulsant by acting upon A1 receptors.36 AMP was able to reduce in vitro epileptiform discharges as well as audiogenic seizure and related mortality. These effects were blocked by DPCPX and potentiated by AMPCP, indicating that AMP per se activates the A1 receptor and is not acting as a metabolic precursor of adenosine.35 However, AMPCP alone had no effect on audiogenic seizures, suggesting that the inhibition of endogenous AMP hydrolysis is not able to restrain seizure development. Furthermore, AMP was unable to reduce seizure score, and a high dose of 250 mg/kg was necessary to increase survival or latency in kainate-treated mice.35
Acute PTZ-induced seizures did not alter ectonucleotidase activities in zebrafish brain membranes.37,38 Furthermore, there were no differences on ectonucleotidase activities after a single PTZ exposure in rats. However, increased ATP hydrolysis was evident in rats that were more resistant to seizures after the PTZ-kindling treatment.39 Changes on ectonucleotidase activities are late and prolonged after recurrent seizure episodes, suggesting these effects are due to an adaptive plasticity.40 Our results have demonstrated that animals pretreated with the ecto-5′nucleotidase inhibitor, AMPCP, reached the tonic-clonic seizure status faster than animals without this pretreatment. These results suggest a decrease in adenosine signaling by inhibition of ecto-5′-nucleotidase after PTZ-induced seizures in zebrafish. These findings corroborate the idea that extracellular AMP hydrolysis by ecto-5′-nucleotidase is an important mechanism to control adenosine levels and, consequently, the development of seizures. Therefore, considering the possible equal ability of AMP and adenosine to activate A1 receptors, currently it is not possible to understand exactly whether AMP and/or adenosine activate A1 receptors within the brain.34–36,41 Therefore, further studies are necessary to elucidate this mechanism.
Under stressful conditions, when adenosine levels are exacerbated, ADA activity is important in adenosine clearance. Studies have shown that successive convulsive episodes decreased the ecto-ADA activity in zebrafish brain membranes.37 However, one single seizure episode significantly increased ADA activity in the zebrafish brain, an effect that was suppressed by AED pretreatments.38 AED pretreatments suppressed the increase in adenosine deamination, which coincides with a longer period to reach the tonic-clonic seizure status.38 Therefore, ADA activity is modulated differently early after a single seizure or successive seizure episodes. Evidence sustaining the approach of combating seizures by inhibiting ADA is limited. There is evidence that seizure protection conferred by the EHNA in genetically seizure-prone epilepsy-like (EL) mice and PTZ infusion confirm that the inhibition of adenosine metabolism by deamination is an effective antiseizure strategy.41 In this study, we have shown that the inhibition of ADA activity modulates acute seizures in zebrafish. When pretreated with the ADA inhibitor, EHNA, animals showed longer latency to reach the tonic-clonic seizure status. These results suggest that the control of adenosine levels by ADA activity has an important role in seizure control in zebrafish.
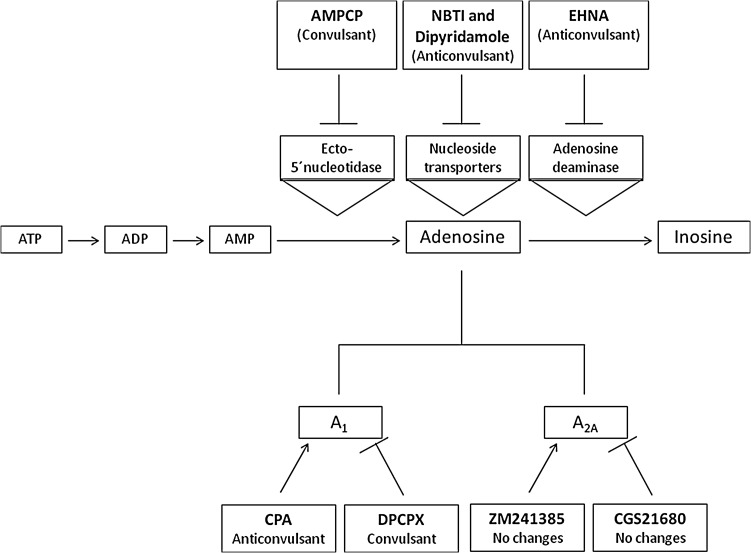
Another important mechanism to control extracellular adenosine levels involves the action of NTs. Our results have also demonstrated that NT inhibition by the nonspecific NT inhibitor, dipyridamole, and by the inhibitor of ENTs, NBTI, has protective effects during seizures in zebrafish. Animals pretreated with dipyridamole and NBTI showed longer latency to reach the tonic-clonic seizure status. Previous studies have demonstrated that NT inhibition by dipyridamole and NBTI retards the adenosine disappearance from extracellular cleft by blocking adenosine uptake into cells, conferring protective effects against seizures.42,43 Therefore, the nucleoside transport is also a mechanism that is able to control adenosine levels and, consequently, the development of seizures in zebrafish. A schematic representation shows the modulation of adenosine signaling in controlling seizures in zebrafish (Fig. 5).
FIG. 5.
Schematic representation of modulation of adenosine signaling in controlling seizures. The scheme shows the action pathway and induced effects of each studied compound.
Our behavioral parameters results have shown that the modulation of adenosine signaling could have an important protective effect against seizures in view of the fact that adenosine levels increase and the A1 activation retards seizure progression. Supporting the behavioral effects observed in our study, the different compounds tested were also able to modulate c-fos regulation in response to seizures. Animals submitted to PTZ-induced seizures showed upregulation of c-fos expression at stage III compared with animals that were not exposed to PTZ. However, animals pretreated with dipyridamole and EHNA did not show c-fos upregulation. In addition, despite having no statistically significant effects, pretreatments with CPA and NBTI present an apparent tendency to establish normal levels of the c-fos gene expression in response to PTZ-induced seizures. The compounds that fasten seizure progression (DPCPX and AMPCP) did not modulate c-fos gene expression in animals submitted to seizures, showing a response pattern similar to animals that were exposed to PTZ without receiving pretreatment. Although we could expect an increase in c-fos expression in animals pretreated with the compounds that fasten seizure progression, we observed a lack of difference in c-fos expression among the vehicle+PTZ, DPCPX+PTZ, and AMPCP+PTZ groups. This result could be explained by the fact that all animals were tested at seizure stage III, the most severe seizure stage, in which the maximum level of c-fos expression could be achieved by the animals. It is interesting to note that animals pretreated with AMPCP at 200 mg/kg showed decreased c-fos expression in comparison with animals that were not treated with AMPCP before PTZ exposure. However, this decrease was not statistically different between these groups, suggesting that c-fos expression was not completely suppressed by AMPCP at this dose. These results reinforce the idea that AMPCP was not able to maintain the c-fos expression at the control level.
In summary, our results demonstrated that anticonvulsant effects of adenosine in zebrafish are promoted mainly through the activation of adenosine A1 receptors. In addition, our findings indicate that the control of adenosine levels by ecto-5′-nucleotidase, ADA, and NTs has an important role in the control of seizure development in this species.
Acknowledgments
This work was supported by DECIT/SCTIE-MS through Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo á Pesquisa do Estado do Rio Grande do Sul (FAPERGS) (Proc. 10/0036-5–PRONEX). CDB and MRB are Research Career Awardees of the CNPq. AMS was recipient of fellowship from CAPES and CNPq. LWK was recipient of fellowship from the CAPES/PNPD Program.
Disclosure Statement
No competing financial interests exist.
References
- 1.Pitkanen A. Therapeutic approaches to epileptogenesis-hope on the horizon. Epilepsia 2010;51:2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov 2010;9:68–82 [DOI] [PubMed] [Google Scholar]
- 3.Boison D. Adenosine and seizure termination: endogenous mechanisms. Epilepsy Curr 2013;13:35–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunwiddie TV. Endogenously released adenosine regulates excitability in the in vitro hippocampus. Epilepsia 1980;21:541–548 [DOI] [PubMed] [Google Scholar]
- 5.Dragunow M, Goddard GV, Laverty R. Is adenosine an endogenous anticonvulsant? Epilepsia 1985;26:480–487 [DOI] [PubMed] [Google Scholar]
- 6.Boison D: Adenosine Augmentation Therapy. In: Jasper's Basic Mechanisms of the Epilepsies. Noebels JL, Avoli M, Rogawski MA, Olsen RW, and Delgado-Escueta AV. (Eds), National Center for Biotechnology Information (US), Bethesda, MD, 2012 [Google Scholar]
- 7.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 2012;8:437–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boison D. Adenosine dysfunction and adenosine kinase in epileptogenesis. Open Neurosci J 2010;4:93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ipata PL, Camici M, Micheli V, Tozz MG. Metabolic network of nucleosides in the brain. Curr Top Med Chem 2011;11:909–922 [DOI] [PubMed] [Google Scholar]
- 10.Bonan CD. Ectonucleotidases and nucleotide/nucleoside transporters as pharmacological targets for neurological disorders. CNS Neurol Disord Drug Targets 2012;11:739–750 [DOI] [PubMed] [Google Scholar]
- 11.Fredholm BB IJ.zerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 2011;63:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol 1992;32:618–624 [DOI] [PubMed] [Google Scholar]
- 13.Berman RF, Fredholm BB, Aden A, O'Connor WT. Evidence for increased dorsal hippocampal adenosine release and metabolism during pharmacologically induced seizures in rats. Brain Res 2000;872:44–53 [DOI] [PubMed] [Google Scholar]
- 14.Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann NY Acad Sci 2001;939:74–84 [DOI] [PubMed] [Google Scholar]
- 15.Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005;131:759–768 [DOI] [PubMed] [Google Scholar]
- 16.Afrikanova T, Serruys AS, Buenafe OE, Clinckers R, Smolders I, de Witte PA, et al. Validation of the zebrafish pentylenetetrazol seizure model: locomotor versus electrographic responses to antiepileptic drugs. Plos One. 2013;8:e54166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pineda R, Beattie CE, Hall CW. Recording the adult zebrafish cerebral field potential during pentylenetetrazole seizures. J Neurosci Methods 2011;200:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong K, Stewart A, Gilder T, Wu N, Frank K, Suciu C, et al. Modeling seizure-related behavioral and endocrine phenotypes in adult zebrafish. Brain Res 2010;1348:209–215 [DOI] [PubMed] [Google Scholar]
- 19.Boehmler W, Petko J, Woll M, Frey C, Thisse B, Thisse C, et al. Identification of zebrafish A2 adenosine receptors and expression in developing embryos. Gene Expr 2009; Patterns 9:144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capiotti KM, Menezes FP, Nazario LR, Pohlmann JB, de Oliveira GM, Fazenda L, et al. Early exposure to caffeine affects gene expression of adenosine receptors, DARPP-32 and BDNF without affecting sensibility and morphology of developing zebrafish (Danio rerio). Neurotoxicol Teratol 2011;33:680–685 [DOI] [PubMed] [Google Scholar]
- 21.Southam E, Stratton SC, Sargent RS, Brackenborough KT, Duffy C, Hagan RM, et al. Broad spectrum anticonvulsant activity of BW534U87: possible role of an adenosine-dependent mechanism. Pharmacol Biochem Behav 2002;74:111–118 [DOI] [PubMed] [Google Scholar]
- 22.Mares P. Anticonvulsant action of 2-chloroadenosine against pentetrazol-induced seizures in immature rats is due to activation of A1 adenosine receptors. J Neural Transm 2010;117:1269–1277 [DOI] [PubMed] [Google Scholar]
- 23.Akula KK, Dhir A, Kulkarni SK. Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor increases pentylenetetrazol seizure threshold in mice: possible involvement of adenosinergic mechanism. Epilepsy Res 2008;78:60–70 [DOI] [PubMed] [Google Scholar]
- 24.Phelps HA, Runft DL, Neely MN. Adult zebrafish model of streptococcal infection. Curr Protoc Microbiol 2009; Chapter 9:Unit 9D.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chawla MK, Penner MR, Olson KM, Sutherland VL, Mittelman-Smith MA, Barnes CA. Spatial behavior and seizure-induced changes in c-fos mRNA expression in young and old rats. Neurobiol Aging 2013;34:1184–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang R, Dodd A, Lai D, Mcnabb WC, Love DR. Validation of zebrafish (Danio rerio) reference genes for quantitative Real-time RT-PCR normalization. Acta Biochim Biophys Sin 2007;39:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosseinmardi N, Mirnajafi-Zadeh J, Fathollahi Y, Shahabi P. The role of adenosine A1 and A2A receptors of entorhinal cortex on piriform cortex kindled seizures in rats. Pharmacol Res 2007;56:110–117 [DOI] [PubMed] [Google Scholar]
- 28.Li M, Kang R, Shi J, Liu G, Zhang J. Anticonvulsant activity of B2, an adenosine analog, on chemical convulsant-induced seizures. PLoS One 2013;8:e67060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marston HM, Finlayson K, Maemoto T, Olverman HJ, Akahane A, Sharkey J, et al. Pharmacological characterization of a simple behavioral response mediated selectively by central adenosine A1 receptors, using in vivo and in vitro techniques. J Pharmacol Exp Ther 1998;285:1023–1030 [PubMed] [Google Scholar]
- 30.Huber A, Güttinger M, Möhler H, Boison D. Seizure suppression by adenosine A(2A) receptor activation in a rat model of audiogenic brainstem epilepsy. Neurosci Lett 2002;329:289–292 [DOI] [PubMed] [Google Scholar]
- 31.Klyuch BP, Dale N, Wall MJ. Deletion of ecto-5'-nucleotidase (CD73) reveals direct action potential-dependent adenosine release. J Neurosci 2012;32:3842–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, et al. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A 2012;109:6265–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005;310:113–116 [DOI] [PubMed] [Google Scholar]
- 34.Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, et al. AMP is an adenosine A1 receptor agonist. J Biol Chem 2012;287:5301–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muzzi M, Coppi E, Pugliese AM, Chiarugi A. Anticonvulsant effect of AMP by direct activation of adenosine A1 receptor. Exp Neurol 2013;250:189–193 [DOI] [PubMed] [Google Scholar]
- 36.Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, et al. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab 2013;33:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siebel AM, Piato AL, Capiotti KM, Seibt KJ, Bogo MR, Bonan CD. PTZ-induced seizures inhibit adenosine deamination in adult zebrafish brain membranes. Brain Res Bull 2011;86:385–389 [DOI] [PubMed] [Google Scholar]
- 38.Siebel AM, Piato AL, Schaefer IC, Nery LR, Bogo MR, Bonan CD. Antiepileptic drugs prevent changes in adenosine deamination during acute seizure episodes in adult zebrafish. Pharmacol Biochem Behav 2013;104:20–26 [DOI] [PubMed] [Google Scholar]
- 39.Bonan CD, Amaral OB, Rockenbach IC, Walz R, Battastini AMO, Izquierdo I, et al. Altered ATP hydrolysis induced by pentylenetetrazol kindling in rat brain synaptosomes. Neurochem Res 2000;25:775–779 [DOI] [PubMed] [Google Scholar]
- 40.Bonan CD, Walz R, Pereira GS, Worm PV, Battastini AMO, Cavalheiro EA, et al. Changes in synaptosomal ectonucleotidase activities in two rat models of temporal lobe epilepsy. Epilepsy Res 2000;39:229–238 [DOI] [PubMed] [Google Scholar]
- 41.Muzzi M, Blasi F, Chiarugi A. AMP-dependent hypothermia affords protection from ischemic brain injury. J Cereb Blood Flow Metab 2013;33:171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George B, Kulkarni SK. Modulation of lithium-pilocarpine-induced status epilepticus by adenosinergic agents. Methods Find Exp Clin Pharmacol 1997;19:329–333 [PubMed] [Google Scholar]
- 43.Zhang G, Franklin PH, Murray TF. Manipulation of endogenous adenosine in the rat prepiriform cortex modulates seizure susceptibility. J Pharmacol Exp Ther 1993;264:1415–1424 [PubMed] [Google Scholar]