Abstract
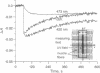
We report a method that allows us to determine the diffusion coefficient of native myoglobin in intact and mechanically unaffected red muscle fibers. The method is based on an optical recording of intracellular diffusion of metmyoglobin, which is produced inside the cells by photooxidation of oxymyoglobin with a UV light pulse. We find a myoglobin diffusivity of 1.2 x 10(-7) cm2/s (22 degrees C), which is only 1/10th of the value measured in very dilute myoglobin solutions and 1/5th of the value obtained from measurements in solutions of myoglobin at 18 g/dl. The latter value often has been used in model calculations of oxygen transport to tissue incorporating myoglobin-facilitated oxygen diffusion. Recalculating facilitated diffusion with the value obtained by us implies that its contribution to total intracellular oxygen transport is of minor importance. Furthermore, it shows that sterical hindrance to myoglobin diffusion is dominated by the muscle-cell architecture rather than by the overall protein concentration of the muscle fiber.
Full text
PDF
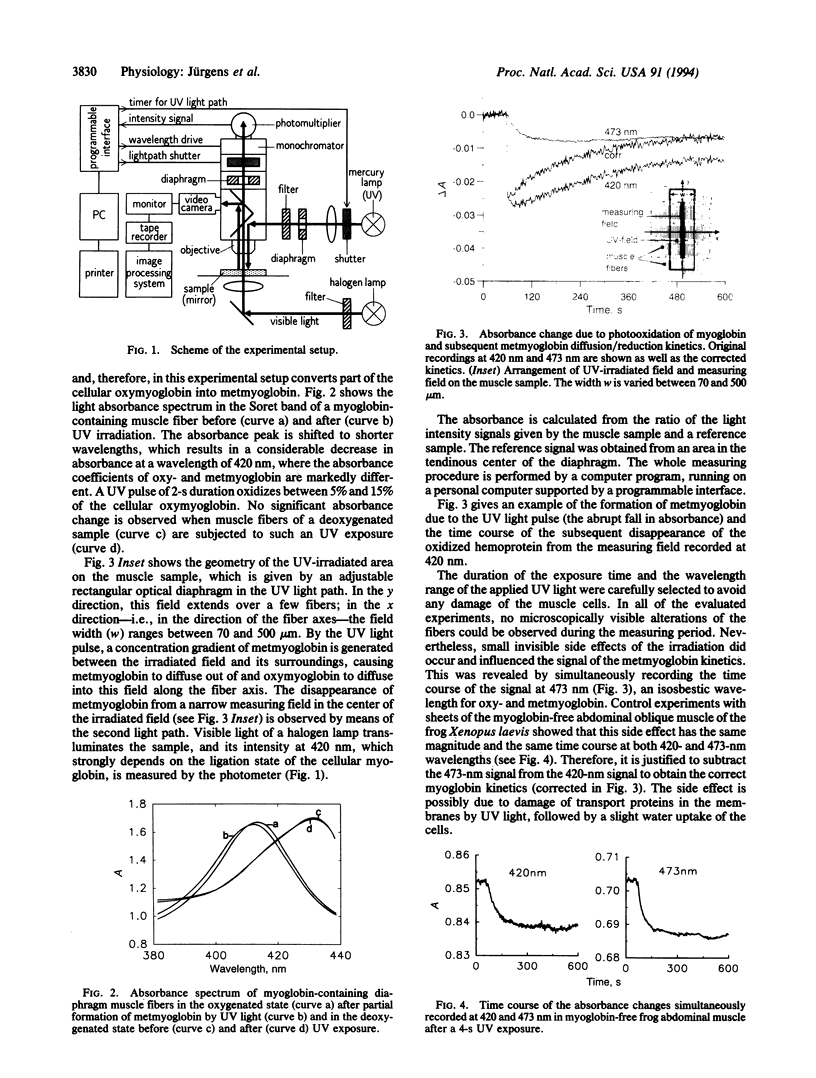
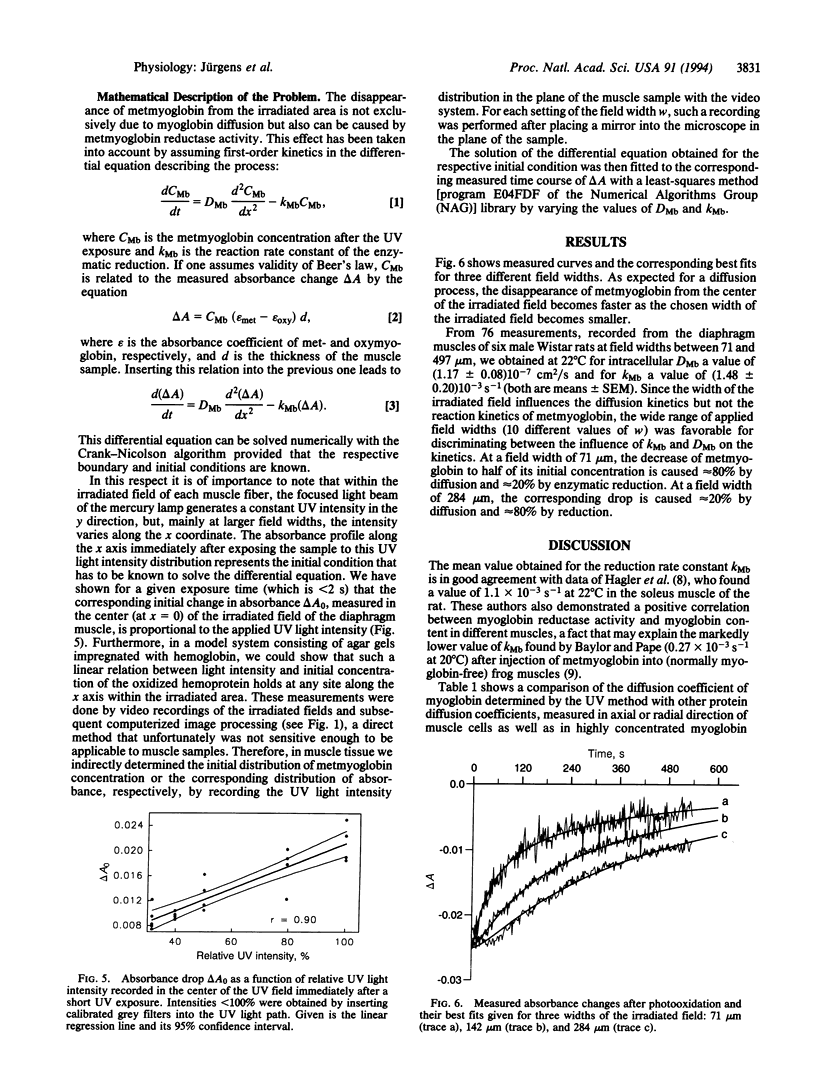
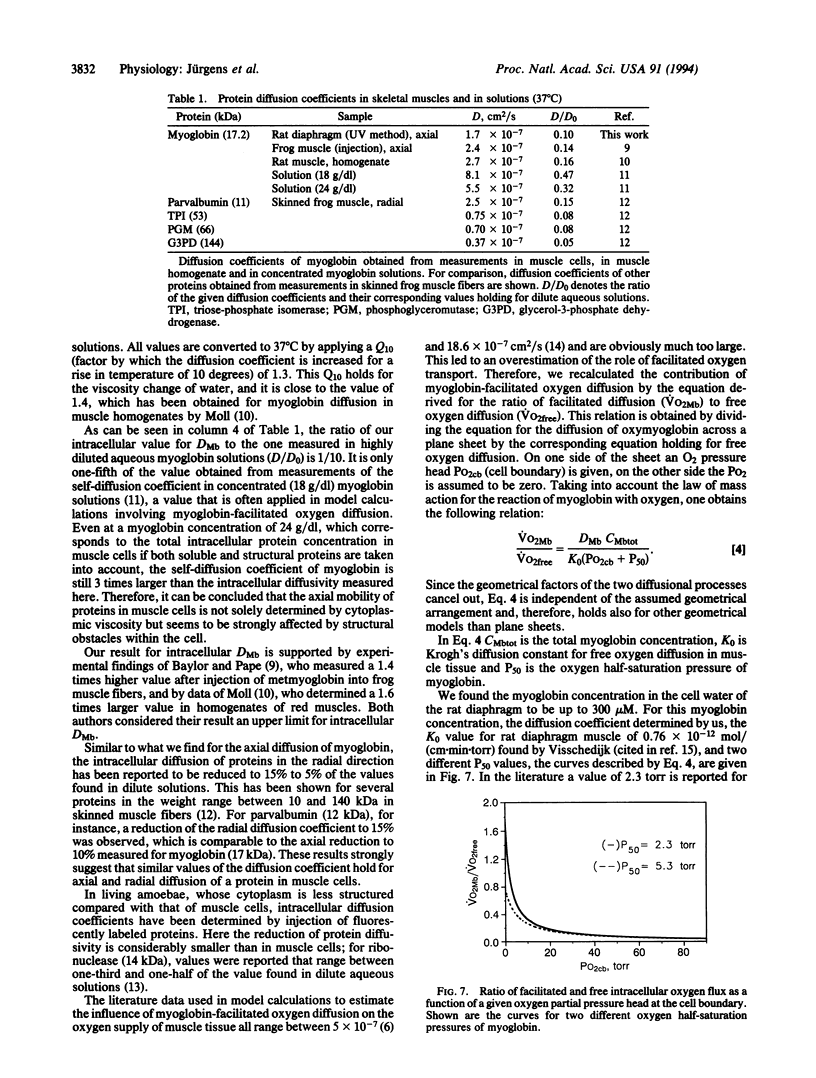

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Pape P. C. Measurement of myoglobin diffusivity in the myoplasm of frog skeletal muscle fibres. J Physiol. 1988 Dec;406:247–275. doi: 10.1113/jphysiol.1988.sp017379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley T. B., Meng H., Pittman R. N. Temperature dependence of oxygen diffusion and consumption in mammalian striated muscle. Am J Physiol. 1993 Jun;264(6 Pt 2):H1825–H1830. doi: 10.1152/ajpheart.1993.264.6.H1825. [DOI] [PubMed] [Google Scholar]
- Bylund-Fellenius A. C., Walker P. M., Elander A., Holm S., Holm J., Scherstén T. Energy metabolism in relation to oxygen partial pressure in human skeletal muscle during exercise. Biochem J. 1981 Nov 15;200(2):247–255. doi: 10.1042/bj2000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demma L. S., Salhany J. M. Direct generation of superoxide anions by flash photolysis of human oxyhemoglobin. J Biol Chem. 1977 Feb 25;252(4):1226–1230. [PubMed] [Google Scholar]
- Doeller J. E., Wittenberg B. A. Myoglobin function and energy metabolism of isolated cardiac myocytes: effect of sodium nitrite. Am J Physiol. 1991 Jul;261(1 Pt 2):H53–H62. doi: 10.1152/ajpheart.1991.261.1.H53. [DOI] [PubMed] [Google Scholar]
- Gayeski T. E., Honig C. R. Intracellular PO2 in long axis of individual fibers in working dog gracilis muscle. Am J Physiol. 1988 Jun;254(6 Pt 2):H1179–H1186. doi: 10.1152/ajpheart.1988.254.6.H1179. [DOI] [PubMed] [Google Scholar]
- Groebe K., Thews G. Calculated intra- and extracellular PO2 gradients in heavily working red muscle. Am J Physiol. 1990 Jul;259(1 Pt 2):H84–H92. doi: 10.1152/ajpheart.1990.259.1.H84. [DOI] [PubMed] [Google Scholar]
- Hagler L., Coppes R. I., Jr, Askew E. W., Hecker A. L., Herman R. H. The influence of exercise and diet on myoglobin and metmyoglobin reductase in the rat. J Lab Clin Med. 1980 Feb;95(2):222–230. [PubMed] [Google Scholar]
- Hartling O. J., Kelbaek H., Gjørup T., Schibye B., Klausen K., Trap-Jensen J. Forearm oxygen uptake during maximal forearm dynamic exercise. Eur J Appl Physiol Occup Physiol. 1989;58(5):466–470. doi: 10.1007/BF02330698. [DOI] [PubMed] [Google Scholar]
- Jones D. P., Kennedy F. G. Intracellular O2 gradients in cardiac myocytes. Lack of a role for myoglobin in facilitation of intracellular O2 diffusion. Biochem Biophys Res Commun. 1982 Mar 30;105(2):419–424. doi: 10.1016/0006-291x(82)91450-4. [DOI] [PubMed] [Google Scholar]
- Kawashiro T., Nüsse W., Scheid P. Determination of diffusivity of oxygen and carbon dioxide in respiring tissue: results in rat skeletal muscle. Pflugers Arch. 1975 Sep 9;359(3):231–251. doi: 10.1007/BF00587382. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S. The effect of myoglobin-facilitated oxygen transport on the basal metabolism of papillary muscle. Biophys J. 1987 Jun;51(6):905–913. doi: 10.1016/S0006-3495(87)83418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan D., Lord C. Protein diffusivities in skinned frog skeletal muscle fibers. Adv Exp Med Biol. 1988;226:75–84. [PubMed] [Google Scholar]
- Moll W. The diffusion coefficient of myoglobin in muscle homogenate. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299(3):247–251. doi: 10.1007/BF00362587. [DOI] [PubMed] [Google Scholar]
- Riveros-Moreno V., Wittenberg J. B. The self-diffusion coefficients of myoglobin and hemoglobin in concentrated solutions. J Biol Chem. 1972 Feb 10;247(3):895–901. [PubMed] [Google Scholar]
- Wang Y. L., Lanni F., McNeil P. L., Ware B. R., Taylor D. L. Mobility of cytoplasmic and membrane-associated actin in living cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4660–4664. doi: 10.1073/pnas.79.15.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B., Caldwell P. R. Role of myoglobin in the oxygen supply to red skeletal muscle. J Biol Chem. 1975 Dec 10;250(23):9038–9043. [PubMed] [Google Scholar]
- Wittenberg J. B. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970 Oct;50(4):559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]