Abstract
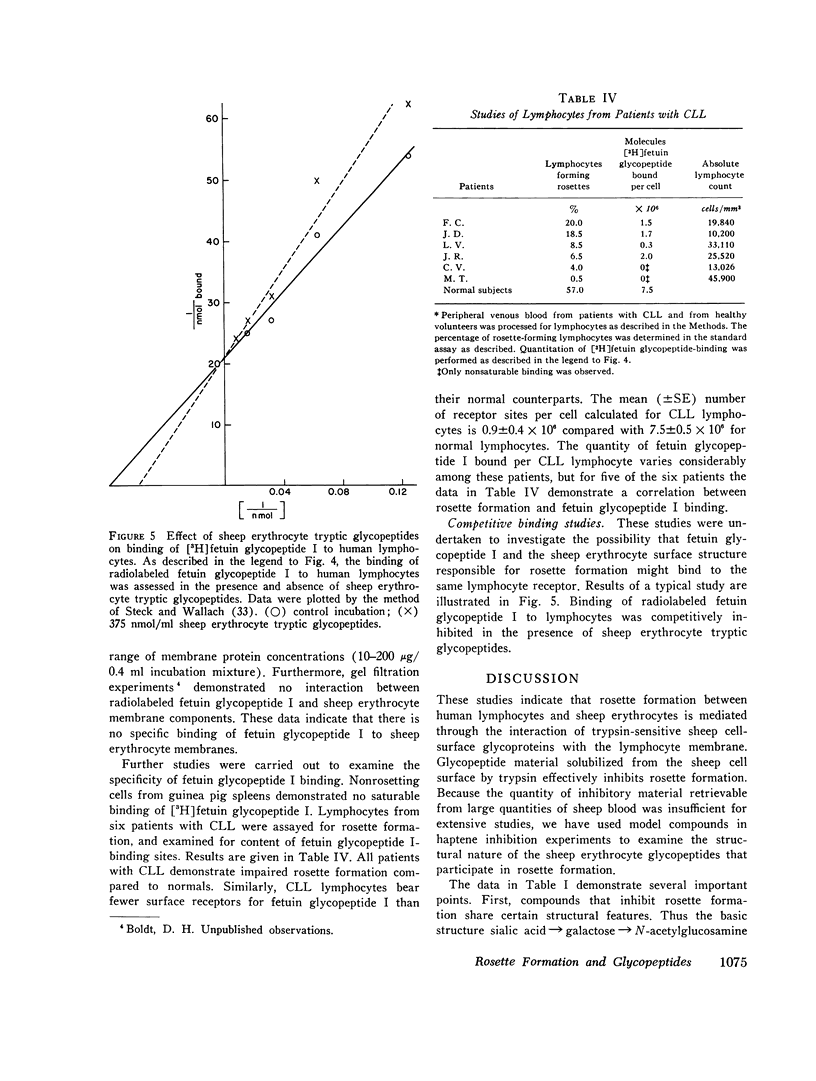
Rosette formation with unsensitized sheep erythrocytes is a characteristic of human thymus dependent lymphocytes. Release of glycopeptides from the sheep erythrocyte by trypsin reduces rosette formation. These tryptic glycopeptides inhibit rosette formation by untrypsinized sheep erythrocytes; this suggests that rosetting is mediated by erythrocyte surface glycopeptides. To investigate the molecular nature of this interaction, we examined the abilities of various model compounds to act as haptenic inhibitors of rosette formation. Inhibition is given by glycopeptides bearing oligosaccharide units rich in sialic acid, galactose, N-acetylglucosamine, and mannose linked to asparagine residues through glycosylamine bonds. Among compounds tested, fetuin glycopeptide is most effective, but human transferrin glycopeptide and human erythrocyte glycopeptide I also inhibit rosette formation. Other compounds including human erythrocyte glycopeptide II, human IgG glycopeptide, lacto-N-neotetraose, 3'- and 6'-sialyllactose show no significant inhibition. Neither sialic acid, galactose, manose, nor N-acetyl-glucosamine alone inhibits rosette formation. Stepwise degradation of fetuin glycopeptide established the galactose residues as important determinants of inhibitory activity. Fetuin glycopeptide blocks rosette formation when added to a suspension of human lymphocytes and sheep erythrocytes or when preincubated with human lymphocytes, but not when preincubated with sheep erythrocytes. Studies of the binding of [3H] fetuin glycopeptide to normal lymphocytes demonstrate 7.5 x 10(6) saturable binding sites per cell. No saturable binding of this compound to sheep erythrocyte membranes is observed. Compared to normals, lymphocytes from patients with chronic lymphatic leukemia demonstrate decreased fetuin glycopeptide binding with a mean of 0.9 x 10(6) sites per cell. This decreased binding correlates with the impaired ability of these cells to form rosettes. The data suggest that fetuin glycopeptide inhibits rosette formation by binding to the thymus-dependent cell where competition occurs with sheep erythrocytes for specific lymphocyte surface receptors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Long J. C., Wilkes B. Chronic lymphocytic leukemia cells: rosette formation and adherence to nylon fiber columns. J Natl Cancer Inst. 1974 Jan;52(1):13–17. doi: 10.1093/jnci/52.1.13. [DOI] [PubMed] [Google Scholar]
- BERGMANN F. H., LEVINE L., SPIRO R. G. Fetuin: immunochemistry and quantitative estimation in serum. Biochim Biophys Acta. 1962 Mar 26;58:41–51. doi: 10.1016/0006-3002(62)90815-6. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dormont J., Dardenne M., Balner H. In vitro rosette inhibition by antihuman antilymphocyte serum. Correlation with skin graft prolongation in subhuman primates. Transplantation. 1969 Sep;8(3):265–280. doi: 10.1097/00007890-196909000-00008. [DOI] [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7270–7281. [PubMed] [Google Scholar]
- Bentwich Z., Douglas S. D., Skutelsky E., Kunkel H. G. Sheep red cell binding to human lymphocytes treated with neuraminidase; enhancement of T cell binding and identification of a subpopulation of B cells. J Exp Med. 1973 Jun 1;137(6):1532–1537. doi: 10.1084/jem.137.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain P., Gordon J., Willetts W. A. Rosette formation by peripheral lymphocytes. Clin Exp Immunol. 1970 May;6(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Brown G., Greaves M. F., Lister T. A., Rapson N., Papamichael M. Expression of human T and B lymphocyte cell-surface markers on leukaemic cells. Lancet. 1974 Sep 28;2(7883):753–755. doi: 10.1016/s0140-6736(74)90945-3. [DOI] [PubMed] [Google Scholar]
- Carvalho A. C., Ellman L. L., Colman R. W. A comparison of the staphylococcal clumping test and an agglutination test for detection of fibrinogen degradation products. Am J Clin Pathol. 1974 Jul;62(1):107–112. doi: 10.1093/ajcp/62.1.107. [DOI] [PubMed] [Google Scholar]
- Catovsky D., Miliani E., Okos A., Galton D. A. Clinical significance of T-cells in chronic lymphocytic leukaemia. Lancet. 1974 Sep 28;2(7883):751–752. doi: 10.1016/s0140-6736(74)90944-1. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., Wilson A. B., Holm G., Lindgren B. Rosette-formation between human lymphocytes and sheep red cells not involving immunoglobulin receptors. Int Arch Allergy Appl Immunol. 1970;39(5-6):658–663. doi: 10.1159/000230390. [DOI] [PubMed] [Google Scholar]
- EYLAR E. H., MADOFF M. A., BRODY O. V., ONCLEY J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962 Jun;237:1992–2000. [PubMed] [Google Scholar]
- Fletcher M. A., Woolfolk B. J. Immunochemical studies on infectious mononucleosis. I. Isolation and characterization of heterophile antigens from hemoglobin-free stroma. J Immunol. 1971 Sep;107(3):842–853. [PubMed] [Google Scholar]
- Galili U., Schlesinger M. The formation of stable E rosettes after neuraminidase treatment of either human peripheral blood lymphocytes or of sheep red blood cells. J Immunol. 1974 May;112(5):1628–1634. [PubMed] [Google Scholar]
- Jett M., Jamieson G. A., DeBernardo S. L. The carbohydrate sequence of the glycopeptide chains of human transferrin. J Biol Chem. 1971 Jun 10;246(11):3686–3693. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Keller J., Baenziger J., Kornfeld S. The structure of the glycopeptide of human gamma G myeloma proteins. J Biol Chem. 1971 May 25;246(10):3259–3268. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- Kornfeld S., Rogers J., Gregory W. The nature of the cell surface receptor site for Lens culinaris phytohemagglutinin. J Biol Chem. 1971 Nov;246(21):6581–6586. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgita R. A., Tomasi T. B., Jr Suppression of the immune response by alpha-fetoprotein on the primary and secondary antibody response. J Exp Med. 1975 Feb 1;141(2):269–286. doi: 10.1084/jem.141.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presant C. A., Kornfeld S. Characterization of the cell surface receptor for the Agaricus bisporus hemagglutinin. J Biol Chem. 1972 Nov 10;247(21):6937–6945. [PubMed] [Google Scholar]
- STECK T. L., HOELZLWALLACH D. F. THE BINDING OF KIDNEY-BEAN PHYTOHEMAGGLUTININ BY EHRLICH ASCITES CARCINOMA. Biochim Biophys Acta. 1965 Mar 8;97:510–522. [PubMed] [Google Scholar]
- Sela B. A., Wang J. L., Edelman G. M. Antibodies reactive with cell surface carbohydrates. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1127–1131. doi: 10.1073/pnas.72.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960 Oct;235(10):2860–2869. [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Winzler R. J., Harris E. D., Pekas D. J., Johnson C. A., Weber P. Studies on glycopeptides released by trypsin from intact human erythrocytes. Biochemistry. 1967 Jul;6(7):2195–2202. doi: 10.1021/bi00859a042. [DOI] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnin S. Fetuin, an inhibitor of lymphocyte transformation. The interaction of fetuin with phytomitogens and a possible role for fetuin in fetal development. J Exp Med. 1975 Jan 1;141(1):242–256. doi: 10.1084/jem.141.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]