The abilities of intracavernous injection of autologous stromal vascular fraction (SVF) and adipose-derived stem cells (ADSCs) to facilitate recovery of erectile function in a rat model of cavernous nerve injury were compared. Intracavernous injection of SVF significantly improved erectile function compared with that in the control group, whereas the ADSC group showed marginally significant improvement. The increases in the smooth muscle/collagen ratio and von Willebrand factor expression were larger in the SVF group than in the ADSC group. Both cell types are equally effective in recovering penile erection, but SVF may be superior to ADSCs in terms of histomorphometric changes.
Keywords: Adipose, Culture, Endothelial cell, Neuropathy, Rat model, Smooth muscle cells
Abstract
The abilities of intracavernous injection of autologous stromal vascular fraction (SVF) and adipose-derived stem cells (ADSCs) to facilitate recovery of erectile function in a rat model of cavernous nerve (CN) injury were compared. Forty male Sprague-Dawley rats were randomly divided into four groups: sham and control groups (intracavernous injection of phosphate-buffered saline), SVF group (intracavernous injection of SVF), and ADSC group (intracavernous injection of ADSCs). Rats in the latter three groups underwent bilateral CN injury prior to injection. The evaluation of erectile function and histomorphometric studies were performed 4 weeks after injection. The ratio of maximal intracavernous pressure to mean arterial pressure was significantly lower in the control group than in the sham group (0.18 vs. 0.56, p < .001). Intracavernous injection of SVF (0.36, p = .035) significantly improved erectile function compared with that in the control group, whereas the ADSC group (0.35, p = .052) showed marginally significant improvement. The smooth muscle/collagen ratio, smooth muscle content, number of neuronal nitric-oxide synthase-positive nerve fibers, and expression of von Willebrand factor were significantly higher in the SVF and ADSC groups than in the control group. Expression of endothelial nitric-oxide synthase was significantly increased in the SVF group. The increases in the smooth muscle/collagen ratio and von Willebrand factor expression were larger in the SVF group than in the ADSC group. Intracavernous injection of SVF or ADSCs was equally effective in recovering penile erection in a rat model of CN injury.
Introduction
Radical prostatectomy (RP) is considered the gold standard for the treatment of clinically localized prostate cancer. Nerve-sparing RP represents the approach of choice in all men with normal erectile function and organ-confined disease [1]. Improvements in equipment and surgical techniques have enabled postoperative erectile function to be preserved, thereby improving postoperative quality of life [2–4]. Phosphodiesterase type 5 inhibitors are commonly used to treat erectile dysfunction (ED) following RP; however, the efficacy is expected to be lower in these patients than in the entire ED patient population [5].
ED following RP typically results from injury to the cavernous nerve (CN), which provides most of the autonomic input to the erectile tissue [6]. Most of CN injury is neuropraxia, that is, failure of nerve conduction in the absence of structural change [7]. Erectile function is lost immediately following RP and then slowly recovers over the following 6–18 months along with restoration of function [8, 9]. Prolonged intracorporal hypoxia, caused by decreased arterial flow and chronic absence of erections, results in apoptosis of corporal smooth muscle cells and deposition of collagen fibers [10, 11]. In affected patients, the imbalance between the numbers of corporal smooth muscle cells and collagen fibers leads to veno-occlusive dysfunction and increased venous leak [12].
Recent research efforts on ED following RP include the use of stem cell-based therapies. Recent studies used adipose tissue as the source of stem cells [13]. However, there are several concerns regarding the implantation of cultured stem cells into humans, including the risks of xenogenic nutritional sources, microbial contamination, and tumorigenesis [14]. To avoid these risks, uncultured/heterogeneous stromal vascular fraction (SVF) has emerged as an easier and safer way to use stem and progenitor cells derived from adipose tissue. Recent clinical trials have focused on use of SVF in various diseases [15], including ED [16, 17].
However, few preclinical studies have assessed the efficacy and safety of such treatment for ED [18–21]. Moreover, no systematic study has compared the use of different cell types derived from adipose tissue. Therefore, we compared the effects of autologous SVF and adipose-derived stem cells (ADSCs) on erectile function recovery in a rat model of CN injury.
Materials and Methods
Animal and Study Design
All aspects of animal care and treatment and the surgical procedures used were approved by our Institutional Animal Care and Use Committee (2013–13–117). Forty 8-week-old male Sprague-Dawley rats were purchased from Orient Bio Inc. (Gyeonggi, Korea, http://www.orient.co.kr) and housed for 1 week to acclimatize. During experiments, rats were maintained under a 12-hour:12-hour light/dark cycle (lights on at 8 a.m. and off at 8 p.m.), at a temperature of 22°C ± 2°C, and at a humidity level of 50%–55%, with ad libitum access to food and water.
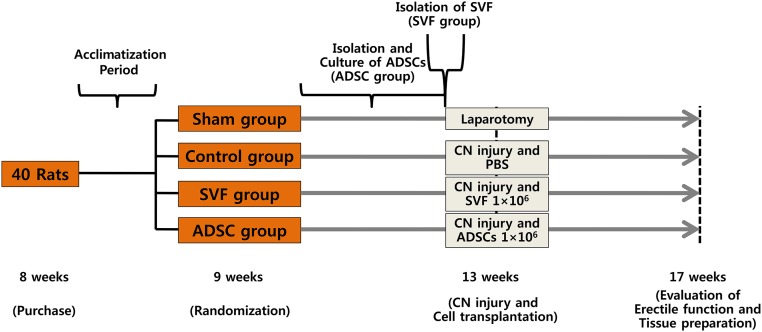
At 9 weeks old, the rats were randomly divided into 4 groups (10 animals per group): sham group, control group, SVF group, and ADSC group. Rats in the ADSC group underwent bilateral paratesticular fat excision via scrotal incision to isolate and culture ADSCs. Four weeks later, rats in the SVF group underwent bilateral paratesticular fat excision via scrotal incision to isolate SVF. At the same time, laparotomy was performed in the sham group, and bilateral CN injury was performed in the other three groups. Phosphate-buffered saline (PBS), SVF, and ADSCs were injected intracavernously into rats in the control, SVF, and ADSC groups, respectively. At 17 weeks old, all rats underwent erectile function evaluation and then sacrificed. Their tissues were prepared for histological examination. The study design is outlined in Figure 1.
Figure 1.
Study design. Abbreviations: ADSC, adipose-derived stem cell; CN, cavernous nerve; PBS, phosphate-buffered saline; SVF, stromal vascular fraction.
Isolation of SVF and Culture of ADSCs
Adipose tissue was harvested from paratesticular fats via scrotal incision when rats had been randomized (ADSC group) or following CN injury (SVF group). ADSCs were cultured according to a standardized protocol [22, 23]. Adipose tissue was enzymatically digested for 30 minutes at 37°C in 0.075% collagenase type I (Thermo Fisher Scientific Inc., Waltham, MA, http://www.thermofisher.com). The cell suspension was passed through a 100-μm pore-sized filter to remove undissociated tissue, neutralized by addition of Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), and centrifuged at 800g for 5 minutes. The stromal cell pellet was resuspended in DMEM containing 10% FBS and 1% antibiotic-antimycotic solution. Cultures were maintained at subconfluent levels (80%) at 37°C in 5% CO2. Cells were passaged using trypsin/EDTA (Thermo Fisher Scientific Inc.) as required. The resuspended cells were plated at a density of 2 × 10 3 cells per cm2 and cultured until passage 5 before being injected.
To determine whether the processed lipoaspirate cells were characteristic of SVF or ADSCs, flow cytometry were performed with a FACScan argon laser cytometer (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) according to a previous study [24]. Briefly, cells were harvested in 0.25% trypsin/EDTA and fixed for 30 minutes in ice-cold 2% formaldehyde. The fixed SVF was washed in flow cytometry buffer (2% bovine serum albumin, 0.1% sodium azide in PBS) and incubated for 30 minutes in flow cytometry buffer containing anti-CD45PE-Cy7, CD31PE, CD73APC, and CD90FITC (BD Biosciences). The fixed ADSCs were washed in flow cytometry buffer and incubated for 30 minutes in flow cytometry buffer containing anti-CD29FITC, CD34FITC, CD44FITC, CD45FITC, CD73APC, and CD90FITC (BD Biosciences).
Differentiation of ADSCs
The ADSCs at the fourth passage were incubated with a StemPro Adipogenesis Differentiation Kit and StemPro Osteogenesis Differentiation Kit for 14 days and a StemPro Chondrogenesis Differentiation kit (Thermo Fisher Scientific Inc.) for 21 days, and the medium was changed every 3–4 days. The adipogenic differentiation was confirmed using Oil Red O (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), the osteogenic differentiation was confirmed using 2% alizarin red S (ScienCell, Carlsbad, CA, https://sciencellonline.com), and the chondrogenic differentiation was confirmed using Alcian blue. The cells were photographed with an inverted microscope (Olympus, Tokyo, Japan, http://www.olympus-global.com)
Labeling for Tracking
SVF and ADSCs were labeled with the fluorescent dye PKH-26 (Sigma-Aldrich). Cells were placed in a conical polypropylene tube at concentrations of 1 × 106 cells per ml, mixed with a solution of PKH-26 dye, and incubated at 25°C for 5 minutes. An equal volume of serum was added to stop the staining reaction. The sample was diluted with an equal volume of the Diluent C and centrifuged. The cells were resuspended in saline buffer and injected into the corpus cavernosum.
CN Injury and Cell Transplantation
Each rat was intramuscularly anesthetized with 0.2 ml of tiletamine (Zoletil; Virbac Laboratories, Carros, France, http://www.virbac.com). Using a lower abdominal midline incision, the bilateral major pelvic ganglia (MPG) were identified and exposed on the posterolateral side of the prostate. Bilateral CNs running to the lateral side of the prostate were identified and separated from the prostate using microscissors. Thereafter, bilateral CNs were damaged by compression using two mosquito clamps for 2 minutes.
For intracavernous injection of SVF or ADSCs, 50-μl amounts of a cell suspension in PBS (1 × 106 cells) was injected into the corpus cavernosum using a 30-gauge insulin syringe. Immediately prior to injection, drainage via the dorsal vein was halted by circumferential compression of the base of the penis using a bulldog clamp. The compression was released 1 minute after injection of cells or PBS.
Evaluation of Erectile Function and Tissue Preparation
At 17 weeks old, all rats were anesthetized with 0.2 ml of tiletamine given intramuscularly. A midline incision was created from the neck to the upper thorax, and the right carotid artery was cannulated using a heparinized 24-gauge silastic cannula. The cannula was connected to a real-time continuous pressure transducer to measure mean arterial pressure (MAP). The skin of the penis was stripped off, and the corpus cavernosa was exposed. Using the previously created lower abdominal midline incision, the MPG posterolateral to the prostate were dissected and exposed. A heparinized 23-gauge butterfly needle was inserted into the corpus cavernosum to measure intracavernous pressure (ICP). Electrical stimulation (5 V at 10 Hz for 1 minute) was applied to the MPG using a bipolar hook electrode, and the ICP change was measured. Electrical stimulation was performed at least twice, and the interval between stimulations was more than 10 minutes. To compare erectile function among the groups, the maximal ICP and total ICP (area under the curve) were divided by the MAP and designated ICPmax/MAP and ICPtotal/MAP, respectively. Measurements were conducted in a blinded fashion. After evaluation of erectile function, the penis of each rat was harvested for histomorphometric analysis.
Immunohistochemistry and Masson’s Trichrome Staining of Penis Tissue
Penile sections 5 μm in thickness for immunohistochemistry were prepared were prepared from rats of each group. Tissue sections placed on microslides were deparaffinized in xylene, hydrated in serial alcohol solutions of decreasing dilution, and next immersed in 3% H2O2 to quench endogenous peroxidase activity. To permit antigen retrieval, all sections were next microwaved for 15 minutes in Tris-EDTA buffer (pH 9.0) prepared in distilled water. The sections were next incubated for 2 hours at ambient temperature with an anti-von Willebrand factor (vWF) primary antibody (1:400; Abcam, Cambridge, U.K., http://www.abcam.com). After washing, incubation with a biotin-free polymeric horseradish peroxidase-linker antibody conjugate system (Lab Vision, Fremont, CA, http://www.labvision.com) for 30 minutes at ambient temperature followed. The slides were washed, and chromogen development next proceeded for 10 minutes. The slides were counterstained with Mayer’s hematoxylin and mounted using Immu-Mount (Thermo Shandon, Pittsburgh, PA, http://www.thermo.com) prior to examination.
The sections were incubated for 5 minutes with Biebrich scarlet-acid fuchsin solution to stain fibers red. After washing, to prepare the sections for the uptake of aniline blue stain, the sections were incubated in a phosphotungstic/phosphomolybdic acid solution for 5 minutes. The slides were drained on tissue paper and incubated for 3 minutes with aniline blue solution to stain collagen blue. The sections were then incubated for 3 minutes in 1% glacial acetic acid to render the colors more delicate and transparent. After washing in distilled water, rapid dehydration using ethanol solutions decreasing in dilution, and clearing, the slides were mounted in dibutyl phthalate xylene (Sigma-Aldrich).
For image analysis, four randomly selected penile fields per each animal were photographed. For analysis of cavernous smooth muscle and endothelial cells, the corpus cavernosum was photographed at an original magnification of ×20 using an Olympus U-LH100L-3 camera and Olympus Ix 71 software. The smooth muscle/collagen ratio within the tunica albuginea and the ratio of the area staining positive for vWF to total area within the tunica albuginea were used for statistical analysis. All images were analyzed using ImagePro-Plus 5.1 software (Media Cybernetics, Rockville, MD, http://www.mediacy.com) to quantify signals.
Immunofluorescence Staining of Penis Tissue
Samples were fixed in 4% paraformaldehyde and blocked using normal goat serum. Anti-α smooth muscle actin (ASMA) (1:400; Sigma-Aldrich), anti-neuronal nitric-oxide synthase (nNOS) (1:100; Novus Biologicals, Littleton, CO, http://www.novusbio.com), anti-endothelial nitric-oxide synthase (eNOS) (1:100; Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com), anti-vWF (1:400; Abcam), and anti-isolectin B (1:100; Enzo Life Sciences, Inc., Farmingdale, NY, http://www.enzolifesciences.com) antibodies were added. Incubation with these primary antibodies proceeded overnight in a humidified chamber at 4°C. Secondary antibodies, goat anti-mouse and goat anti-rabbit (1:400; Thermo Fisher Scientific Inc.), were added, and incubation at room temperature for 1 hour followed. Nuclear staining was performed using 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com). For image analysis, four randomly selected penile fields from each animal were photographed. The images were recorded at an original magnification of ×200 (ASMA) or ×400 (nNOS and eNOS) using fluorescence microscopy (Carl Zeiss, Jena, Germany, http://www.zeiss.com). The ratio of the area staining positive for nNOS to the total area of the dorsal nerve and the ratio of the area staining positive for ASMA or eNOS to the total area within the tunica albuginea were used for statistical analysis. All images were analyzed using Adobe Photoshop CS2 (Adobe Systems Inc., San Jose, CA, http://www.adobe.com) to quantify the signals.
Cross-sections of the penis injected with PKH-26-labeled SVF or ADSCs were observed using the fluorescent microscope. Cells positive for ASMA, vWF, or isolectin B were examined to investigate whether SVF or ADSCs transdifferentiate into smooth muscle or endothelial cells in vivo.
Statistical Analysis
All results are expressed as means ± SEs. Multiple groups were compared using one-way analysis of variance followed by the Tukey’s honest significant difference test for post hoc comparisons. All statistical tests were two-sided, and p < .05 was considered significant. The data were analyzed using SPSS Statistics, version 21 (IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/).
Results
Characterization of SVF and ADSCs
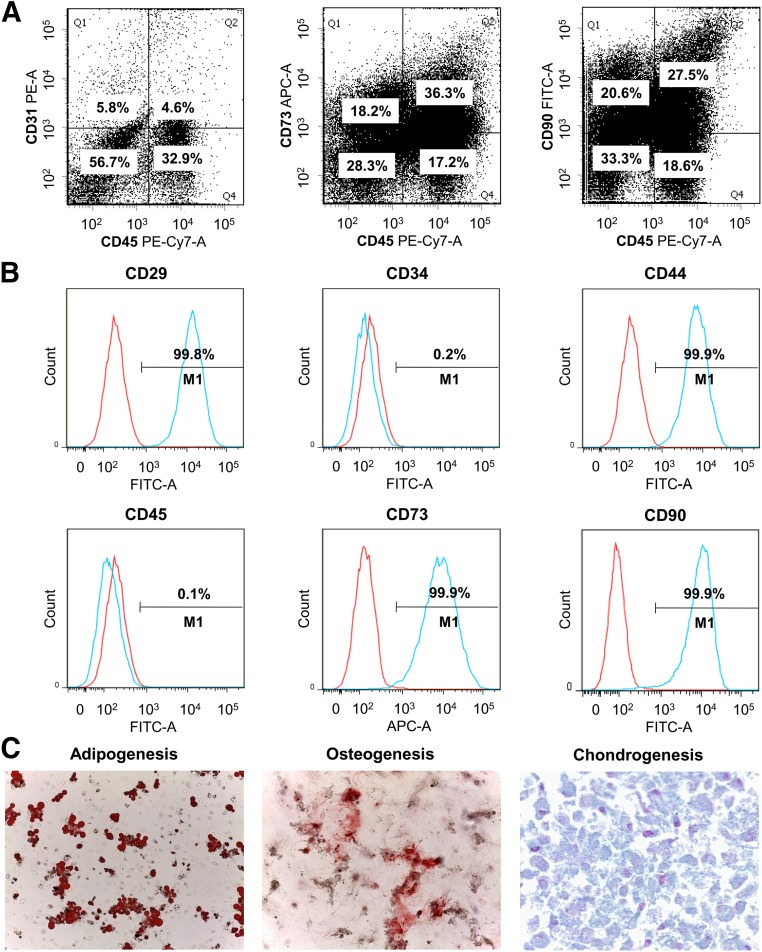
To characterize SVF and ADSCs, CD marker profiles characteristic of SVF and ADSCs were examined using flow cytometry. Flow cytometric analysis showed that SVF was made up of approximately 33% CD45+ cells (blood- and tissue-derived leukocytes), 6% CD45−/CD31+ (endothelial cells) and 57% cells that expressed neither CD45 nor CD31. CD73 and CD90 were expressed by almost two fifths of CD45− cells (stromal cells). Flow cytometric analysis showed that of cultured ADSCs, 99.8% expressed CD29, 0.2% expressed CD34, 99.9% expressed CD44, 0.1% expressed CD45, 99.9% expressed CD73, and 99.9% expressed CD90. The multilineage differentiation capacity was confirmed by observation of adipogenic, osteogenic, and chondrogenic differentiations after 3 weeks of culture in appropriate induction media (Fig. 2).
Figure 2.
Representative flow cytometry histograms. (A): Representative flow cytometry histograms of stromal vascular fraction. (B): Representative flow cytometry histograms of adipose-derived stem cells (ADSCs). (C): Appearance of ADSCs after 3 weeks of induction of adipogenic, osteogenic, and chondrogenic differentiation. Magnification, ×100 in adipogenesis and osteogenesis and ×400 in chondrogenesis. Abbreviations: APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Effects of SVF and ADSCs on Erectile Function
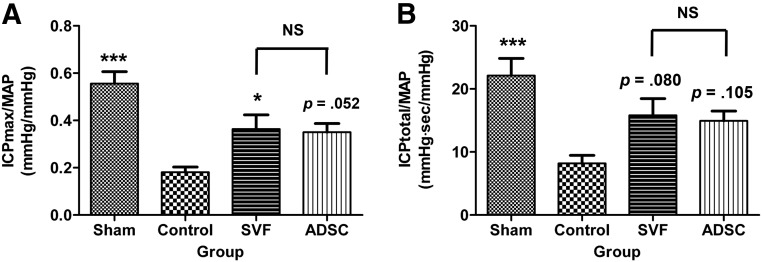
The MAP did not differ among the groups during MPG stimulation. The ICPmax/MAP and ICPtotal/MAP ratios in response to MPG stimulation were markedly lower in the control group than in the sham group (0.18 ± 0.05 vs. 0.56 ± 0.05, p < .001 and 8.18 ± 1.28 vs. 22.12 ± 2.71, p < .001, respectively). Intracavernous injection of SVF significantly improved erectile function compared with that in the control group (ICPmax/MAP and ICPtotal/MAP ratios of 0.36 ± 0.06, p = .035 and 15.76 ± 2.48, p = .080, respectively). Intracavernous injection of ADSCs improved erectile function compared with the control group, although this was only marginally significant (ICPmax/MAP and ICPtotal/MAP ratios of 0.35 ± 0.04, p = .052 and 14.93 ± 1.55, p = .105, respectively). There were no differences in erectile function between the SVF and ADSC groups (Fig. 3).
Figure 3.
Intracavernous pressure/mean arterial pressure ratios. (A): Maximal intracavernous pressure/mean arterial pressure ratios. (B): Total intracavernous pressure/mean arterial pressure ratios. NS indicates p > .05 between the SVF and ADSC groups. ∗, p < .05; ∗∗∗, p < .001 compared with the control group. Abbreviations: ADSC, adipose-derived stem cell; ICP, intracavernous pressure; MAP, mean arterial pressure; NS, not significant; sec, second; SVF, stromal vascular fraction.
Cavernous Smooth Muscle Cells
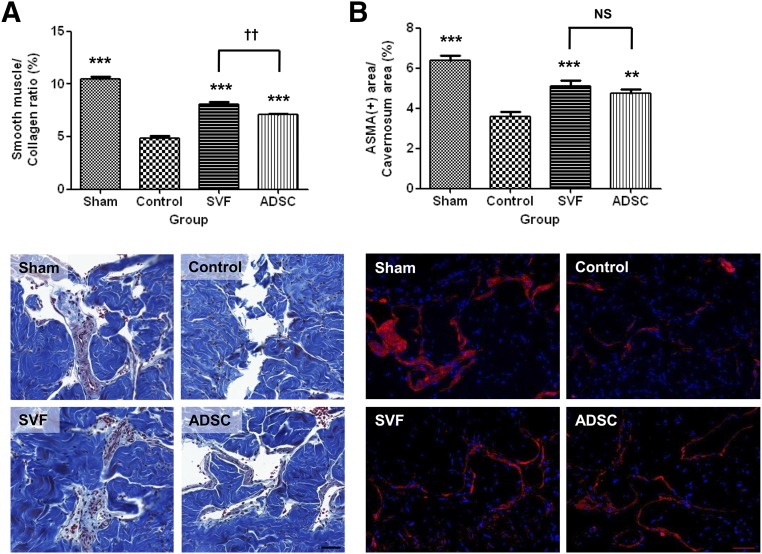
Masson’s trichrome staining and immunofluorescence staining for ASMA revealed that the smooth muscle/collagen ratio and smooth muscle content were significantly lower in the control group than in the sham group, whereas they were significantly increased in the SVF and ADSC groups. The smooth muscle/collagen ratio was significantly higher in the SVF group than in the ADSC group. There were no differences in smooth muscle content between the SVF and ADSC groups (Fig. 4).
Figure 4.
Smooth muscle/collagen ratio and smooth muscle content. (A): Graph showing the smooth muscle/collagen ratio obtained by quantitative image analysis of Masson’s trichrome staining data (top). Bottom: Representative photomicrographs of corpus cavernosum tissue stained with Masson’s trichrome stain. Original magnification, ×20. (B): Graph showing smooth muscle content as the ratio of the area stained positive for ASMA to the total area within the tunica albuginea (top). Bottom: Representative photomicrographs of smooth muscle content in the corpus cavernosum tissue. Original magnification, ×200. NS indicates p > .05 between the SVF and ADSC groups. ∗∗, p < .01; ∗∗∗, p < .001 compared with the control group; ††, p < .01 between the SVF and ADSC groups. Abbreviations: ADSC, adipose-derived stem cell; ASMA, α smooth muscle actin; NS, not significant; SVF, stromal vascular fraction.
CNs
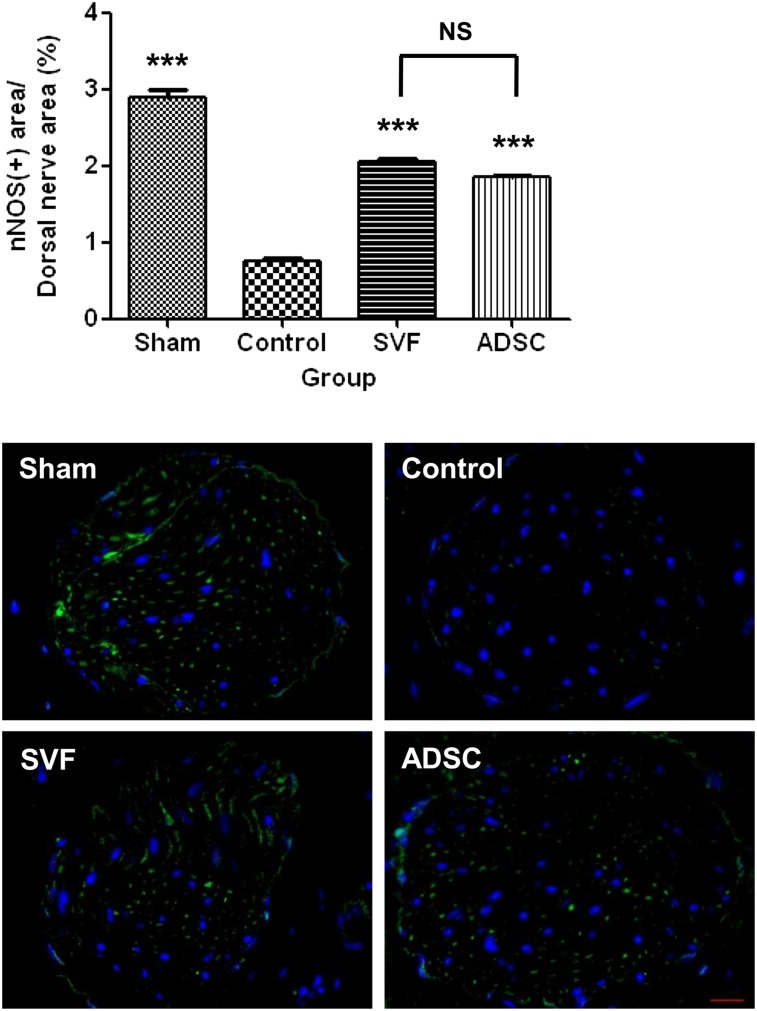
The nNOS-positive percentage area was lower in the control group than in the sham group, whereas it was significantly increased in the SVF and ADSC groups. There were no differences in the number of nNOS-positive nerve fibers between the SVF and ADSC groups (Fig. 5).
Figure 5.
Neuronal nitric-oxide synthase. Top: Graph showing cavernous nerve function as the ratio of the area stained positive for neuronal nitric-oxide synthase to the total area within dorsal nerves. Bottom: Representative photomicrographs of neuronal nitric-oxide synthase-positive area in the dorsal nerve. Original magnification, ×400. NS indicates p > .05 between the SVF and ADSC groups. ∗∗∗, p < .001 compared with the control group. Abbreviations: ADSC, adipose-derived stem cell; nNOS, neuronal nitric-oxide synthase; NS, not significant; SVF, stromal vascular fraction.
Endothelial Cells
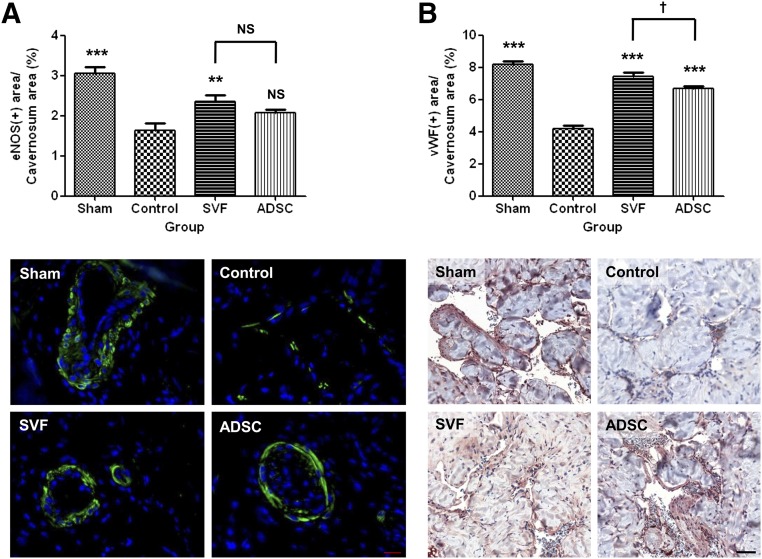
Immunofluorescence staining for eNOS and immunohistochemical staining for vWF revealed that endothelial cell content was significantly lower in the control group than in the sham group. According to this labeling, the endothelial cell content was higher in the SVF group than in the control group. Staining for vWF revealed that the endothelial cell content was higher in the SVF group than in the ADSC group. Staining for vWF also revealed that the endothelial cell content was higher in the ADSC group than in the control group (Fig. 6).
Figure 6.
Endothelial cell content. (A): Graph showing endothelial cell content as ratio of the area stained positive for endothelial nitric-oxide synthase to the total area within the tunica albuginea (top). Bottom: Representative photomicrographs of endothelial cell content in the corpus cavernosum tissue. Original magnification, ×400. (B): Graph showing endothelial cell content as the ratio of the area stained positive for von Willebrand factor to the total area within the tunica albuginea (top). Bottom: Representative photomicrographs of endothelial cell content in the corpus cavernosum tissue. Original magnification, ×20. NS indicates p > .05 compared with the control group, or between the SVF and ADSC groups. ∗∗, p < .01; ∗∗∗, p < .001 compared with the control group; †, p < .05 between the SVF and ADSC groups. Abbreviations: ADSC, adipose-derived stem cell; eNOS, endothelial nitric-oxide synthase; NS, not significant; SVF, stromal vascular fraction; vWF, von Willebrand factor.
Survival, Localization, and Differentiation of SVF and ADSCs
Four weeks after intracavernous injection, neither PKH-26-labeled SVF cells nor ADSCs were found in the corpus cavernosum. In addition, neither PKH-26-labeled SVF cells nor ADSCs colocalized with ASMA-, vWF-, or isolectin B-positive cells, suggesting that differentiation had occurred in to smooth muscle or endothelial cells (data not shown).
Discussion
Recently, many studies have investigated the use of stem cells isolated from several sources to treat ED caused by various factors. ED following RP is difficult to treat and is therefore the focus of many efforts, at the forefront of which is stem cell therapy [5, 13]. ADSCs are an attractive source of stem cells for tissue repair and regeneration for several reasons. First, humans have abundant subcutaneous fat deposits, and ADSCs can be easily harvested using minimally invasive liposuction [24, 25]. Second, ADSCs have similar characteristics to bone marrow-derived mesenchymal stem cells (MSCs) in terms of self-renewal and multipotency [26, 27]. Third, the proportions of MSCs in bone marrow are between 1 in 25,000 and 1 in 100,000 cells, whereas ADSCs constitute approximately 2% of lipoaspirated cells [28]. Furthermore, ADSCs enter senescence later than bone marrow-derived MSCs, which may be beneficial for the treatment of chronic or persistent conditions [29].
However, the cultured/relatively homogeneous ADSCs have some limitations, including the cost and time of culturing, the potential risk of contamination, changes in cell characteristics during culturing procedures, and tumorigenesis [14]. Because of these limitations, ED research using uncultured/heterogeneous SVF has accelerated [18–21], and several clinical trials are underway to examine the efficacy and safety of the use of SVF to treat ED [16, 17]. However, no study has reported side-by-side comparisons of the effects of different types of adipose tissue-derived cells on ED [13]. This led us to perform this study, which investigated the differences of intracavernous injection of SVF or ADSCs on ED in terms of effectiveness and possible mechanisms of action.
We confirmed that intracavernous injection of autologous SVF or ADSCs restored erection function in a rat model of CN injury. More importantly, both cell types were equally effective, and SVF was superior to ADSCs in terms of the smooth muscle/collagen ratio and endothelial cell content. Also, both cell types significantly increased the number of nNOS-positive nerve fibers, suggesting that they stimulated nerve regeneration. Unlike the many studies investigating the use of ADSCs therapy, few studies have investigated the use of SVF therapy to recover erectile function in a rodent model of CN injury [13]. Qiu et al. [18] reported that intracavernous injection of autologous SVF (2 × 106 cells) improved erectile function in a rat model of CN injury via promoting nerve regeneration and preventing corporeal fibrosis. Recently, Song et al. [21] reported that intracavernous injection of syngeneic SVF (3 × 105 cells) resulted in the complete recovery of erectile function in a mouse model of CN injury via promoting endothelial cell regeneration and restoring the endogenous nitric-oxide pathway. They suggested that various angiogenic factors secreted by SVF, such as vascular endothelial growth factor-A, angiopoietin-1, and hepatocyte growth factor, play important roles in the recovery of erectile function.
The SVF is composed of heterogeneous cell populations including blood-derived cells, endothelial (progenitor) cells, pericytes, and adipose-derived stromal/stem cells [24]. M2 macrophages have an important anti-inflammatory function that decreases immune reactions by stimulating the release of cytokines. T-regulatory cells modulate the immune system and maintain tolerance to self-antigens. Endothelial progenitor cells have the ability to differentiate into endothelial cells, which make up the lining of blood vessels in a process referred to as angiogenesis [30].
Earlier studies focused on cultured stem cells including ADSCs because they were thought to differentiate into local functional cells, including smooth muscle and endothelial cells [26]. However, the scarcity of evidence supporting such differentiation has led most researchers to suggest that paracrine action by growth factors secreted from ADSCs is the principal underlying mechanism [31]. The paradigm shifts in understanding of the roles of stem cells has raised concern about uncultured/heterogeneous SVF. Thus, the number of clinical trials using SVF is rapidly increasing [32]. The present study bridges the gap between previous ADSC studies and ongoing clinical trials using SVF.
The present study has some limitations. First, the exact composition of the rat SVF was not examined. Second, the rats were followed for only 4 weeks. A more long-term study might yield different results, especially considering the possibility of ADSC transdifferentiation. Such a study would allow broader questions to be addressed and would allow the evaluation of both functional and histomorphometric changes over a longer period. Third, rats were injected only once. If repeat injections are shown to have additional effects, ADSCs would be better because they can be kept in a freezer without a marked loss of cell viability and can be expanded by culturing, meaning there is no need for additional liposuction. Further research is now ongoing to determine the optimal protocol for cellular therapy of ED following CN injury.
Nevertheless, this study is the first to compare SVF and ADSC treatments in a rat model of CN injury. The findings suggest that intracavernous injection of SVF holds promise as a simple therapeutic approach for the treatment of postprostatectomy ED and replicates the already reported beneficial effects obtained using ADSCs.
Conclusion
The current study confirms that intracavernous injection of autologous SVF or ADSCs results in recovery of penile erection in a rat model of CN injury. Both cell types are equally effective in recovering penile erection, but SVF may be superior to ADSCs in terms of histomorphometric changes.
Acknowledgment
This work was supported by Grant 2013-576 from the Asan Institute for Life Sciences (Seoul, Korea).
Author Contributions
D.Y.: conception and design, financial support, data analysis and interpretation, manuscript writing; M.J.J.: provision of study material or patients, collection and/or assembly of data, manuscript writing; B.H.K.: provision of study material or patients, collection and/or assembly of data; G.S. and C.-S.K.: conception and design, final approval of manuscript; C.L.: administrative support; N.S., I.G.J., and T.Y.A.: final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: A systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Chabert CC, Merrilees DA, Neill MG, et al. Curtain dissection of the lateral prostatic fascia and potency after laparoscopic radical prostatectomy: A veil of mystery. BJU Int. 2008;101:1285–1288. doi: 10.1111/j.1464-410X.2008.07595.x. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–430. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Montorsi F, McCullough A. Efficacy of sildenafil citrate in men with erectile dysfunction following radical prostatectomy: A systematic review of clinical data. J Sex Med. 2005;2:658–667. doi: 10.1111/j.1743-6109.2005.00117.x. [DOI] [PubMed] [Google Scholar]
- 6.Dean RC, Lue TF. Neuroregenerative strategies after radical prostatectomy. Rev Urol. 2005;7(suppl 2):S26–S32. [PMC free article] [PubMed] [Google Scholar]
- 7.Dorland, NW. Dorland's Illustrated Medical Dictionary, 27th ed. Philadelphia, PA: W.B. Saunders Co, 1988:1126 [Google Scholar]
- 8.Quinlan DM, Epstein JI, Carter BS, et al. Sexual function following radical prostatectomy: Influence of preservation of neurovascular bundles. J Urol. 1991;145:998–1002. doi: 10.1016/s0022-5347(17)38512-9. [DOI] [PubMed] [Google Scholar]
- 9.Walsh PC, Marschke P, Ricker D, et al. Use of intraoperative video documentation to improve sexual function after radical retropubic prostatectomy. Urology. 2000;55:62–67. doi: 10.1016/s0090-4295(99)00363-5. [DOI] [PubMed] [Google Scholar]
- 10.Leungwattanakij S, Bivalacqua TJ, Usta MF, et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24:239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 11.Klein LT, Miller MI, Buttyan R, et al. Apoptosis in the rat penis after penile denervation. J Urol. 1997;158:626–630. [PubMed] [Google Scholar]
- 12.Davila HH, Rajfer J, Gonzalez-Cadavid NF. Corporal veno-occlusive dysfunction in aging rats: Evaluation by cavernosometry and cavernosography. Urology. 2004;64:1261–1266. doi: 10.1016/j.urology.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Lin CS, Xin Z, Dai J, et al. Stem-cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2013;13:1585–1597. doi: 10.1517/14712598.2013.847085. [DOI] [PubMed] [Google Scholar]
- 14.Ning H, Liu G, Lin G, et al. Identification of an aberrant cell line among human adipose tissue-derived stem cell isolates. Differentiation. 2009;77:172–180. doi: 10.1016/j.diff.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casteilla L, Planat-Benard V, Laharrague P, et al. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evaluate the Use of Liposuction and Cell Separation Devices for Autologous Fat (Adipose) Derived Cells to Treat the Symptoms of Erectile Dysfunction. Available at http://www.clinicaltrials.gov/ct2/show/NCT01601353. Accessed July 26, 2014.
- 17.Safety and Clinical Outcomes Study: SVF Deployment for Orthopedic, Neurologic, Urologic, and Cardio-pulmonary Conditions. Available at http://www.clinicaltrials.gov/ct2/show/NCT01953523. Accessed July 26, 2014.
- 18.Qiu X, Fandel TM, Ferretti L, et al. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;62:720–727. doi: 10.1016/j.eururo.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu JK, Tumurbaatar M, Jin HR, et al. Intracavernous delivery of freshly isolated stromal vascular fraction rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. J Sex Med. 2012;9:3051–3065. doi: 10.1111/j.1743-6109.2012.02962.x. [DOI] [PubMed] [Google Scholar]
- 20.Das ND, Song KM, Yin GN, et al. Xenogenic transplantation of human breast adipose-derived stromal vascular fraction enhances recovery of erectile function in diabetic mice. Biol Reprod. 2014;90:66. doi: 10.1095/biolreprod.113.115113. [DOI] [PubMed] [Google Scholar]
- 21.Song KM, Jin HR, Park JM, et al. Intracavernous delivery of stromal vascular fraction restores erectile function through production of angiogenic factors in a mouse model of cavernous nerve injury. J Sex Med. 2014;11:1962–1973. doi: 10.1111/jsm.12597. [DOI] [PubMed] [Google Scholar]
- 22.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 23.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson A, Butler PE, Seifalian AM. Adipose-derived stem cells for clinical applications: A review. Cell Prolif. 2011;44:86–98. doi: 10.1111/j.1365-2184.2010.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin G, Banie L, Ning H, et al. Potential of adipose-derived stem cells for treatment of erectile dysfunction. J Sex Med. 2009;6(suppl 3):320–327. doi: 10.1111/j.1743-6109.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ning H, Liu G, Lin G, et al. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrilleaux B, Phinney DG, Prockop DJ, et al. Review: Ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007–3019. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 29.Kokai LE, Marra K, Rubin JP. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163:399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Riordan NH, Ichim TE, Min WP, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albersen M, Fandel TM, Lin G, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: An update on the transition to clinical translation. Regen Med. 2012;7:225–235. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]