Abstract
Pseudoloma neurophilia is a microsporidium of zebrafish (Danio rerio) that preferentially infects neural tissue. It is one of the most common pathogens of zebrafish in research laboratories based on diagnostic data from the Zebrafish International Resource Center diagnostic service (Eugene, OR). Five hundred fifty-nine zebrafish infected with P. neurophilia submitted to ZIRC from 86 laboratories between the years 2000 and 2013 were examined via histopathology to develop a retrospective study of the features of neural microsporidiosis. Parasite clusters (PCs) occurred in distinct axonal swellings, frequently with no associated inflammation. Inflammation was observed in viable cell bodies distant from PCs. Multiple PCs occasionally occurred within a single axon, suggesting axonal transport. PCs occurred most frequently in the spinal cord ventral white matter (40.3% of all PCs) and the spinal nerve roots (25.6%). Within the rhombencephalon, PCs were most common in the primary descending white matter tracts. Within the rhombencephalon gray matter, PCs occurred most frequently in the reticular formation and the griseum centrale (61% and 39%, respectively). High numbers of PCs within brain and spinal cord structures mediating startle responses and anxiety suggest that related behaviors could be altered by neural microsporidiosis. Infection could, therefore, introduce unacceptable variation in studies utilizing these behaviors.
Introduction
Pseudoloma neurophilia is a common microsporidian parasite of zebrafish (Danio rerio) that can infect nearly every tissue in the body.1,2 It has an overwhelming preference for neural tissue, particularly the nerve roots, spinal cord, and hindbrain.3 First described in 2001, P. neurophilia has since been identified as one of the most common infectious organisms diagnosed in laboratory zebrafish. Clinical infection is most frequently characterized by emaciation and spinal deformities.4,5 Transmission occurs through the consumption of environmentally resistant spores either free in the water or embedded in fish carcasses. Maternal transmission occurs, either by shedding spores at spawning or by parasites within the egg.6 Spores are resistant to bleaching at a concentration of 25–50 ppm for 10 min, which makes transmission prevention difficult even when spores are adhered to the exterior egg surface.5 The Zebrafish International Resource Center (ZIRC) located in Eugene, OR, provides a diagnostic service to the zebrafish community by performing histopathologic, molecular, and bacteriologic analyses of samples from both clinically ill and routine sentinel fish. From the years 2006 to 2013, this service diagnosed P. neurophilia in an average of 50% of submitting facilities (range 19%–74%).5,7,8 Recently, a survey of the ZIRC diagnostic service database revealed that only 26% of fish submitted between the years 1999 and 2013 diagnosed with P. neurophilia were submitted due to clinical disease, while the rest were submitted as routine sentinel cases with no reported clinical disease.7,8 Taken together, these facts indicate that P. neurophilia is prevalent throughout zebrafish facilities and that most cases of P. neurophilia infection are subclinical, making this disease both widespread and insidious.5
Diagnosis of P. neurophilia infection is usually made via histopathologic examination of fixed tissues or by polymerase chain reaction analysis. Microscopic lesions in fish include myositis, meningitis/meninxitis, myelitis, encephalitis, granulomatous inflammation, and, commonly, the presence of parasite clusters (PCs) in nervous and other tissues without inflammation.4,5 PCs were first described as xenomas,4 but more extensive ultrastructural analysis by Cali et al.9 showed that they are not xenomas as defined by Lom and Dykovà.10 Although a particular name has not been officially suggested for these structures created by P. neurophilia, we will refer to them simply as “parasite clusters” in this article, because they contain all stages of the life cycle but are not surrounded by a distinct membrane.
Because most P. neurophilia infections are often subclinical and because the zebrafish is still a relatively new addition to the laboratory animal stable, the drive to screen for and to eliminate P. neurophilia within zebrafish facilities has been relatively low compared with the efforts made with pathogens that cause acute mortality, such as Mycobacterium marinum, Mycobacterium haemophilum, and Edwardsiella ictulari.5
P. neurophilia has been extensively studied with regard to life cycle, transmission, microscopic and ultrastructural features, as well as the effects of stress on infection.3–6,9,11 Zebrafish are now extensively used in behavior research,12–17 and it is possible that these neural infections by P. neurophilia may introduce nonprotocol-induced variation to these experiments.
Based on a relatively small sample size from one facility, Matthews et al.4 first associated severity of infection and concurrent myositis with clinical disease (i.e., emaciation). Ramsay et al.11 later showed that infected fish that were subjected to crowding stress had an increased incidence of myositis. We took advantage of the large collection of histologic slides, including both clinical and apparently normal infected zebrafish at ZIRC, to conduct a more comprehensive retrospective study evaluating the overarching trends of P. neurophilia infection with respect to grossly observable clinical disease, inflammation, anatomic location, and the simultaneous presence of other potentially complicating diseases. The purpose of our detailed study was to further characterize the features of P. neurophilia infections with an eye toward developing a hypothesis as to which specific behaviors or other research endpoints are most likely to be influenced by this common infection.
Materials and Methods
Fish were selected based on data reviewed from the ZIRC diagnostic service database. These archival samples ranged from the years 1999 to 2014.8 To determine whether any microscopic features of infection were correlated with grossly visible clinical disease, infected fish that were submitted as “clinical” were examined (n=175). To compare the features of cases submitted as “routine” or nonclinical, a set of 175 fish from this group was arbitrarily selected. As the study progressed, we decided to match the number of clinical and routine cases for each year to help reduce potential effects of submission date as a confounding factor. This resulted in the examination of a total of 559 fish, with 175 noted as clinical and 384 noted as routine.
Samples examined were glass slides made from paraffin-embedded, fixed tissues. The vast majority were stained with hematoxylin and eosin (H&E), although a small number of slides were stained by utilizing the Giemsa, Gram, acid fast, and Luna techniques utilizing standard procedures. All specimens examined were sectioned sagitally. Also included in our analysis were the year of submission, the submitting laboratory, the sex of the fish, and the presence of other potential causes of primary disease besides P. neurophilia.
A negative binomial regression was used to determine whether the total PC numbers were significantly different between male and female fish, and between routine and clinical fish while controlling for other factors: year, whether or not fish displayed myositis, and whether or not the fish had other diseases. A logistic regression was used to assess the effects of sex, clinical disease, the presence of other diseases, meninxitis, and encephalitis on the prevalence of myositis, while controlling for the effect of the year in which each case was submitted. An ordered logistic regression was used to determine the effects of sex, the presence of other diseases, and encephalitis on the severity of meninxitis. At each of the anatomic locations under study (brain and spinal cord), a negative binomial regression was used to determine whether the total PC numbers observed in white matter versus gray matter were significantly different while controlling for other factors: year of submission, sex, clinical disease, myositis, and the presence of other diseases.
All analyses were conducted using the statistical software R (version 3.1). The R function glm.nb() was used for negative binomial regression analysis, polr() was used for ordered logistic regression analysis, and glm() with a logit link was used for the logistic regression analysis.
Results
Although extraneuromuscular lesions were noted during the study (spores or associated granulomas in the viscera), their observation was rare. Furthermore, Sanders et al.3 and Peterson et al.18 showed that light P. neurophilia infections, particularly those in the viscera, frequently require special staining to highlight small numbers of spores. Because this was a retrospective study ranging from 1999 to 2013 and because the Luna stain was not used for zebrafish diagnotics until ∼2008, we focused on neural and muscular infections observable by H&E staining alone.
To provide an objective analysis of the data to complement the descriptive aspects of the study, the number of PCs in various anatomic locations throughout the nervous system were counted (Fig. 1). Similarly, to provide an objective assessment of the extent of inflammation (meninxitis and encephalitis/myelitis), a rubric was developed based on the character and extent of inflammation within the central nervous system (Fig. 2).
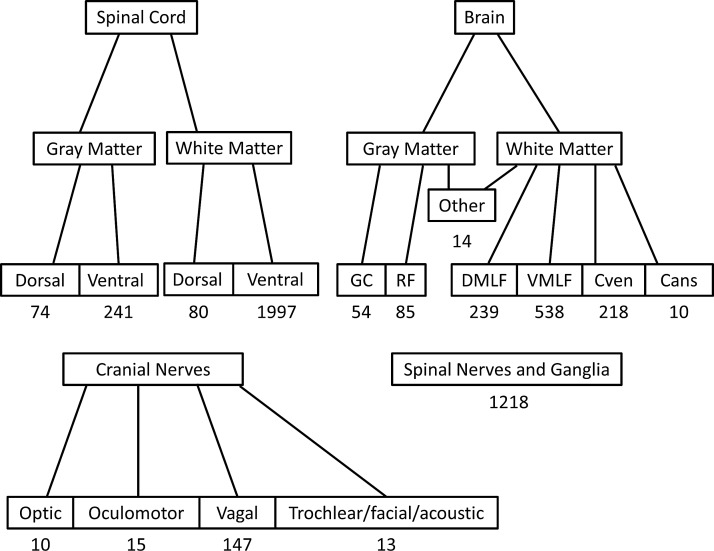
FIG. 1.
Anatomic locations and spore cluster numbers in the nervous system. PCs were most commonly observed in the ventral spinal cord white matter. Spinal nerves and ganglia were a close second. Spores observed in the brain were most common in the VMLF. For both the spinal cord and the brain, PCs were overwhelmingly more commonly observed in the white matter than in the gray matter. Within gray matter in the hindbrain, spores were most commonly observed in the RF and the GC. A strikingly large number of PCs were observed in the vagal nerve. Because of the sectioning of archival samples, it was difficult to distinguish between the trochlear, facial, and acoustic nerves and so these are grouped in single category. The “Other” category applies to all locations in the brain in which less than 10 PCs were observed out of all fish examined. This category includes both white and gray matter structures. It is important to note that no PCs were observed in any gray matter structure farther rostral than the hypothalamus. PCs were never encountered in the telencephalon. The vast majority of the PCs were observed in the rhombencephalon. DMLF, dorsal medial longitudinal fascicle; Cven, commisura ventralis rhombencephali; Cans, commisura ansulata; PCs, parasite clusters; VMLF, ventral medial longitudinal fascicle; RF, reticular formation; GC, griseum centrale.
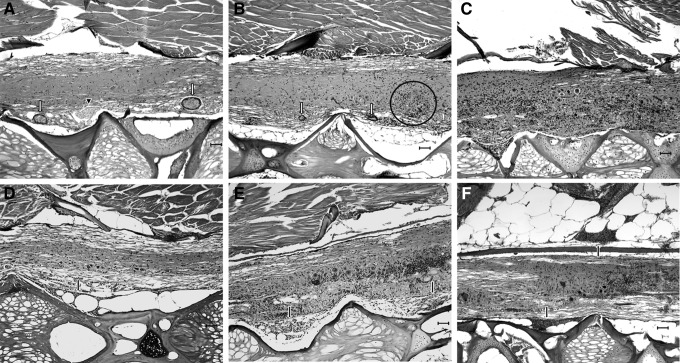
FIG. 2.
Visual grading scheme for encephalitis/myelitis and meninxitis. H&E. Scale bars=50 μM. (A) Grade 1 myelitis: commonly, encephalitis/myelitis in P. neurophilia-infected fish were characterized by localized inflammation surrounding discrete PC in the neuropil. These PC were encapsulated by a layer of flattened, epithelioid cells that are most likely macrophages or microglial cells (white arrows). If an animal's encephalitis or myelitis was confined entirely to these inflammation-encapsulated PC, it was given a grade of 1. These could be present alongside uninflamed PCs (arrowhead). (B) Grade 2 myelitis: animals with grade 2 encephalitis/myelitis had multifocal to coalescing areas of gliosis within the neuropil that are commonly associated with scattered piecemeal neuronal necrosis and/or sattelitosis and/or neuronophagia (circled area). Inflammation-encapsulated PC may have been observed occasionally, as well as PC that appeared to have ruptured (white arrows). The latter PC were characterized by dense clusters of macrophages, presumptive microglial cells, granulocytes, and lymphocytes that surround and separate densely packed, but poorly organized clusters of spores and pre-sporogonic stages. (C) Grade 3 myelitis: animals with grade 3 encephalitis/myelitis had changes similar to those described in animals with grade 2 inflammation, but the encephalitis and myelitis was more severe and extensive, affecting more than 20% of the observable spinal cord and possibly involving granulocytes and amorphous cellular debris as well as large numbers of presumptive microglial cells. (D) Grade 1 meninxitis was characterized by occasional (up to three in a single animal) multifocal mats of granulocytic meninxitis (black arrow), confined to either the dorsal or ventral visible aspect of the spinal cord. (E) Grade 2 meninxitis was characterized by moderate numbers (up to one dozen in a single animal) of discrete inflammatory mats that are generally thicker than grade 1 mats (black arrow). Inflammation is limited to either the dorsal or ventral aspect of the visible cord in grade 2 meninxitis. (F) Grade 3 meninxitis was characterized by thick, widespread mats of inflammation that were frequently circumferential (observed both dorsal and ventral to the spinal cord). In extreme cases, the inflammation will extend past the ectomeninx to surround nerve roots (black arrows). H&E, hematoxylin and eosin.
Characteristics of neuronal infection
When PCs of P. neurophilia were present within the white matter or within nerve roots, they were always observed within the axon rather than within neuron cell bodies, glial cells, or capillary endothelial cells. In certain views, PCs of P. neurophilia were distinctly observed within the axonoplasm, producing gradual to abrupt swellings in the axon itself (Fig. 3).
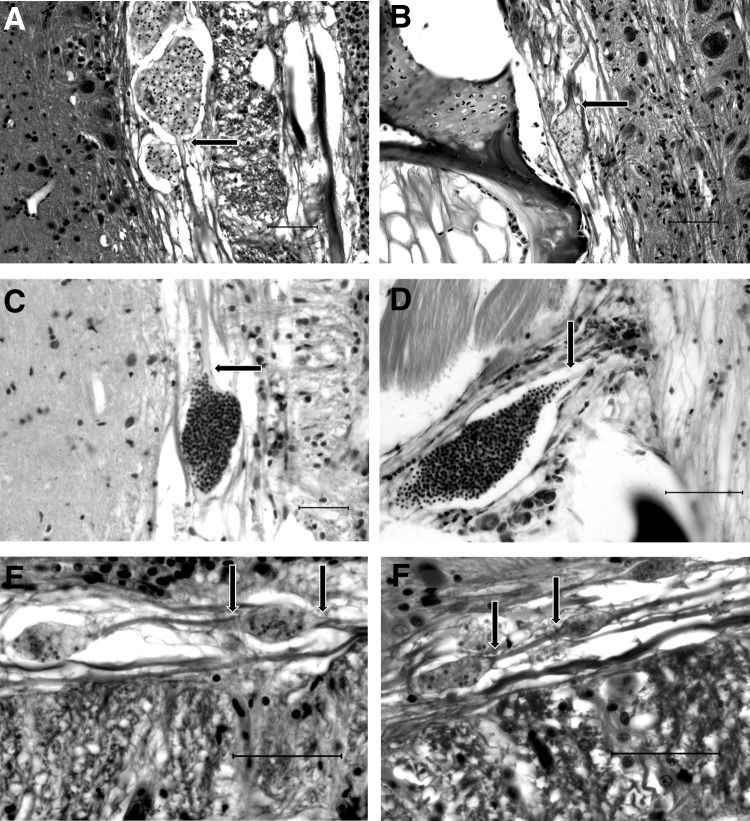
FIG. 3.
Intra-axonal PCs. (A and B) Frequently, PCs are observed in distinct axonal swellings with no associated inflammation. There appears to be an abrupt swelling of the axonoplasm before the PC distends the axon. There is a distinct absence of axonal degeneration (Wallerian degeneration). H&E. Scale bar=50 μM. (C) Occasionally, spores can be seen trailing off at the base of the axonal swelling. Luna. Scale bar=50 μM. (D) Within a nerve root, a distinct line of spores can be seen trailing past the axonal swelling and up the axon. Giemsa. Scale bar=50 μM. (E and F). Occasionally, multiple PC are observed in a single axon, forming a “string of pearls” arrangement. H&E. Scale bar=50 μM. Bases of axonal swellings are indicated by arrows.
Strikingly, in most cases, there was no directly observable evidence of axonal (Wallerian) degeneration (axonal swelling, vacuolation, or digestion chambers) either cranial or caudal to the intra-axonal PCs. Frequently, multiple PCs were observed along a single axon separated by segments of normal-appearing axon, forming a “string of pearls” configuration (Fig. 3). Also of note is the fact that PCs definitively observed within neurons were always present in the axons: No PCs or spores were ever identified in neuron cell bodies. When present in gray matter, as with the white matter, PCs were observed in the neuropil and not in glial cells, endothelial cells, or neuron cell bodies.
Despite the fact that intraneuronal PCs were only ever observed in axons, neuron cell bodies were not spared the consequences of infection. In cases with varying PC numbers throughout the spinal cord, nerve roots, and hind brain, multifocal individual necrotic neurons characterized by cell shrinkage, pyknosis or karyolysis, and cytoplasmic vacuolation were observed within either hindbrain or spinal cord gray matter distant from observable PC. This piecemeal neuronal necrosis was occasionally associated with encephalitis or myelitis of varying severity and was occasionally present when no other inflammation was observed proximal to the neuron cell bodies. PCs were usually not observed within the affected gray matter (Figs. 4 and 5).
FIG. 4.
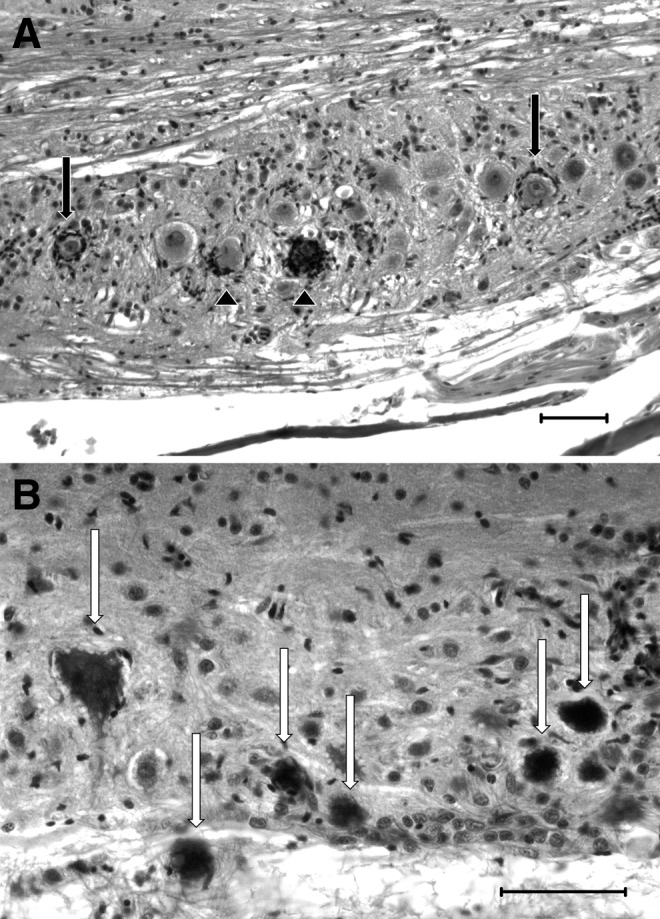
Patterns of encephalitis and myelitis distal to visible PCs. H&E. Scale bars=50 μM. (A) Neuron cell bodies (in the reticular formation, here) appear to be targeted by inflammation, primarily composed of presumptive microglial cells. While some neurons undergoing neuronophagia appear degenerate (chromatolysis, pyknosis, shrunken, and hypereosinophilic; black arrowheads) many neurons that appear completely healthy are surrounded by presumptive microglial cells (black arrows). (B) Inflammation in the spinal cord (myelitis) is characterized primarily by presumptive microglial cells admixed with occasional lymphocytes. Multifocally, there may be individual necrotic neurons (white arrows) characterized by a shrunken, angular profile, karyolysis, and severe hypereosinophilia.
FIG. 5.
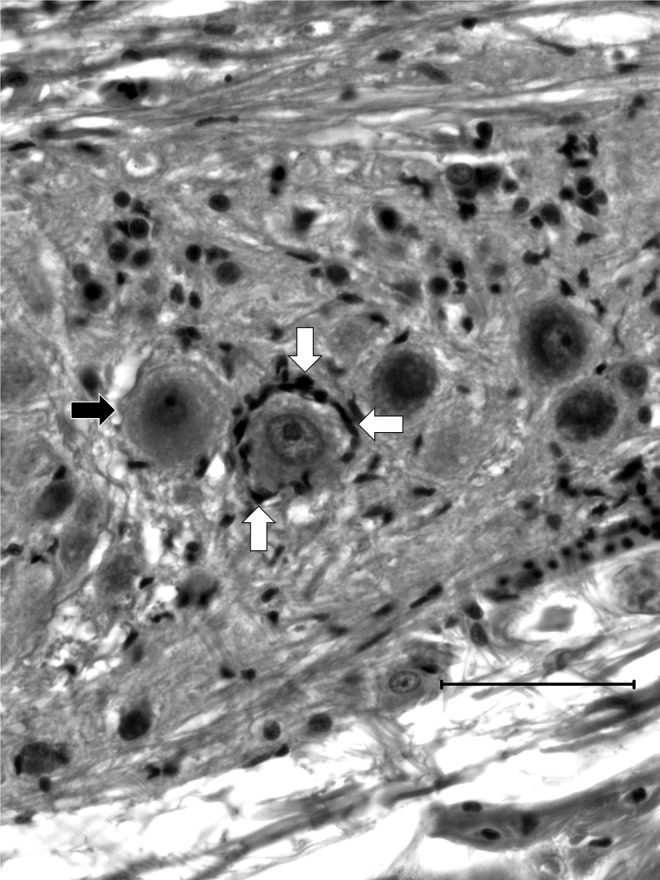
Detailed features of perineuronal inflammation. Frequently, neuron cell bodies with no sign of degeneration including chromatolysis, vacuolation, or pyknotic nuclei, were surrounded by what appeared to be microglial cells (white arrows) when PCs were observed at sites distal to the inflammation. Frequently, affected neurons were directly adjacent to normal, healthy-looking neurons (black arrow).
Multifocal scattered neuron cell bodies surrounded by putative microglial cells that either lined the periphery of the cell (satellitosis) or were observed within the cytoplasm of these cell bodies (neuronophagia) were frequently observed in gray matter distal to observable PCs. This suggests that the PCs were the cause of these lesions (particularly in the absence of any other neuronal disease). To clarify, neuronophagia is defined as the invasion, destruction, and/or consumption of neurons by phagocytic cells (commonly microglial cells in mammals). These neuron cell bodies frequently had viable, non-pyknotic nuclei and no indication of necrosis or degeneration beyond the inflammatory changes of satellitosis and neuronophagia. Satellitosis and/or neuronophagia of visibly necrotic neurons (shrinkage, hypereosinophilia, vacuoloation, pyknosis, karyolysis) was also common.
Overall, the spinal cord ventral white matter, nerve roots, and ganglia were the most common sites for PCs. Regarding the brain, nearly all PCs observed were found in the rhombencephalon. Within the hind brain, there was a consistently striking pattern of infection that appeared to closely follow the primary descending white matter tracts. PCs were frequently found in the dorsal and ventral medial longitudinal fascicle (MLF) as well as the commissura ventralis rhombencephali where fibers were associated with the dorsal and ventral MLF decussate (Fig. 6). When PCs were present in gray matter, they were most commonly found in the reticular formation and the griseum centrale (GC).
FIG. 6.
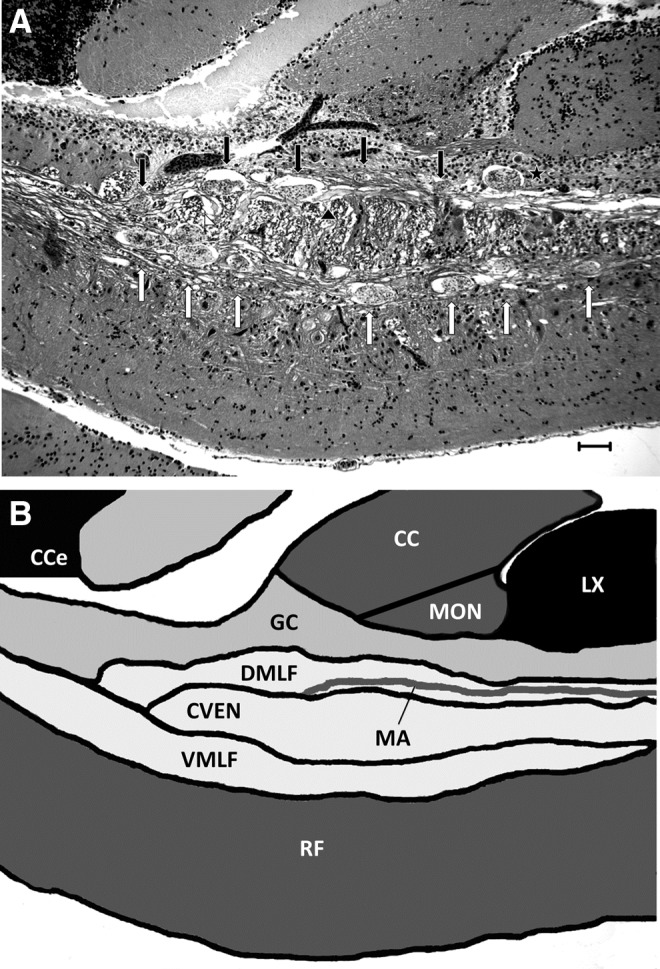
PCs in the rhombencephalon. (A) PCs in the rhombencephalon tended to follow a distinct anatomic distribution, being most commonly found in the major descending white matter tracts: The DMLF (black arrows) in which a PC can be seen impinging on (but not directly infecting) the MA (black arrowhead) and the VMLF (white arrows) along with the Cven (white arrowhead) in which fibers of the MLF decussate. PC are also observed in rhombencephalic gray matter, including the GC (black star). H&E. Scale bar=50 μM. (B) Hindbrain anatomy schematic for comparison to Figure 3A. Cce, corpus cerebelli; CC, Crista cerebrallis; MON, medial octavolateralis nucleus; MA, Mauthner axon.
MLF and the ventral spinal white matter
Most PCs were observed in the ventral spinal cord white matter (40% of the total observed PCs) and in the spinal nerves and ganglia (25%). A total of 23% of all observed PCs were found in the hindbrain. Of these PCs, 12% were observed in the gray matter and 88% were observed in the white matter. Of PCs observed in the hindbrain gray matter, 61% were found in the reticular formation and the rest were observed in the GC. Of PCs observed in the hindbrain white matter, 53% were observed in the ventral medial longitudinal fascicle (VMLF). A total of 4% of all observed PCs were found in the cranial nerves. Of these, the vast majority (79%) were observed in the vagus (Cranial nerve X).
Inflammation
Inflammation associated with P. neurophilia infection was localized based on the anatomical structure in which it was present and categorized as encephalitis/myelitis when present in the neuropil of the brain or spinal cord, meninxitis when associated with the membranes surrounding the central nervous system, and myositis when present in the muscle.
Encephalitis/myelitis
Of the 561 infected fish, 59% had this lesion. Of these, 21% received a grade of 1, 55% received a grade of 2, and 24% received a grade of 3. Among fish submitted without clinical disease, 66% had encephalitis or myelitis. Of these, 24% were grade 1, 54% were grade 2, and 22% were grade 3. Among fish submitted with clinical disease, 54% had encephalitis/myelitis. Of these, 12% were grade 1, 58% were grade 2, and 30% were grade 3.
PCs were frequently observed in neuronal tissue with no visible inflammation either directly associated with the PCs or scattered throughout the neuropil (Figs. 2, 4, 5, and 7). Fish with these features were given an encephalitis/myelitis grade of 0. Inflammation was never associated with the vasculature, and there was no evidence of perivascular inflammation or vasculitis. Gliosis and inflammation in animals with grade 2 and 3 inflammation was randomly scattered throughout the neuropil or closely associated with apparently ruptured PCs (Fig. 8). Distinct perivascular patterns were never identified.
FIG. 7.
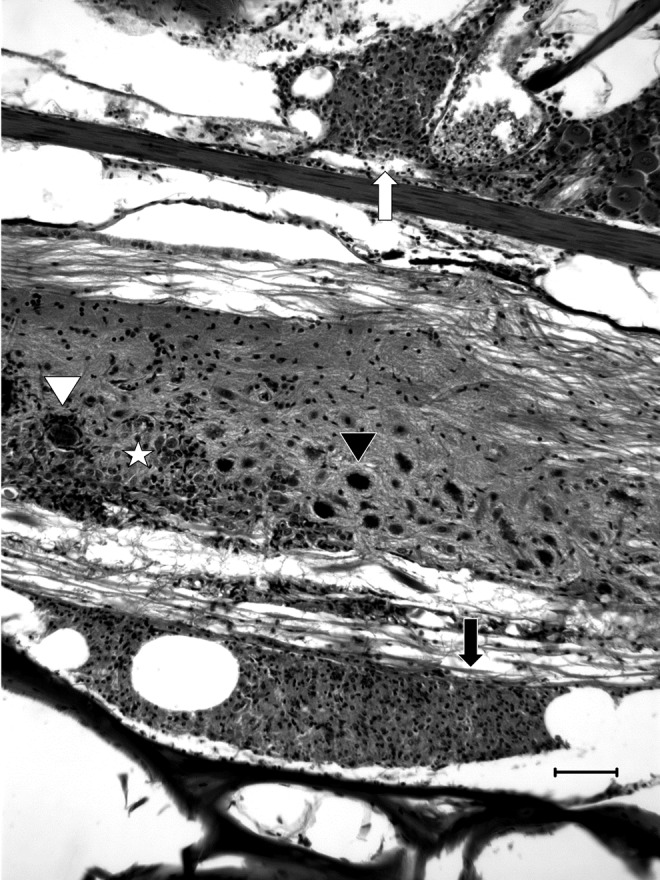
General patterns of inflammation. Meninxitis was frequently composed of granulocytes associated with free spores (arrows). In grade 3 meninxitis, inflammation appeared both ventral (black arrow) and dorsal (white arrow) to the spinal cord. In severe cases, inflammation frequently extended through the ectomeninx to cause periradiculoneuritis (white arrow). In this image, grade 2 myelitis is observed with generalized gliosis (star), neuronophagia (white arrowhead) and piecemeal neuronal necrosis with peripheral vacuolation (black arrowhead). H&E. Scale bar=50 μM.
FIG. 8.
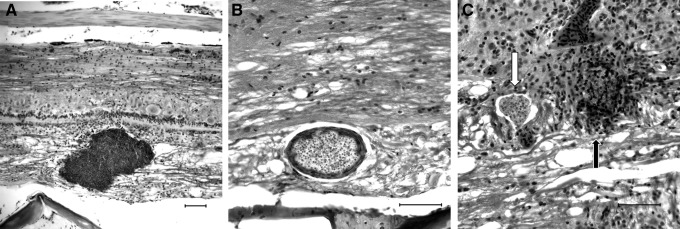
Patterns of inflammation associated directly with PCs in the nervous system. (A) PC can grow to massive sizes, in this case, almost half the diameter of the spinal cord, while inducing only a minimal inflammatory response. Luna. Scale bar=50 μM. (B) Even smaller PC may induce a localized inflammatory response wherein epithelioid macrophages (likely transformed microglial cells) form a layer around the PC presumably in an attempt to “wall it off.” H&E. Scale bars=50 μM. (C) Inflammation associated with individual PC is highly variable, even when they are directly adjacent to each other. One can be completely intact and have no associated inflammation (white arrow), while another can be completely effaced by inflammation separating individual spores and presporogonic stages (black arrow). The latter cluster is presumed to have ruptured. H&E. Scale bar=50 μM.
Meninxitis
The severity of these inflammatory changes was graded by extent of the lesions (1–3), as described in Figure 2. Of the 561 infected fish, 50% showed this lesion. Of these, 22% received a grade of 1, 53% received a grade of 2, and 25% received a grade of 3. Of the 384 fish submitted as routine cases, 52% had meninxitis. Of these, 20% were grade 1, 54% were grade 2, and 26% were grade 3. Of the 177 fish submitted as clinical cases, 45% had meninxitis. Of these, 26% were grade 1, 49% were grade 2, and 25% were grade 3.
About 50% of the fish exhibited inflammation that was associated with the membranes surrounding the brain and spinal cord. In the zebrafish studied here, granulocytic meninxitis frequently formed discrete, well-demarcated mats within the perimeninxial space that contained granulocytes, cellular debris, and, frequently, free P. neurophilia spores (but no observable presporogonic stages) (Fig. 7). This granulocytic meninxitis frequently followed nerve roots for a short distance outside of the vertebral canal and was frequently associated with periganglioneuritis and periradiculoneuritis (Fig. 7).
Myositis
The most commonly observed extraneural sequelae of microsporidiosis are detailed in Figures 9 and 10. Myositis was far rarer than expected, occurring in only 13% of all examined fish. This lesion was observed in 16% of fish submitted with clinical disease and 7% of the fish submitted without clinical disease. Relatively few fish had myositis, which was generally characterized by multifocal to coalescing areas of granulocytic to granulomatous inflammation. Because the locations of myositis were inconsistent between fish and relative severity was difficult to determine, we decided to simply note the presence or absence of myositis rather than producing an ordered grading system. The presence or absence of myodegeneration without inflammation was similarly treated.
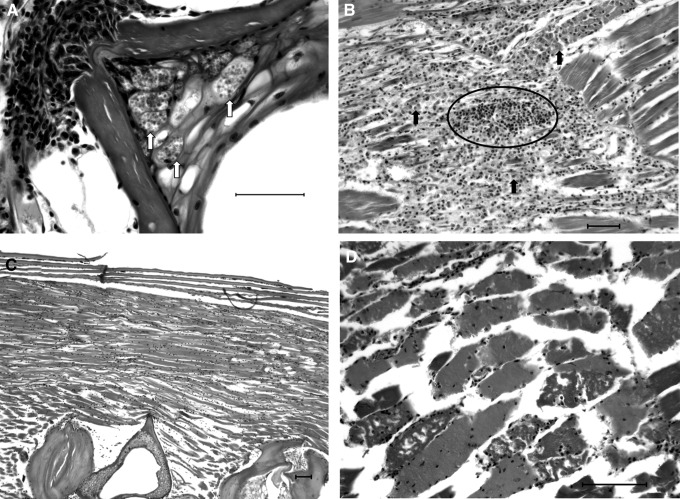
FIG. 9.
Extraneural manifestations of P. neurophilia infection. H&E. Scale bar=50 μM. (A) Notochord remnants in vertebrae contain large intracytoplasmic PC (white arrows). This is an uncommon, but not rare, presentation of P. neurophilia infection. (B) Granulocytic myositis occasionally contains free spores (circled cluster of black spots) and is commonly adjacent to nerve roots either containing PC or surrounded by granulocytic inflammation (black arrows) extending from a meninxitis. (C) Animals with P. neurophilia infection will frequently have noninflammatory myodegeneration. This manifestation is chronic, resulting in muscle atrophy and loss, with increased space between myocytes. (D) Myodegeneration without inflammation can also occur acutely or subacutely with the loss of cross-striation in myocytes, the formation of contraction bands, and cytoplasmic fragmentation.
FIG. 10.

Myositis with intralesional spores. Acute to subacute granulocytic myositis was occasionally associated with spores free in the inflammatory infiltrate (arrow). H&E. Scale bar=50 μM.
Other diseases
This study focuses on P. neurophilia infection. However, potentially clinically significant (health-affecting) histopathologic lesions were frequently observed concurrent with neural microsporidiosis. These lesions included, but were not limited to, aerocystitis (inflammation of the swim bladder), oophoritis and ovarian granulomas, mycobacteriosis, nephrocalcinosis (mineral deposits in renal tubules), and neoplasias such as seminomas and ultimobranchial gland adenomas. For the purposes of this study, all histopathologic lesions (besides neural microsporidiosis) that could have produced clinical disease were categorized as “positive for other disease.”
Statistical analysis
There were significant differences among years of submission in terms of the PC number per fish (p<0.01), total PCs in the brain (p<0.01), in the spinal cord (p<0.01), in the nerve roots (p<0.01), in the brain white matter (p<0.01), in the spinal cord white matter (p<0.01), and in the spinal cord gray matter (p<0.01). The submission year was then accounted for in the next analyses to correct for the effect of this variable. There was a significant difference between females and males in terms of total number of PCs/fish (p<0.01). The corresponding estimated coefficient was 0.35, which indicated that male fish had significantly more total PCs compared with the females. There was, however, no significant difference between clinical and normal fish (p=0.45) in terms of total number of PCs/fish.
The presence of clinical disease was a significant predictor for the prevalence of myositis (p<0.01), as the odds of having myositis for fish submitted as routine cases were only 27.51% of the odds for fish that were submitted as clinical cases. There was strong evidence that sex and encephalitis were significant predictors for meninxitis and males tended to have more severe meninxitis than females (estimated coefficient 0.41, p<0.01), and fish with more severe encephalitis also tended to have more server meninxitis (estimated coefficient for encephalitis categories 1, 2, and 3 are 0.47, 1.51, and 2.41, respectively). However, clinical disease was not a significant predictor of either meninxitis or encephalitis/myelitis (p=0.4 and p=0.08, respectively. There were significantly more PCs observed in the white matter than in the gray matter of both the brain and spinal cord (p<0.01 for each). When combined, total PCs in the gray and white matter of the brain were significantly associated with the presence of other diseases (p=0.047).
Surprisingly, there were no statistically significant associations between total PC number and clinical disease (p=0.45); total parasite number in any specific anatomic location and clinical disease (p>0.05); and either meninxitis or encephalitis/myelitis and clinical disease (p>0.05 for each).
We also explored whether or not the submitting laboratory had an effect on infection patterns. Because there were so many submitting laboratories, we decided that it would be best to focus on the four laboratories submitting the most samples. Using 163 samples from these laboratories, we found that certain correlations that were previously not statistically significant became so. When accounting for submitting laboratory: total PCs in the brain were significantly associated with the presence of other diseases (p=0.047); total PCs in the nerve roots were significantly associated with myositis (p=0.01); total PCs in brain white matter were significantly associated with the presence of other disease (p=0.019); total PCs in spinal cord white matter was significantly associated with the presence of other disease (p=0.041); total PCs in the gray and white matter of the brain were highly correlated with the presence of other diseases (p=0.0091); total PCs in the white matter of the spinal cord were correlated with the presence of other diseases (p=0.041); and parasites in the combined gray and white matter of the spinal cord were strongly correlated with the presence of other disease (p=0.011).
Moreover, when submitting laboratory was taken into account, myositis was significantly correlated with the presence of other diseases (p=0.0033 compared with p=0.079 when laboratory was not taken into account). Following this trend, when laboratory was taken into account, myositis was no longer significantly correlated with clinical disease (p=0.89 compared with 0.0089 when submitting laboratory was ignored).
Discussion
Sanders et al.3 described the entry of P. neurophilia spores into the gut and their sequential distribution to viscera surrounding the gut followed by infection of the central nervous system. The initial distribution into the viscera adjacent to the gut is likely due to the launching of the polar tubule through the gut wall and into the surrounding viscera. Because it appears that this must occur before the appearance of parasites in the CNS, there is an unidentified step between the first occurrence of parasites in the viscera and their transport to the CNS because P. neurophilia has no capacity for motility beyond the use of the polar tubule and, as seen in this study and others, there is a very specific tropism for the CNS. Hematogenous spread is a potential mechanism. However, the consistent presence of PCs in nerve roots and white matter along with the fact that PCs are present within axonal swellings could suggest a simultaneous or alternative method of transport to the CNS; retrograde axonal transport.
Retrograde axonal transport is the movement of viruses, toxins, organelles, and proteins along microtubules in the axon toward the cell body via the action of molecular motors.19 Prions, the rabies virus, and herpesviruses utilize this method to spread from their points of entry in the peripheral tissues to the CNS. Currently, the only reported infectious moieties that move via axonal transport are viruses and prions; however, theoretically, the size of an object that can be transported via the axon is limited only by the diameter of the axon itself.19 Vesicles and organelles as large as mitochondria are regularly transported along the axon.20 Adult zebrafish axons (besides the Mauthner axon) are generally at least 7–8 μm in diameter and all developmental stages of P. neurophilia are considerably smaller than this,9 making axonal transport of at least certain stages of the parasite theoretically possible. The presence of PCs within the axoplasm rather than within the myelin sheath,9 along with the presence of multiple PCs in a single axon and the fairly specific tropism of the parasite for the commissura ventralis rhombencephali and MLF, both of which are continuous with the spinal cord ventral white matter, makes axonal transport highly plausible (Figs. 3 and 6). This provides an explanation of how an organism with no known method of motility besides its polar tubule would be able to infect such specific structures as the MLF and commissura ventralis rhombencephali, which happen to be white matter tracts.
If this were found to be true in the case of P. neurophilia, it would be the first documented case of any organism more complex than a virus utilizing retrograde axonal transport to move through the nervous system. Axonal transport of mitochondria is well documented,20 as is the close association between microsporidia and mitochondria. Microsporidia are obligate intracellular parasites and in certain species, including Encephalitozoon cuniculi, parasitophorous vacuoles of the meront stage bind to host mitochondria using a special transmembrane pore to siphon host ATP out of the organelle. Electron microscopy has revealed that the parasitophorous vacuoles of E. cuniculi are partially coated by host mitochondria.21 Because axonal transport of mitochondria is well documented and because microsporidia tend to associate closely with host mitochondria, axonal transport of mitochondria could be a method by which P. neurophilia “hitches a ride” down the axon if it cannot hijack axonal molecular motors on its own.
Intracellular PCs were occasionally observed within multiple vertebrae distending the cytoplasm of notochord remnant cells (Fig. 9). This pattern of intravertebral infection could be explained if spores, consumed when the fish are larvae (or even as embryos in the case of vertical transmission), launch their polar tubules and hit the notochord.3 This would allow for both the spread of the microsporidium throughout notochord cells and their retention within the notochord remnants of adjacent vertebrae in adult fish.
Satellitosis and neuronophagia of otherwise healthy-appearing neuron cell bodies could indicate that P. neurophilia antibodies are expressed in neuron cell bodies associated with infected axons. Observation of the parasite within the axonoplasm is corroborated by transmission electron microscopy of P. neurophilia that showed ultrastructurally that spores and pre-sporogonic stages were present within the axonoplasm.9 Inflammation of neuron cell bodies in the absence of directly observable pathogens in the cell body has some precedent in mammals: Human neurons express atypical MHC-I molecules in response to infection by rabies or herpesviruses, and rodent neurons in vitro can internalize and express foreign ovalbumin antigens via MHC-I complexes that leads to cytotoxic T-cell-mediated killing.22,23 Histopathologically, most of the inflammatory cells surrounding cell bodies in zebrafish with P. neurophilia resembled microglial cells rather than T cells (Figs. 4 and 5). Of course, it is impossible to definitively identify these cells without proper immunohistochemical labeling, which does not exist at this stage in the development of the zebrafish as a laboratory animal.
It is also possible that neuron cell bodies in these fish may be damaged in ways that are not observable via light microscopy and that this, rather than antigen expression, is the reason for the presumptive microglial attack of neuron cell bodies. Regardless of the specific mechanism, this piecemeal neuronal necrosis, satellitosis, and neuronophagia suggest that P. neurophilia infection of axons may have retrograde effects on neuron cell bodies. The caveat to this suggestion is that, during this retrospective study, there was no clear identification of a single inflamed or necrotic neuron cell body connected directly to a PC-containing axon.
Perhaps the existence of PCs in the axon protects the organisms from an immune reaction, providing an explanation for why PCs were often indirectly associated with inflammation and why a subset of fish evaluated had encephalitis/myelitis had scores of zero. Similarly, Loma salmonae, a microsporidium of salmon, infects the pillar cells and endothelium of gills, and elicits essentially no tissue reaction when within intact xenomas. Severe chronic inflammation associated with free spores generally occurs only after xenoma rupture.24 A similar phenomenon appears to occur with P. neurophilia—that is, inflammation is enhanced when PCs rupture their host axon, exposing organisms to the immune system.
Interestingly, some of the largest PCs observed in this study were associated with minimal to no inflammation (Fig. 8). Perhaps PCs may be able distend the axon to astounding dimensions without rupturing them, as seen with xenomas formed by other microsporidian species. If axon rupture is indeed the cause of inflamed PCs, the cause of rupture is unclear, particularly because size itself does not seem to be a factor. A possible explanation for this could be the rate of PC growth within the axon: Slow growth may allow for progressive stretching of the axon, but if PCs expand too quickly, the axon may be unable to compensate and may rupture.
About 50% of the fish exhibited inflammation associated with the membranes surrounding the brain and spinal cord. Teleosts generally do not have a distinct arachnoid, pia, or dura mater.25 Cyprinids have an ectomeninx, composed of mucinous tissue, collagen fibers, and blood vessels, that is most comparable to the mammalian dura mater. Directly opposed to the brain is the endomeninx, composed of an outer, intermediate, and inner layer, which is comparable to the leptomeninges in mammals.25 Between the ectomeninx and the endomeninx is a layer of fatty tissue called the perimenixial space.26 Meninxitis associated with P. neurophilia infection in this study was primarily granulocytic, and it was located largely within the perimeninxial space. The pattern of meninxitis in these fish was strikingly different from that generally observed in mammals with meningitis.27 In mammals, due the fact that the pia mater follows blood vessels deep into the neuropil, leptomeningitis usually results in a perivascular pattern of inflammation with the expansion of the leptomeninges by inflammatory cells.28
Myositis and extraneural infections
Once outside of the ectomeninx, inflammation associated with P. neurophilia infections was generally unrestricted. Frequently, it produced perivertebral myositis and, in some cases, interstitial nephritis. Myositis, however, is not pathognomic for P. neurophilia, as only 13% of infected fish had this lesion. As reported by Matthews et al.4 in the first histopathologic description of P. neurophilia, myositis was associated with clinical disease: Fish with clinical disease were twice more likely to have this lesion than apparently healthy fish in our study. It may be that the presence of myositis makes it more likely for fish to become visibly emaciated due to a decreased ability to find and acquire food. It may also predispose these fish to grossly visible vertebral abnormalities, due to early infection of the notochord or due to the chronic effects of differential muscle tension on vertebrae. Ramsay et al.11 showed that zebrafish fish under subjected to stressors and with increased cortisol had an increased incidence of P. neurophilia-associated myositis. Interestingly, many cases of granulocytic myositis contained extracellular spores free within the inflammation. It is possible that these incidents of myositis are largely due to the rupture of peripheral nerves and release of spores outside of the plane of section with spread into the surrounding muscle (Fig. 10).
When the submitting laboratory was ignored, we found a positive correlation between the presence of diseases other than microsporidiosis and myositis. The presence of these other diseases could increase the severity of P. neurophilia infection due to stress and subsequent immunosuppression.11 However, other infections may cause these lesions in the absence of P. neurophilia. Infections with Mycobacterium spp. are the second most common disease diagnosed in laboratory zebrafish,29 and mycobacteriosis may cause myositis. Conversely, taking into account the submitting laboratory, it appeared that multiple factors, including the parasite number in several anatomic locations, became significantly correlated with the presence of other diseases. Myositis was significantly correlated with the presence of other diseases, but not with clinical disease when submitting laboratory was taken into account. One explanation for this could be the following: If one of the laboratories under study submitted a large number of fish with mycobacteriosis that were co-infected with P. neurophilia, we would likely see a strong association between myositis and the presence of other diseases (mycobacteriosis).
The fact that myositis was no longer correlated with the presence of clinical disease caused by P. neurophilia when the submitting laboratory was taken into account illustrates one of the most difficult aspects of working with a large number of facilities when constructing a retrospective study: Not all labs are created equal, and even when they are, variations are bound to occur over time. Most zebrafish facilities are similar in that they maintain their fish at about 28°C in large, recirculating systems and use similar breeding methods.30 The zebrafish as a laboratory animal is still in its infancy relative to mice, and although standards of care exist, there is marked variation between individual labs in terms of stocking densities and individual system dynamics. With the infinite variations in genetic knockouts and the wide variety of strains available, the interaction between host genetics and the parasite may be very different between individual laboratories. In addition, pathogen profiles vary dramatically between laboratories, as researchers use fish with a wide range of pathogen status and history, from pet store fish reared in ponds and exposed to other fishes, to fish from ZIRC with documented disease histories, to SPF fish from Oregon State University.1 Compounding this issue is the fact that only a small fraction of zebrafish research facilities submit moribund fish for pathogen diagnoses,30 and thus their pathogen profiles are unknown. As we have seen in this study, the interaction between microsporidiosis and other co-infecting pathogens can dramatically influence the health of the animal and the incidence of other diseases varies between submitting laboratories.
Perhaps even P. neurophilia strains may differ between laboratories. The rapid reproductive cycle of both zebrafish and the parasite, along with different modes of transmission of the parasite (e.g., vertical and horizontal), could drive the rapid evolution of P. neurophilia toward more or less virulent strains. Each laboratory is its own ecosystem and each tank is its own microenvironment, both of which could nudge the parasite into various evolutionary directions. These same conditions and the development of individual laboratories along with intermittent changes in housing conditions could also provide an explanation for the statistically significant effects of submission year on infection patterns.
Inflammation patterns and clinical disease
The driving forces behind inflammation severity, parasite load, and easily observable clinical disease are as of yet unclear. While we may suggest that variability in these factors may be due to differences in stress and subsequent immunosuppression in individual fish, there may be more to the story such as differential behavioral predelictions to increased parasite intake (increased carcass consumption) or heritable differences in resistance or susceptibility. Further complicating the issue is that, while increased cortisol levels and increased parasite load can be experimentally correlated with weight loss and decreased fecundity,11 in this particular study, there was no statistical correlation between particular features of infection and the reporting of clinical disease by submitting personnel. This discrepancy highlights the need for further study of specific study of subtle changes produced by a chronic, seemingly subclinical parasite.
Binomial pattern
A well-recognized paradigm in parasitology is that macroparasites, such as nematodes, in wild animals generally exhibit a negative binomial distribution, with relatively few hosts harboring most parasites.31,32 This type of distribution extends to fish as well as to domestic terrestrial hosts,33 and we actually recently demonstrated this phenomenon with Pseudocapillaria tomentosa in zebrafish that were experimentally infected in the laboratory.34 Interestingly, P. neurophilia, which would be considered a “microparasite” as it replicates in its host, also showed a binomial distribution of infection in our study. This resulted in an overdispersion of infection severity, in which relatively few fish had very large numbers of PCs and most fish had relatively low numbers.
Sex
Sex may also influence infection patterns. Chow et al.,8 using essentially the same data set as was used in this study, reported that males had a higher prevalence of infection by P. neurophilia compared with females, in both clinical and routinely submitted fish. Here, we expand these observations, showing that males also tend to have more total PCs than females. Males also tended to have more severe encephalitis/myelitis and meninxitis than females. Zebrafish in aquaria establish hierarchies, which are more pronounced in males. Filby et al.35 and Spence and Smith36 showed that subordinate males exhibited a greater rise in cortisol than subordinate females. Thus, Chow et al.8 suggested that a possible reason for the increased likelihood of clinical disease in males could be increased stress due to hierarchical inter-fish competition correlating with elevated cortisol levels resulting in more severe infections. Another possible explanation is that sexual differences in hormones may differentially influence the immune system and responses to stress.
Behavior
Over the past three decades, the zebrafish has become an important animal model for behavioral research.27,37–40 The potential influence of P. neurophilia on these studies should be considered, given that it infects the CNS and is widespread in research facilities. Both the Mauthner axon and reticular formation are important for the functioning of the startle response in zebrafish.27,37–41 It is possible that the infection may adversely affect behavioral studies that rely on the startle response, including the tap test, due to the frequent presence of P. neurophilia spores in the MLF proximal to and compressing the Mauthner axon, and the frequent infection and associated inflammation of reticular formation cell bodies. This is particularly plausible, because most experiments studying anxiety or the startle response use motor reflexes and movement as an experimental endpoint.27,37–40 Because P. neurophilia infects nerve roots and the ventral spinal cord (both containing motor neurons) and because infections can be associated with myositis and muscle atrophy, there could be impairments at every step of the startle response.40
The GC receives input from the medial habenula, which has been implicated in anxiety and habituation in relation to stress.41 The frequent presence of PCs in this structure could, therefore, have effects on experiments that interrogate anxiety and habituation, which are frequently examined in zebrafish behavioral studies.13,15,41 In fact, even studies that attempt to evaluate higher functions, such as learning and comprehension, utilize noxious stimuli and habituation in response to stress, both of which could be affected by neural microsporidiosis.13,15,41
Myxobolus arcticus infections in the hindbrain have been associated with decreased swimming speed of naturally infected salmon.42 Because this myxozoan parasite exhibits a similar pattern of infection as P. neurophilia (mostly in the hind brain, often with no inflammation), this suggests that the latter infection could affect experiments with zebrafish that involve swimming or other motor functions. Another Myxobolus species, M. balantiocheili of tricolor sharks (Balantiocheilos melanopterus), causes prominent CNS infections that extend into the meninxes. Although it was not reported to cause significant inflammation, it was associated with uncoordinated darting, rolling and pitching.43
Infections of the central nervous system by various pathogens that do not elicit significant histopathologic lesions probably have effects beyond their presence as mere space occupying lesions. These parasites are living organisms, and there is active chemical “cross talk” between the host and parasite. For example, chronic infections with Toxoplasma gondii cysts in the brain have been associated with unusual and profound behavior changes infected rats.44,45 Although T. gondii is an apicomplexan and P. neurophilia is a microsporidium, the parasites share some similarities: Both are spread via the fecal-oral route with the eventual spread of the parasite from the gut to the central nervous system. Similar to P. neurophilia, T. gondii has a noninflammatory stage in tissues (encysted bradyzoites).
In conclusion, our retrospective study confirmed that many apparently healthy zebrafish from research facilities have significant P. neurophilia infections at a histological level, particularly in the CNS. Close examination of numerous histological slides revealed evidence for axonal transport of the parasite throughout the nervous system. The anatomic locations of parasite-associated lesions indicates that the use of such infected fish may adversely affect in vivo experiments with zebrafish beyond merely inducing morbidity, emaciation, and reducing fecundity. Experiments are underway in our laboratory to elucidate the influence of the infection on certain behavioral endpoints.
Acknowledgments
This research was funded in part by NIH grants T32 RR023917: Training of veterinarians in aquatic animal research and 2R24OD010998-11 to Michael L. Kent.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kent ML, Buchner C, Watral VG, Sanders JL, LaDu J, Peterson TS, et al. Development and maintenance of a specific pathogen free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Org 2011;95:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders GE. Zebrafish housing, husbandry, health and care: IACUC considerations. ILAR J 2012;53:205–207 [DOI] [PubMed] [Google Scholar]
- 3.Sanders J, Peterson T, Kent M. Early development and tissue distribution of Pseudoloma neurophilia in the zebrafish, Danio rerio. J Eukaryot Microbiol 2014;61:238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews JL, Brown AM, Larison K, Bishop-Steward JK, Rogers P, Kent ML. Pseudoloma neurophilia n.g., n. sp., a new microsporidium from the central nervous system in the zebrafish (Danio rerio). J Eukaryot Microbiol 2001;48:227–233 [DOI] [PubMed] [Google Scholar]
- 5.Murray KN, Dreska M, Nasiadka A, Rinne M, Matthews JL, Carmichael C, et al. Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities. Comp Med 2011;61:322–329 [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders J, Watral V, Clarkson K, Kent ML. Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the zebrafish, Danio rerio. PLoS One 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent ML, Spitsbergen JM, Matthews JM, Fournie JW, Murray KN, Westerfield M. Diseases of Zebrafish in Research facilities. ZIRC Health Services Zebrafish Disease Manual 2012. Available at http://zebrafish.org/zirc/health/diseaseManual.php
- 8.Chow FW, Xue L, Kent ML. Retrospective study of the prevalence of Pseudoloma neurophilia shows male gender bias in zebrafish Danio rerio (Hamilton-Buchanan). J Fish Dis. 2015. [Epub ahead of print]; DOI: 10.1111/jfd.12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cali A, Kent M, Sanders J, Pau C, Takvorian PM. Development, ultrastructural pathology, and taxonomic revision of the Microsporidial genus, Pseudoloma and its type species Pseudoloma neurophilia, in skeletal muscle and nervous tissue of experimentally infected zebrafish Danio rerio. J Eukaryot Microbiol 2012;59:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lom J, Dykovà I. Microsporidian xenomas in fish seen in wider perspective. Folia Parasitol (Praha) 2005;69–81 [PubMed] [Google Scholar]
- 11.Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia infections in zebrafish Danio rerio: effects of stress on survival, growth, and reproduction. Dis Aquat Organ 2009;88:69–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris JA. Zebrafish: a model system to examine the neurodevelopmental basis of schizophrenia. Prog Brain Res 2009;179:97–106 [DOI] [PubMed] [Google Scholar]
- 13.Pittman JT, Lott CS. Startle response memory and hippocampal changes in adult zebrafish pharmacologically-induced to exhibit anxiety/depression-like behaviors. Physiol Behav 2014;123:174–179 [DOI] [PubMed] [Google Scholar]
- 14.Stewart A, Wong K, Cachat J, Gaikwad S, Kyzar E, Wu N, et al. Zebrafish models to study drug abuse-related phenotypes. Rev Neurosci 2011;22:95–105 [DOI] [PubMed] [Google Scholar]
- 15.Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV. Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacol 2012;62:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart AM, Nguyen M, Wong K, Poudel KW, Kalueff AV. Developing zebrafish models of autism spectrum disorder (ASD). Prog Neuropsychopharmacol Biol Psychiatry 2014;50:27–36 [DOI] [PubMed] [Google Scholar]
- 17.Willemsen R, Hasselaar W, van der Linde H, Bonifati V. Zebrafish as a new model organism for Parkinson's disease. Proc Measuring Behav 2008;50–51 [Google Scholar]
- 18.Peterson TS, Spitsbergen JM, Feist SW, Kent ML. Luna stain, an improved selective stain for detection of microsporidian spores in histologic sections. Dis Aquat Organ 2001;95:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaVail JH, Tauscher AN, Aghaian E, Harrabi O, Sidhu SS. Axonal transport and sorting of herpes simplex virus components in a mature mouse visual system. J Virol 2003;77:6117–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salinas S, Schiavo G, Kremer EJ. A hitchiker's guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol 2010;9:645–655 [DOI] [PubMed] [Google Scholar]
- 21.Hacker C, Howell M, Bhella D, Lucocq J. Strategies for maximizing ATP supply in the microsporidian Encephalitozoon cuniculi: direct binding of mitochondria to the parasitophorous vacuole and clustering of the mitochondrial porin VDAC. Cell Microbiol 2014;16:565–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cebrián C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, et al. MHC-I expression renders catecholaminergic neurons susceptible to T-cell mediated degeneration. Nat Commun 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mégret F, Prehaud C, Lafage M, Moreau P, Rouas-Freiss N, Carosella ED, et al. Modulation of HLA-G and HLA-E expression in human neuronal cells after rabies virus or herpes virus simplex type 1 infections. Hum Immunol 2007;68:294–302 [DOI] [PubMed] [Google Scholar]
- 24.Kent ML, Dawe SC, Speare DJ. Resistance to reinfection in chinook salmon Oncorhynchus tshawytscha to Loma salmonae (Microporidia). 1999;37:205–208 [DOI] [PubMed] [Google Scholar]
- 25.Caruncho HJ. and Pinto Da Silva P. Alterations in the intermediate layer of goldfish meninges during adaptation to darkness. J Anat 1994;184:355–362 [PMC free article] [PubMed] [Google Scholar]
- 26.Ariens Kappers CU. The meninges in Cyclostomes, Selachians, and Teleosts, compared with those in man. Proc R Acad Amst 1924;28:71–80 [Google Scholar]
- 27.Hatta K, Korn H. Physiological properties of the Mauthner system in the adult zebrafish. J Comp Neurol 1998;395:493–509 [PubMed] [Google Scholar]
- 28.Maxie MG, Youssef S: Nervous system. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals Volume 1 Maxie MG. (ed), pp. 281–298, Elsevier Limited, Philadelphia, PA, 2007 [Google Scholar]
- 29.Whipps CM, Lieggie C, Wagner R. Mycobacteriosis in zebrafish colonies. ILAR J 2012;53:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence C, Ennis DG, Harper C, Kent ML, Murray K, Sanders GE. The challenges of implementing pathogen control strategies for fishes used in biomedical research. Comp Biochem Physiol C Toxicol Pharmacol 2012;155:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crofton HD. A model of host-parasite relationships. Parasitology 1971;63:343–364 [DOI] [PubMed] [Google Scholar]
- 32.Poulin R. Explaining variability in parasite aggregation levels among host samples. Parasitology 2013;140:541–546 [DOI] [PubMed] [Google Scholar]
- 33.Lester R. A review of methods for estimating mortality due to parasites in wild fish populations. Helgol Mar Res 1984;37:53–64 [Google Scholar]
- 34.Collymore C, Watral V, White JR, Colvin ME, Rasmussen S, Tolwani RJ, et al. Tolerance and efficacy of Emamectin benzoate and Ivermectin for the treatment of Pseudocapillaria tomentosa in laboratory zebrafish (Danio rerio). Zebrafish 2014;11:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Filby AL, Paull GC, Bartlet EJ, Van Look KJ, Tyler CR. Physiological and health consequences of social status in zebrafish (Danio rerio). Physiol Behav 2010;101:576–587 [DOI] [PubMed] [Google Scholar]
- 36.Spence R, Smith C. Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behav Ecol 2006;17:779–783 [Google Scholar]
- 37.Eaton RC, Bombardieri RA, Meyer DL. The Mauthner-initiated startle response in teleost fish. J Exp Biol 1977;66:65–81 [DOI] [PubMed] [Google Scholar]
- 38.Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron 1999;23:325–335 [DOI] [PubMed] [Google Scholar]
- 39.Liu DW, Westerfield M. Function of identified motoneurones and coordination of primary and secondary motor systems during zebrafish swimming. J Physiol 1988;403:73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zottoli SJ, Newma BC, Rieff HI, Winters DC. Decrease in occurrence of fast startle responses after selective Mauthner cell ablation in goldfish (Carassius auratus). J Comp Physiol A 1999;184:207–218 [DOI] [PubMed] [Google Scholar]
- 41.Mathuru AS, Jesuthusasan S. The medial habenula as a regulator of anxiety in adult zebrafish. Front Neural Circuits 2013;7:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moles A, Heifetz J. Effects of the brain parasite Myxobolus arcticus on sockeye salmon. J Fish Biol 1998;52:146–151 [Google Scholar]
- 43.Levsen A, Alvik T, Grotmol S. Neurological symptoms in tricolor sharkminnow Balantiocheilos melanopterus associated with Myxobolus balantiocheili n. sp. infecting the central nervous system. Dis Aquat Org 2004;59:135–140 [DOI] [PubMed] [Google Scholar]
- 44.Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci 2000;267:1591–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol 1998;28:1019–1024 [DOI] [PubMed] [Google Scholar]