Abstract
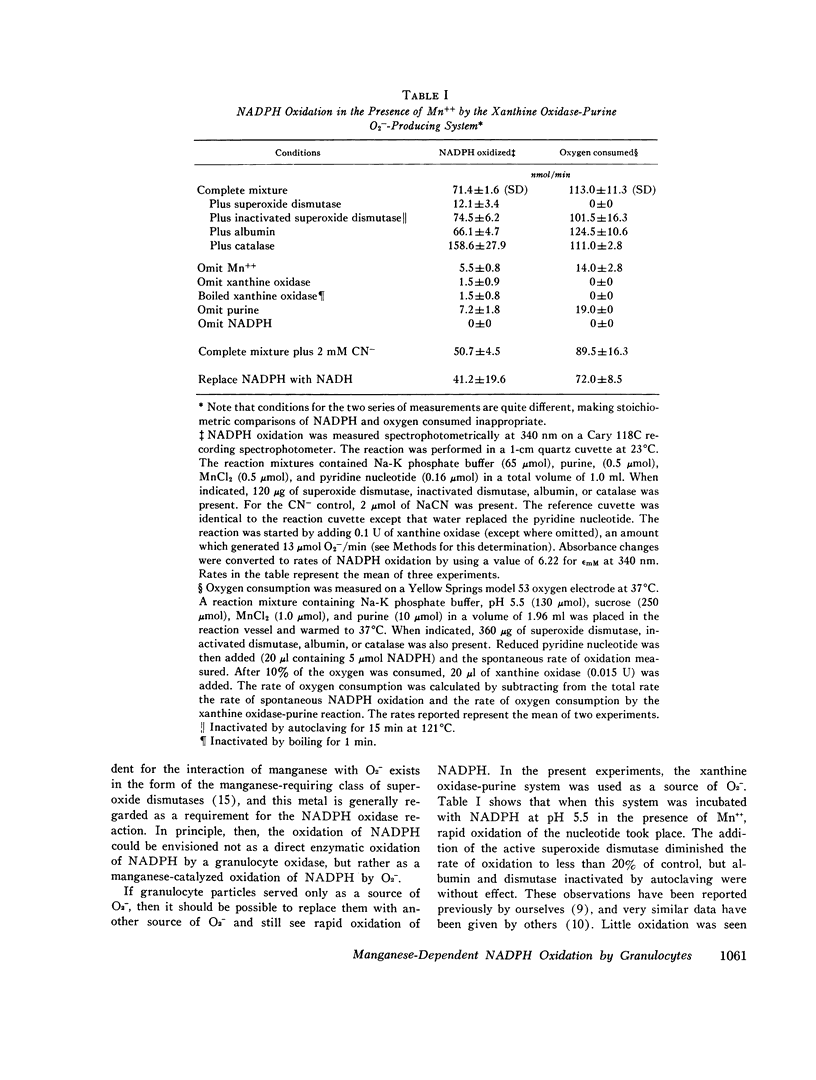
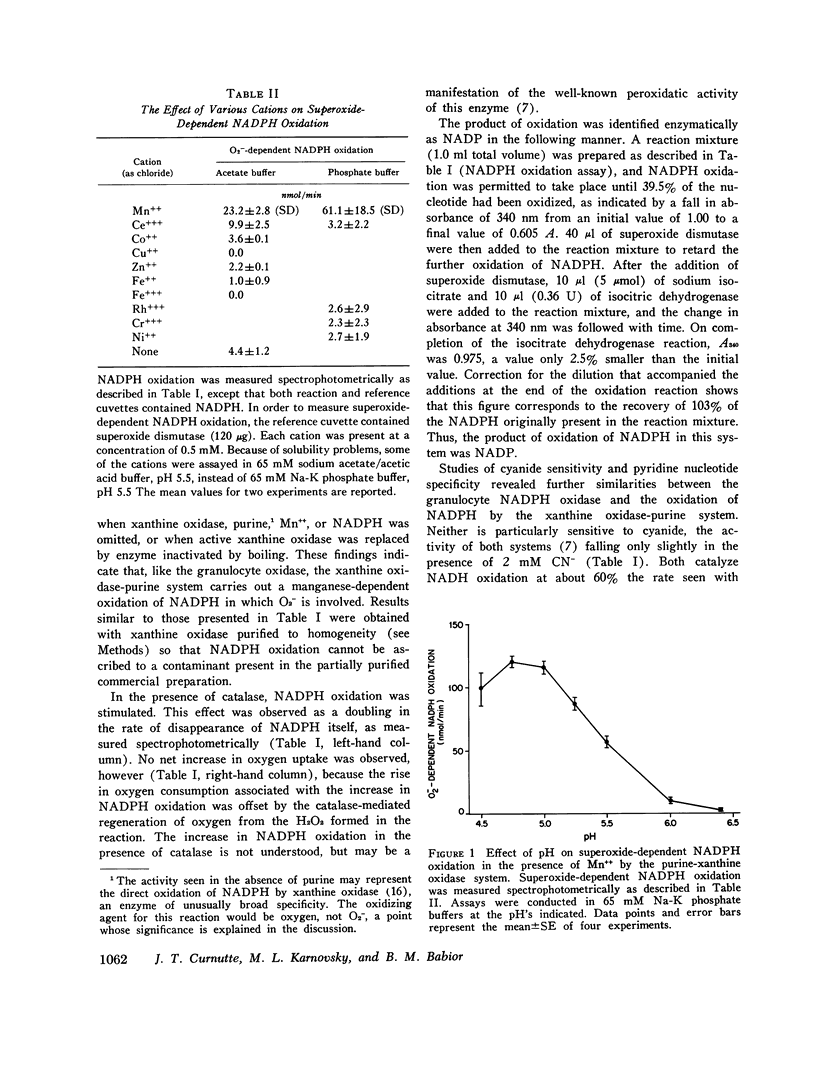
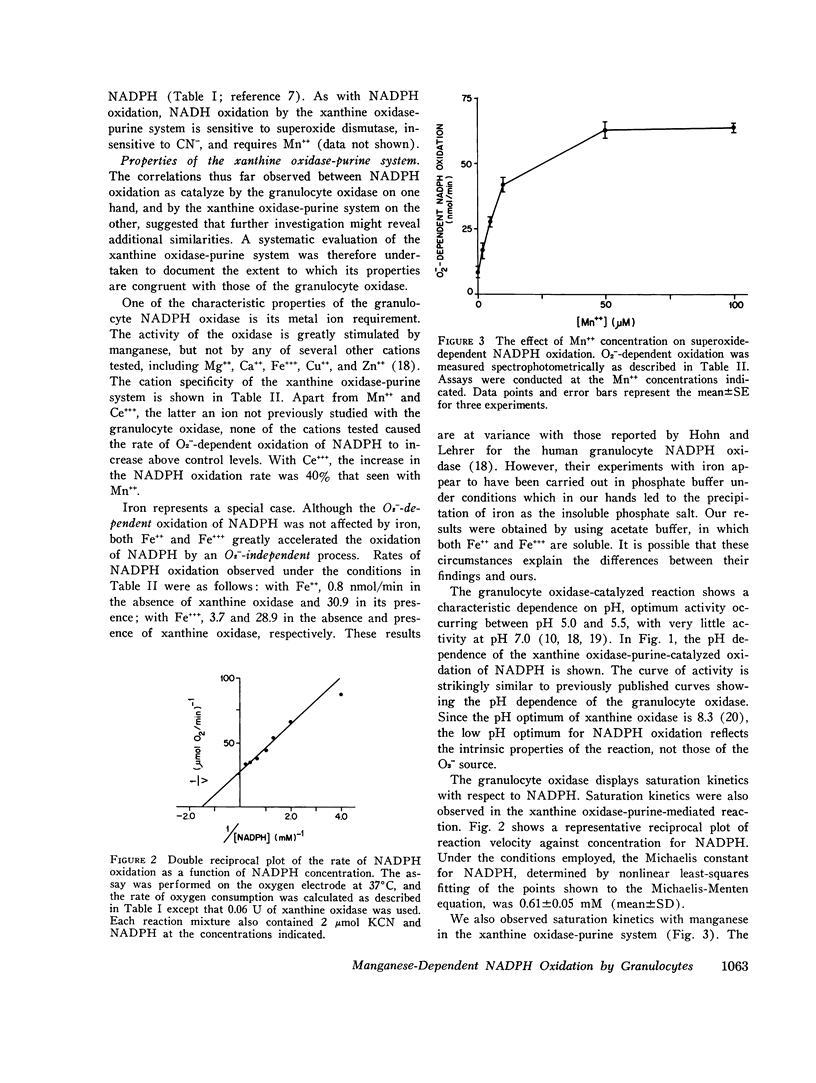
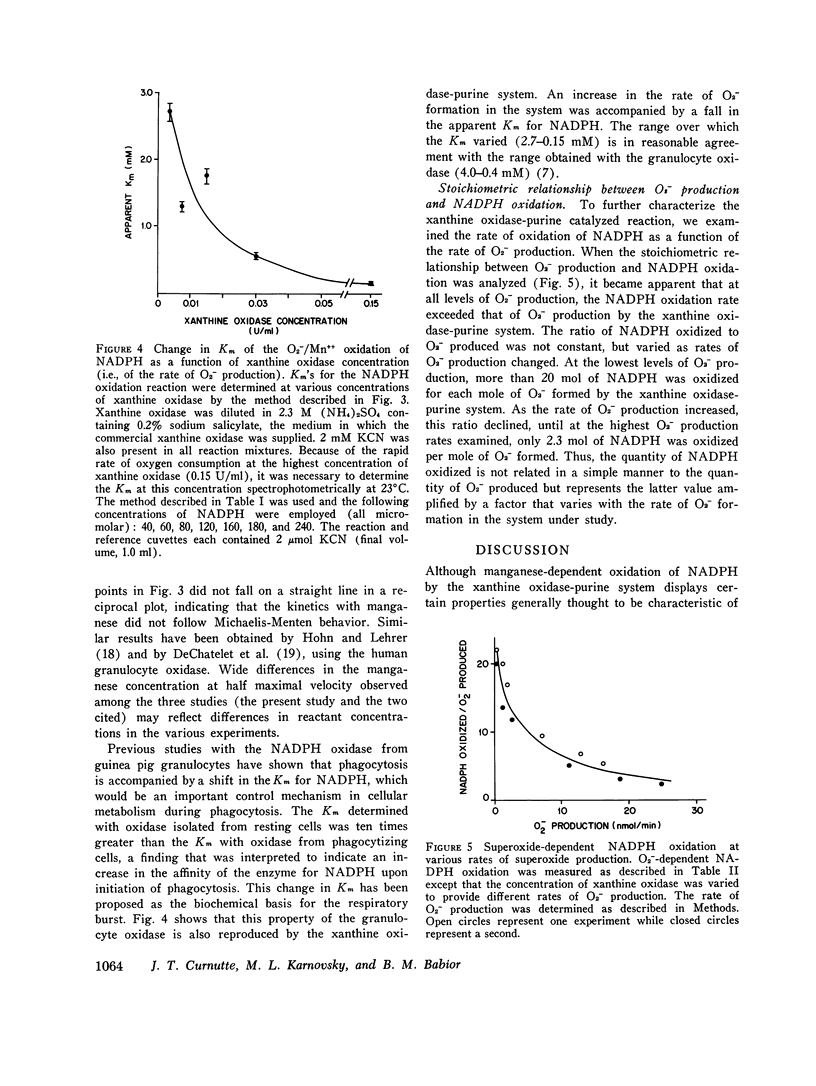
Recent work has indicated that superoxide is involved in the manganese-stimulated oxidation of NADPH by crude granule preparations of guinea pig neutrophils. The characteristics of a model manganese-requiring NADPH-oxidizing system that employs a defined O2-generator have now been compared to the original neutrophil-granule system. With respect to pH dependence, cyanide sensitivity, and reduced pyridine nucleotide specificity, the properties of the two systems are very similar. Additional information has been obtained concerning cation specificity and the kinetics of the metal-catalyzed NADPH oxidation. From the similarities between the properties of the model and neutrophil particle systems, we postulate that the manganese-dependent NADPH oxidation observed in the presence of neutrophil granules represents in large part of nonenzymatic free radical chain involving the oxidation of NADPH to NADP, with O2- as both the chain initiator and one of the propagating species. In this reaction, the neutrophil particles serve only as a source of O2-. Further, the same changes in kinetics (decrease in apparent Km for NADPH) observed previously when granules from phagocytizing rather than resting cells were employed could be mimicked by varying the rate of O2-generation by the model system. We conclude from these results that it is unnecessary to invoke a manganese-requiring enzyme as a component of the phagocytically stimulated respiratory system of the neutrophil.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielski B. H., Chan P. C. Kinetic study by pulse radiolysis of the lactate dehydrogenase-catalyzed chain oxidation of nicotinamide adenine dinucleotide by HO2 and O2-RADICALS. J Biol Chem. 1975 Jan 10;250(1):318–321. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., McPhail L. C., Mullikin D., McCall C. E. An isotopic assay for NADPH oxidase activity and some characteristics of the enzyme from human polymorphonuclear leukocytes. J Clin Invest. 1975 Apr;55(4):714–721. doi: 10.1172/JCI107981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- GREEN D. E., BEINERT H. Xanthine oxidase, a molybdo-flavoprotein. Biochim Biophys Acta. 1953 Aug;11(4):599–600. doi: 10.1016/0006-3002(53)90111-5. [DOI] [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER G. Y., QUESTEL J. H. NADPH and NADH oxidation by guinea pig polymorphonuclear leucocytes. Can J Biochem Physiol. 1963 Feb;41:427–434. [PubMed] [Google Scholar]
- Johnson J. L., Waud W. R., Cohen H. J., Rajagopalan K. V. Molecular basis of the biological function of molybdenum. Molybdenum-free xanthine oxidase from livers of tungsten-treated rats. J Biol Chem. 1974 Aug 25;249(16):5056–5061. [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Intraphagosomal pH of human polymorphonuclear neutrophils. Proc Soc Exp Biol Med. 1970 Jun;134(2):447–449. doi: 10.3181/00379727-134-34810. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Dri P., Kakinuma K., Tedesco F., Rossi F. Studies on the mechanism of metabolic stimulation in polymorphonuclear leucocytes during phagocytosis. I. Evidence for superoxide anion involvement in the oxidation of NADPH2. Biochim Biophys Acta. 1975 Apr 7;385(2):380–386. doi: 10.1016/0304-4165(75)90367-0. [DOI] [PubMed] [Google Scholar]
- RICHERT D. A., WESTERFELD W. W. The relationship of iron to xanthine oxidase. J Biol Chem. 1954 Jul;209(1):179–189. [PubMed] [Google Scholar]
- Rossi F., Zatti M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia. 1964 Jan 15;20(1):21–23. doi: 10.1007/BF02146019. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]



