The hypothesis that autologous stromal vascular fraction (SVF) cells therapeutically modulate peripheral artery vasoactivity in syngeneic mouse models of small artery function was tested. Freshly isolated, adipose SVF cells promoted vasomotor relaxation in vasoactive arteries via a hydrogen peroxide-dependent mechanism that required CD11b+ cells (most likely macrophages). The intravenous delivery of this therapeutic cell preparation would significantly improve tissue perfusion, in particular, in disease with diffuse vascular involvement.
Keywords: Cell therapy, Adipose stromal vascular fraction, Small artery, Vasomotor tone, Macrophage
Abstract
Vasoactivity, an important aspect of tissue healing, is often compromised in disease and tissue injury. Dysfunction in the smaller vasoactive arteries is most impactful, given the role of these vessels in controlling downstream tissue perfusion. The adipose stromal vascular fraction (SVF) is a mix of homeostatic cells shown to promote tissue healing. Our objective was to test the hypothesis that autologous SVF cells therapeutically modulate peripheral artery vasoactivity in syngeneic mouse models of small artery function. Analysis of vasoactivity of saphenous arteries isolated from normal mice 1 week after intravenous injection of freshly isolated SVF cells revealed that pressure-dependent artery vasomotor tone was decreased by the SVF cell isolate, but not one depleted of CD11b+ cells. Scavenging hydrogen peroxide in the vessel wall abrogated the artery relaxation promoted by the SVF cell isolate. Consistent with a CD11b+ cell being the relevant cell type, SVF-derived F4/80-positive macrophages were present within the adventitia of the artery wall coincident with vasorelaxation. In a model of artery inflammation mimicking a common disease condition inducing vasoactive dysfunction, the SVF cells potentiated relaxation of saphenous arteries without structurally remodeling the artery via a CD11b+ cell-dependent manner. Our findings demonstrate that freshly isolated, adipose SVF cells promote vasomotor relaxation in vasoactive arteries via a hydrogen peroxide-dependent mechanism that required CD11b+ cells (most likely macrophages). Given the significant impact of small artery dysfunction in disease, we predict that the intravenous delivery of this therapeutic cell preparation would significantly improve tissue perfusion, particularly in diseases with diffuse vascular involvement.
Introduction
The non-adipocyte stromal vascular fraction (SVF) of adipose contains an array of tissue cells that function to establish adipose tissue homeostasis. Typically harvested via enzymatic digestion, the freshly isolated adipose SVF contains endothelial cells, perivascular cells (e.g., smooth muscle cells, pericytes), fibroblasts, mesenchymal cells, and resident immune cells, such as regulatory and natural killer T lymphocytes, B lymphocytes, dendritic cells, and macrophages [1–3]. These cells facilitate provascularizing therapies by promoting angiogenesis through the release of angiogenic factors and formation of new vessel segments via a profound vasculogenic-like activity [1–6]. In a rat model of myocardial infarction, adipose SVF cells, applied as an epicardial patch, improved coronary functional flow reserve, independent of increases in vessel density [7]. Whether these vascular-associated SVF cells from the patch acted directly on the vessels to influence vessel dilation or constriction was not determined.
Perfusion to a tissue bed is regulated by the vasoactive feed and resistance arteries (arteries 350 μm or less in diameter [8]) supplying the tissue. These upstream small arteries normally exist in a preconstricted state (termed “tone”) enabling dynamic increases or decreases in downstream blood flow to meet tissue demands. Increases in vasomotor tone dampens vasodilation, thereby limiting the perfusion capacity. In many diseases, particularly those associated with tissue inflammation, the ability of these small arteries to properly dilate and constrict is compromised [9–11]. In inflamed tissues, such as those with chronic ischemia or metabolic disorders (e.g., diabetes, obesity), the vessel tone is elevated, in part because of the elevated oxidant stress [12], contributing to vasoactive dysfunction [13–15]. Persistent increases in small artery tone can lead to subsequent, long-lasting, undesired structural changes [16], further exacerbating tissue perfusion and compromising tissue repair.
To explore the possible impact of adipose SVF cells on vascular function in normal and inflamed tissue conditions, we tested the hypothesis that freshly isolated adipose SVF cells might act directly to influence vasoactivity when used therapeutically. Thus, we examined the vasoactivity of isolated saphenous arteries from mice injected intravenously with freshly isolated, syngeneic, adipose SVF cells. Additionally, because tissue macrophages (MΦs) reside within adipose tissue [4, 17] and regulate the vasoactivity of arteries as normal constituents of the artery wall [18], we examined the SVF-derived macrophage as a potential important cell type mediating the possible vascular responses. Finally, we determined the effect of the SVF cells on vessel diameter, as a surrogate indicator of vascular tone, in a focal model of artery inflammatory insult.
Materials and Methods
All animal studies were performed under protocols approved by the University of Louisville Institutional Animal Care and Use Committee and according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The mouse strains used in the present study included FVB/n, FVB-Tg(CAG-luc,-GFP)L2G85Chco/J [19], and Tg(TIE2GFP)287Sato/J [20], either purchased from Jackson Laboratories (Bar Harbor, MN, http://jaxmice.jax.org) or obtained from in-house colonies. All reported findings were from male mice (donors and recipients) aged 10–23 weeks.
Isolation of Adipose SVF Cells
For SVF isolations, epididymal fat pads were collected, weighed, minced until paste-like, and digested with a filter-sterilized solution of lot-tested 0.1% (wt/vol) collagenase plus 0.05% (wt/vol) DNase in 5-(and-6)-choromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF)-phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA-DCF-PBS) at 37°C for 35 minutes [4]. The digestate was centrifuged, and the SVF cell pellet was suspended and washed twice in 0.1% BSA-DCF-PBS The washed pellet, suspended in 10 ml of 0.1% BSA-DCF-PBS, was passed through a sterile 20-µm nylon screen prewetted with 0.1% BSA-DCF-PBS to remove any incompletely digested matrix fragments and clumped cells. The flow through of single cells was collected and an aliquot counted with a NucleoCounter (LI-COR, Lincoln, NE, http://www.licor.com). The total SVF cell yields averaged 2.58 × 106 ± 0.46 × 106 cells per gram of fat.
Magnetic Depletion of SVF Cell Isolates
The depletion experiments used the Miltenyi MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com) according to the manufacturer’s instructions. In brief, up to 1 × 107 screened SVF cells were suspended in 90 µl of MACS buffer (degassed solution of PBS, pH 7.2; 0.5% BSA, and 2 mM EDTA) and incubated with 10 µl of anti-mouse CD11b antibody conjugated to iron particles (Miltenyi Biotec, catalog no. 130-049-601) at 4°C for 15 minutes. An additional 3 ml of MACS buffer was added to the cell suspension followed by centrifugation at 400g for 4 minutes. The cell pellet was resuspended with 1.0 ml of MACS buffer and loaded 0.5 ml at a time onto a Miltenyi Biotec MACS column prewetted with 0.5 ml of MACS buffer within the magnet chamber. The cell-loaded column was gravity drained and then flushed with 0.5 ml of MACS buffer at least 3 times to remove any additional cells. The effluent was collected and considered to be the CD11b+-depleted fraction (SVF-11bΔ), which represented a 38.1% ± 1.65% reduction in the total cell numbers. The column was removed from the magnet and flushed as before to collect the CD11b+-enriched fraction (11bΔ).
Flow Cytometry
Aliquots of SVF cells, CD11b+-depleted SVF cells (SVF-11bΔ), and CD11b+-enriched SVF cells (11bΔ) isolated from FVB/n tie2:GFP-expressing transgenic mice were divided into polypropylene tubes for flow cytometry at a concentration of 5 × 105 to 1 × 106 cells in 100 µl of wash buffer (Dulbecco’s PBS containing 1% BSA and 0.025 M HEPES) per tube. Aliquots of the following antibodies (at optimized antibody dilutions) were added to label the cell surface markers: CD2-PE (catalog no. 553112; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), CD45-PerCP (catalog no. 557235; BD Biosciences), CD11b-APC (catalog no. 130-098-088; Miltenyi Biotec), Gr-1-PE (catalog no. 12-5931-83; eBioscience, San Diego, CA, http://www.ebioscience.com), FεR1-PerCP (catalog no. 46-5898-82; eBioscience), CD11b-PE (catalog no. 130-098-087; Miltenyi Biotec), CD80-APC (catalog no. 17-0801-82; eBioscience), F4/80-PerCP-Cy5.5 (catalog no. 45-4801; eBioscience), and CD301-Alexa Fluor 647 (catalog no. MCA2392A647T; AbD Serotec, Raleigh, NC, http://www.abdserotec.com). The green fluorescent protein (GFP) fluorescence (i.e., tie2 expression) was used to mark the endothelial cells. Species-matched isotypes were added to separate tubes of wild-type FVB/n SVF cell isolates. Additionally, single color tubes of FVB/n SVF were used as compensation controls. The cells were incubated in antibodies at 4°C for 30 minutes protected from light, lysed with PharmLyse (catalog no. 555899; BD Biosciences) for 3 minutes at 37°C, washed twice with 2 ml wash buffer, spun at 350g for 5 minutes to pellet, suspended in 400 µl wash buffer per tube, and analyzed using an LSRII flow cytometer (BD Biosciences) using FACS Diva software. Postacquisition data analyses were performed using FlowJo, version 7.6.2, software (FlowJo, Ashland, OR, http://www.flowjo.com).
Tail Vein Injection of Cells
SVF cells (1 × 106 cells per mouse) and SVF-11bΔ cells (0.8 × 106 cells per mouse), suspended in 0.2 ml of sterile saline, were injected into the tail vein using a 30-gauge needle as a single bolus.
Saphenous Artery Vasoactive Responses
Saphenous arteries were explanted, taking care to remove the extraneous connective tissue, from anesthetized (5% isoflurane/O2 balance) recipient mice into cold, filtered physiological saline solution (PSS) (pH 7.4; containing 145 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, 3.0 mM 4-morpholinepropanesulfonic acid buffer, and 1% BSA). The arteries were cannulated on size- and resistance-matched glass pipettes in a Lucite chamber containing warm (37°C) PSS and assessed as previously described [21]. The arteries were preconstricted with phenylephrine (2 µM) to approximately 30% of the resting diameter at 50 mmHg; those that did not constrict were discarded. To assess the pressure-dependent responses, the intraluminal pressure was decreased to 1 mmHg and sequentially increased (waiting 3 minutes at each step), while simultaneously recording the luminal diameters throughout the procedure. The intraluminal pressure was then returned to 50 mmHg, the chamber was washed with fresh PSS and refreshed with phenylephrine, and, on tone establishment, the lumen diameter changes were recorded during drug dose-response curves for acetylcholine (doses ranged from 1 × 10−9 to 1 × 10−4 M, 3 minutes per dose) and, after washing, for sodium nitroprusside (doses ranged from 1 × 10−10 to 1 × 10−4 M, 3 minutes per dose). The chamber was then washed twice with PSS without CaCl2 to measure the maximum dilation. The chamber was washed twice again without calcium and exposed to the same pressure-change regimen to assess the passive relaxation to pressure. The intraluminal diameters (measured with electronic calipers) were normalized to the maximum diameter obtained in the absence of calcium and are reported as the percentage of relaxation: (diameter/maximum diameter) ×100.
Polyethylene Glycol-Catalase Myogenic Responses and Reactive Oxygen Species Fluorescence Imaging
After the active myogenic response assessment, the chamber was washed with PSS without albumin, and the vessels were incubated for 10 minutes in the dark with 5-(and-6)-choromethyl-2′,7′-dichlorodihydrofluorescein diacetate (5 µM) and washed with PSS without albumin to fluorescently measure the presence of H2O2 in the vessel walls [22]. DCF fluorescence images were captured with the same exposure times and magnifications from arteries incubated without and then with polyethylene glycol (PEG)-catalase (PEG-CAT; 500 U/ml; catalog no. C4963; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) for 1 hour [23]. The pre- and post-PEG-CAT images were analyzed for fluorescence intensity within a 40 × 150 µm region of interest using Nikon Elements software (Nikon Instruments, Melville, NY, http://www.nikoninstruments.com). A separate group of mice were used to measure the levels of O2− and nitric oxide (NO) using dihydroethidium (DHE) and 4-amino-5-methylamino-2′2′-difluorofluorescein diacetate (DAF), respectively. The vessel was infused intraluminally with DHE (10−4 M) for 10 minutes and washed with PSS without albumin. Images of ethidium bromide and hydroethidine fluorescence were captured and subsequently analyzed using Nikon Elements software by subtracting the hydroethidine fluorescence signal from the ethidium bromide fluorescence signal to determine the relative O2− levels. For NO, the vessels were infused with DAF (5 μM) for 10 minutes, protected from light, and washed with PSS without albumin. The DAF images were analyzed for fluorescence intensity as above.
Confocal Microscopy
Fixed saphenous arteries (2% paraformaldehyde) were washed for 2 × 5 minutes in PBS, and then permeabilized in PBS plus 0.5% Triton-X (catalog no. T8787; Sigma-Aldrich) for 15 minutes at room temperature. The vessels were blocked for 10 minutes at room temperature in DAKO Protein Block (code 0909; Dako, Glostrup, Denmark, http://www.dako.com), washed 2 times for 5 minutes in PBS and then incubated overnight with gentle shaking at 4°C in rat anti-mouse F4/80 antibody (catalog no. 14-4801; eBioscience) at 1:250 dilution in PBS plus 10% normal donkey serum plus 0.5% BSA plus 0.1% Triton-X. The next day, the arteries were washed 3 times for 15 minutes in PBS with gentle shaking at room temperature and protected from light. Following this washing, the vessels were incubated in donkey anti-rat rhodamine (catalog no. 712-026-150; Jackson ImmunoResearch, West Grove, PA, http://www.jacksonimmuno.com) at 1:200 dilution in PBS, 10% normal donkey serum, 0.5% BSA, and 0.1% Triton-X for 1 hour at room temperature, protected from light. After secondary antibody incubation, the vessels were washed for 15 minutes in PBS at room temperature and then incubated for 30 minutes at room temperature in Hoechst nuclear stain (1:2,000 dilution) and subsequently washed 15 minutes in PBS. The stained vessels were placed in Fluoromount-G (catalog no. 0100-01; SouthernBiotech, Birmingham, AL, http://www.southernbiotech.com) on a dimpled slide to allow the vessels to retain their natural shape during imaging. Confocal imaging was performed using an Olympus FV1000 confocal microscope (Olympus America, Central Valley, PA, http://www.olympusamerica.com) equipped with 488 (for visualizing GFP+ SVF cells) and 543 (F4/80) laser lines and a multiphoton laser set to 800 nm to visualize Hoechst staining. Confocal stacks were obtained (1–1.5-µm step size) at ×40 magnification through one half of the vessel and volume rendered using Amira software (Visualization Sciences Group, Burlington, MA, http://www.vsg3d.com). For the cell counts per vessel area, the images were coded, and a blinded observer counted the GFP+ cells, F4/80+ cells, and colabeled cells for each image, ensuring that a Hoechst-positive nucleus was present for each positive count. The vessel area was measured using Amira software by tracing the curvature of the vessel in the x dimension and multiplying by the length of the vessel in the y dimension for each image. To assess the proliferation of cells within the artery adventitia, the recipient mice received 25 µg/g body weight via i.p. injection at 2 hours after cell injection and again on days 2, 4, and 6. 5-Ethynyl-2′-deoxyuridine (EdU) was detected as per the manufacturer’s instructions (Click-iT Plus EdU Alexa 647 Imaging Kit, catalog no. C10640; Life Technologies).
Bioluminescence Imaging
In vivo bioluminescence imaging was performed on mice using a Photon Imager (BioSpace Lab, Paris, France, http://www.biospacelab.com). Each mouse was injected intraperitoneally with d-luciferin potassium salt dissolved in PBS at a dose of 150 mg/kg of body weight. The mice were initially anesthetized with 3% isoflurane at 1 liter/minute, then 2% isoflurane at 500 ml/minute was used to keep the mice anesthetized during imaging. The mice were kept anesthetized with isoflurane and warmed at 37°C for the entire imaging period. The mice were imaged 10 minutes after d-luciferin injection.
Histologic Evaluation and Immunostaining
At explantation, the anesthetized mice were cannulated with a polyethylene (PE)-50 catheter through the left ventricle and perfused with 10 ml of warmed PBS containing a vasodilator cocktail (2.5 μg/ml single nucleotide polymorphism [SNP], 60 μg/ml papaverine, 10 U/ml heparin, and 1 mg/ml adenosine) and then with 12 ml of warmed 4% paraformaldehyde/PBS, all at 100 mmHg pressure using a pressure gauge. The harvested tissue samples were placed in 4% paraformaldehyde/PBS and kept at 4°C until processed into paraffin before sectioning and staining. For morphometry, the sections were stained with hematoxylin and eosin using standard methods. To identify the injected cells in the saphenous arteries and select tissues, the sections were immunostained for luciferase-positive cells using an HRP kit (EnVision+ System HRP, Dako) according to the manufacturer’s instructions. The sections were treated with 3% hydrogen peroxide for 10 minutes at room temperature before incubation with an anti-firefly luciferase antibody (Abcam, Cambridge, U.K., http://www.abcam.com) at a 1:1,000 dilution in 10% goat serum (Sigma-Aldrich) for 1 hour at room temperature. All sections were then counterstained with hematoxylin and cover slipped. Tissue sections from luciferase-positive donor mice and wild-type mice that did not receive cells served as the positive and negative controls, respectively, for immunostaining.
Saphenous Artery Cuffing
All procedures were performed using a sterile technique with modifications to a previously published method [24]. The medial area of the right hindlimb of an anesthetized (isoflurane), supine mouse maintained at 37°C body temperature was depilated and wiped with Nolvasan solution (Zoetis, Florham Park, NJ, http://www.zoetis.com). A 1-cm incision on the calf side of the midline, approximately one third the distance proximal to the knee, was made and deflected to the midline to expose the femoral-saphenous vessels. The overlying fascia was blunt dissected to expose the vascular nerve sheath, which was further opened to access the vessels. The saphenous artery was carefully freed from the saphenous vein for a length of 4 mm. A 2-mm-long section of ethylene oxide-sterilized PE-50 tubing split lengthwise down the middle was placed around the freed artery section and tied closed with 5-0 silk ligature. The skin was placed back into position and closed with a single surgical clip. The sham groups were prepared as described, except that the cuff was not placed.
Results
Cells From Injected SVF Disseminate Into Recipient Peripheral Tissues
Because of the practicalities of treating small arteries owing to their relatively small size, numbers, and disseminated locations, we explored intravenous delivery of the freshly isolated SVF cells as a method to distribute the cells. Our rationale was that the injected SVF cells would disperse throughout the circulation, homing to the small arteries. Whole-animal bioluminescence imaging revealed that luciferase-active SVF cells delivered to syngeneic recipient adult mice were observed throughout the body of the mouse, including in the bone marrow, 1 week and as late as 3 months after injection (Fig. 1). Furthermore, relative luminescence intensities were increased at the later time points, particularly in the visceral fat pads, suggesting SVF cell proliferation had occurred. Consistent with the imaging results, luciferase-positive cells were present within the histological sections of a variety of tissues, including around and within the walls of the small vessels, after i.v. injection (Fig. 1).
Figure 1.
Intravenously injected SVF cells distribute into a variety of tissues. (A): Bioluminescence imaging of mice receiving a single i.v. injection of syngeneic SVF cells constitutively expressing green fluorescent protein (GFP) and luciferase. Shown are the cell distributions 1, 2 (same mouse as in 1-week panel), and 8 weeks after injection. Also shown are GFP+ cells (arrows) within the bone marrow of an SVF cell-injected mouse, 12 weeks after injection. (B): Visualization of luciferase-positive SVF cells within histological paraffin sections of different tissues from SVF-injected mice. Brown stain indicates positive luciferase immunostaining and the presence of SVF cells. Tissues were harvested 1 week after cell delivery. Positive and negative controls involved untreated transgenic luciferase-positive donor and wild-type mice, respectively. Abbreviations: Neg., negative; Pos., positive; SVF, stromal vascular fraction; VFP, visceral fat pad.
CD11b+ Cells Constitute a Relatively Large Fraction of the SVF Isolate
To define the cell constituents in our SVF preparation and possibly identify the cell types distributing to peripheral tissues, we used flow cytometry to characterize the fresh SVF isolates. Using a donor mouse expressing GFP behind the tie2 promoter [20], we identified the prevalent cell types expressing markers common to innate immune cells (CD11b), endothelial cells (tie2-GFP), tissue macrophages (F4/80), myeloid cells (Gr-1), and lymphocytes (CD2) (Fig. 2; supplemental online Figs. 1–3). The largest fractions of the SVF cells appeared to be endothelial cells (∼25%), CD11b+ cells (∼20%), and myeloid cells (∼22%). We have previously shown that mesenchymal stem cells represent a relatively small proportion of the cells in the fresh SVF isolate [4]. Although differences were present in the isolation methods, similar distributions have been described in lean adipose tissue from C57B/6 mice by others [25].
Figure 2.
The cellular composition of adipose SVF cell preparations is heterogeneous. (A): Percentage of nucleated cells expressing select markers in fresh adipose SVF isolates, CD11b+ cell-depleted adipose SVF cells (SVF-11bΔ), and the removed CD11b+ cell-enriched fraction (11bΔ) as assessed by flow cytometry. Percentage of CD11b+ (B) and F4/80+ (C) cells expressing select markers from the same three SVF groups. Data are mean ± SEM; n = 3 for each group. ∗∗∗, p < .001, all groups different from each other; ∗∗, p < .001, difference between 11bΔ versus others, and p < .05, SVF versus SVF-11bΔ; ∗, p < .001, 11bΔ group differs from the others; ‡, p < .01, SVF-11bΔ differs from the others; and #, p < .001, SVF-11bΔ differs from the others, as determined by analysis of variance. Dot plots showing the gating used to generate these graphs are shown in supplemental online Figures 1–3. Abbreviations: 11bΔ, removed CD11b+ cell-enriched fraction; SVF-11bΔ, CD11b+ cell-depleted adipose SVF cells; SVF, stromal vascular fraction.
Because the CD11b+ cells represented a relatively large fraction of the fresh SVF isolate, and MΦs, a prominent CD11b+ cell type [26], are dynamic regulators of vascular function in arteries [18], we further processed the SVF cells to create a preparation depleted of CD11b+ cells as a method to investigate the role of this SVF-derived subset of cells in the hypothesized vascular activity. To do this, we used a selective magnetic separation approach (Miltenyi Biotec) targeting CD11b-expressing cells. Flow cytometry of the different fractions after magnetic depletion confirmed that ∼94% of the CD11b+ cells were removed from the SVF isolate (Fig. 2; supplemental online Figs. 1–3). Furthermore, ∼80% of the CD11b+ cells were composed of F4/80+ cells (i.e., MΦs), of which, a significant majority (∼70%) were also positive for the homeostatic, M2-class MΦ phenotype marker CD301 [27]. Endothelial cells, lymphocytes, and other hematopoietic cells (CD45) were not significantly lowered by the selection in the CD11b-depleted SVF fraction (SVF-11bΔ). As expected, the CD11b-enriched fraction (11bΔ; the cells captured on the column) was highly enriched for macrophages (Fig. 2; supplemental online Figs. 1–3). The presence of tie2-GFP+ cells in this CD11b-enriched fraction resulted from small capillary fragments that were nonspecifically trapped within the column matrix used in the depletion process (data not shown). Similar to MΦs, but to a lesser extent, Gr-1+ cell distributions between the different fractions were also affected by the depletion process (Fig. 2). Gr-1 is a commonly used marker for myeloid cells [28]. However, a significant proportion of the Gr-1+ cells in the adipose SVF isolate were double-positive for CD11b (Fig. 2B), a marker combination recently described for myeloid suppressor cells, a cell type thought to mediate lymphocyte activity [29]. Thus, although a significant majority (>80%) of the cells removed by the CD11b selection were macrophages, other cell types (likely hematopoietic in origin given the high prevalence of CD45+ cells) were also removed.
Intravenously Injected SVF Cells Reduce Artery Vasomotor Tone in a CD11b+ Cell-Dependent Manner
To evaluate the potential of the SVF cells and the CD11b+ cell-depleted subfraction to modulate vascular function, we directly measured the vasodilation responses in the saphenous arteries from syngeneic recipient mice that received a single intravenous injection of these preparations. Although the saphenous artery in the mouse is in a conduit vessel position within the vascular tree, its size, medial structure and vasoactivity functionally reflect more the vasoactive, small distal arteries in humans. In an isolated, pressurized vessel preparation, saphenous arteries from untreated, SVF cell-injected, or SVF-11bΔ cell-injected mice exhibited comparable endothelium-dependent (acetylcholine) and -independent (SNP) vasodilation responses 1 week after injection (Fig. 3). However, vessel relaxation to the sequentially increasing changes in intravascular pressure was significantly enhanced in the saphenous arteries from mice injected with the SVF cells, but not those with the SVF depleted of CD11b+ cells (Fig. 3). These differences in active tone were not observed in the absence of extravascular Ca2+ (Fig. 3), consistent with an absence of any structural remodeling of the artery wall (supplemental online Fig. 4). The influence on pressure-dependent, but not agonist-dependent, relaxation reflects changes in vessel wall tension or vasomotor tone.
Figure 3.
SVF cells relax vasomotor tone in normal saphenous arteries. Vessel relaxation curves of isolated, normal saphenous arteries from mice untreated or injected with SVF cells or SVF-11bΔ cells in response to endothelium-dependent (acetylcholine) (A) or endothelium-independent (nitroprusside) (B) dilators, progressively increased intravascular pressures in the presence (C) or absence (D) of extravascular calcium, or untreated vessels (n = 3) exposed to increasing pressures in the presence of L-NAME (to block nitric oxide production) (E) or the H2O2 scavenger polyethylene glycol (PEG)ylated-CAT (F). H2O2 in SVF-injected vessels was scavenged using PEGylated-CAT (C). In all cases, the measured internal vessel diameters were normalized to maximal diameters to account for intervessel variability. ∗, p < .05, determined by t test between the untreated and SVF-injected vessels at each pressure. Abbreviations: 11bΔ, removed CD11b+ cell-enriched fraction; ACh, acetylcholine; CAT, catalase; L-NAME, L-NG-nitroarginine methyl ester; SNP, sodium nitroprusside; SVF, stromal vascular fraction; SVF-11bΔ, CD11b+ cell-depleted adipose SVF cells.
Changes in Vasomotor Tone Are Hydrogen Peroxide-Dependent
Although potentially many pathways mediate vasomotor tone, one prevalent mechanism involves vessel wall reactive oxygen species (ROS) [30]. H2O2, one such ROS, is a potent vasodilator [31], and the reduction in super oxide anions (O2−) associated with its production preserves nitric oxide, thereby further promoting vessel relaxation [32]. Scavenging H2O2 with tissue permeant PEGylated-catalase [23] (Fig. 3C, 3D) in saphenous arteries from mice injected with the SVF cells eliminated the cell-dependent decrease in vasomotor tone, mimicking the pressure responses with CD11b-depleted SVF cell treatment. The H2O2 activity is unique to the SVF cell therapy, because baseline saphenous artery tone is mediated by NO but not H2O2 (Fig. 3E, 3F).
Also consistent with an SVF-induced H2O2 production, the saphenous arteries from mice injected with SVF cells had higher H2O2 levels, but not nitric oxide levels, in the vessel wall compared with the levels in the untreated arteries (Fig. 4). The super oxide levels were concomitantly increased (Fig. 4). The H2O2 and O2− levels in the CD11b-depleted SVF cell vessel group were not elevated (Fig. 4). Also, higher numbers of DCF-bright (H2O2-producing [33]) cells were present in the walls of SVF cell-injected arteries than in the untreated or SVF-11bΔ-injected arteries (Fig. 4). All these findings are consistent with an SVF-derived, CD11b+ cell-dependent, H2O2-dependent mechanism of small artery relaxation.
Figure 4.
SVF cells alter reactive oxygen species within the walls of saphenous arteries. (A): DCF fluorescence in isolated saphenous arteries to visualize the presence of H2O2. Arteries were from untreated, SVF-injected, or SVF-11bΔ-injected mice. Bottom row: Images of the same vessels in the top row after treatment with the H2O2 scavenger polyethylene glycolylated-CAT. All images were acquired at the same camera exposure and after acquisition image processing settings. Top graph shows measurement of the intensity of DCF fluorescence for the different vessels shown at the left. Bottom graph shows measurement of the density of DCF-bright cells within the vessel wall for the different vessels shown in the top graph. (B): Normalized DHE fluorescence intensities of isolated saphenous arteries from untreated and SVF-injected mice (n = 3 for each) to visualize the presence of O2−. DHE (B) and DAF (C) fluorescence intensities of isolated saphenous arteries from untreated and SVF-injected mice (n = 3 for each) to visualize the presence of superoxide and nitric oxide, respectively. All data are shown as the mean ± SEM, n ≥ 3. p value was determined by one-way ANOVA within each pre- and post-catalase groups for each of the experimental groups. Abbreviations: CAT, catalase; DAF, 4-amino-5-methylamino-2′2′-difluorofluorescein diacetate; DCF, 5-(and-6)-choromethyl-2′,7′-dichlorodihydrofluorescein diacetate; DHE, dihydroethidium; SVF, stromal vascular fraction; SVF-11bΔ, CD11b+ cell-depleted adipose SVF cells.
SVF-Derived Macrophages Populate the Saphenous Artery Adventitia
The presence of luciferase-positive SVF cells surrounding peripheral arteries after i.v. injection and the additional presence of H2O2-producing cells in the artery wall after SVF delivery suggested that the injected SVF cells were populating the saphenous artery walls. To address this directly, we examined isolated saphenous arteries via confocal microscopy for the presence and location of the SVF cells by imaging for GFP fluorescence (expressed via the GFP transgene within the SVF cells). Using rhodamine-tagged GS-1 lectin and hydrazide to simultaneously label the endothelial cells of the vaso vasorum and the elastic laminae, respectively [34], we observed GFP+ SVF cells within the artery wall near the vaso vasorum of the artery adventitia, with nearly 75% of these cells positive for the tissue macrophage marker, F4/80 (Fig. 5; supplemental online Fig. 5). GFP+ cells were also present within the adventitia of arteries from mice injected with the CD11b+ cell-depleted SVF preparation (SVF-11bΔ) (Fig. 5; supplemental online Fig. 5). However, additional analysis determined that the fraction of SVF-derived MΦs (GFP-F4/80 double-positive cells) was ∼50% lower (Fig. 5B, 5C), indicating a depletion of SVF-derived MΦs in the arterial walls of the mice injected with the CD11b+ cell-depleted fraction. No difference was found in macrophage density in the walls between the two experimental groups. However, considerably more of the adventitial macrophages were derived from SVF in the SVF-treated arteries (supplemental online Fig. 5).
Figure 5.
SVF-derived macrophages spontaneously populate artery walls. (A): Visualization (cross-section) of GFP+ SVF cells (green) within regions of saphenous artery walls from untreated, SVF-injected, or SVF-11bΔ-injected mice. Macrophages were identified via F4/80 immunostaining (red) and cell nuclei by Hoechst dye (blue) (corresponding en face views are shown in supplemental online Fig. 5). In other instances, lectin GS-1 (red) and hydrazide (blue) was used to identify the endothelium and elastic laminae, respectively, to locate the vaso vasorum relative to the IEL and EEL. Also shown are the density of GFP+ cells (B) and the percentage of the fraction of those cells also positive for F4/80 (C) in arteries from SVF-injected or SVF-11bΔ-injected mice. Data are shown as the mean ± SEM, n = 3 for all groups. p value determined by t test. Abbreviations: EEL, external elastic lamina; GFP, green fluorescent protein; IEL, internal elastic lamina; SVF, stromal vascular fraction; SVF-11bΔ, CD11b+ cell-depleted adipose SVF cells.
Intravenously Injected SVF Cells Promote Dilation in a Vessel Insult Model
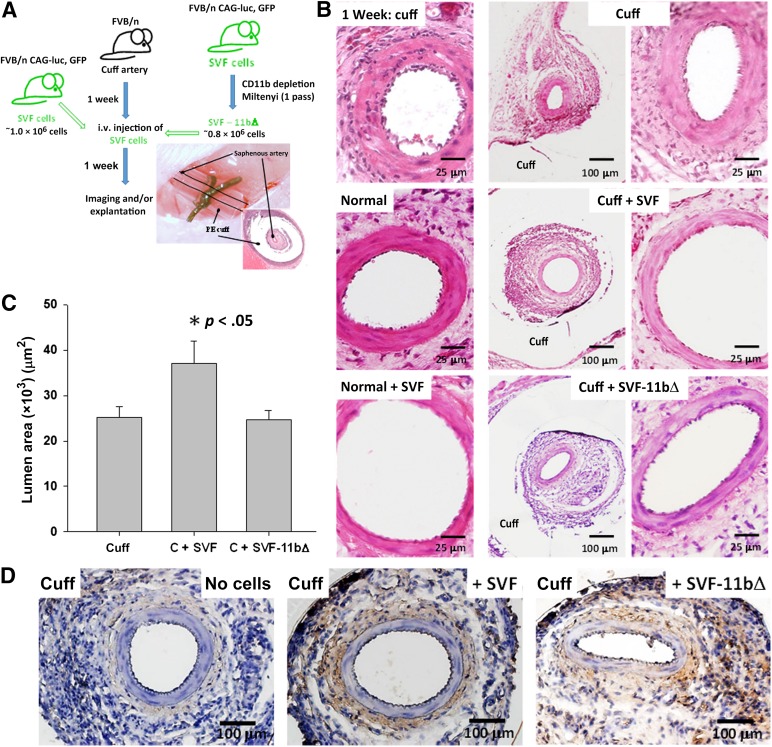
The findings to this point indicate that SVF cells populate the adventitia of vasoactive arteries and relax vasomotor tone by modulating wall reactive oxygen species. To explore whether the SVF cells are also capable of modulating tone in a condition involving vascular insult, we used a model of focal vascular injury [35] in which a small region of the right medial saphenous artery is loosely cuffed with PE tubing to induce a materials-driven, tissue inflammation around the artery (Fig. 6). The intent with this model was to mimic the type of insult experienced by an artery present within in a chronically inflamed tissue while simultaneously minimizing any confounding systemic influences (e.g., cytokines). After inducing the insult, we intravenously delivered freshly isolated SVF cells as before and assessed the vessel morphology 1 week later. As anticipated, and consistent with a more relaxed artery wall phenotype, SVF cell delivery significantly increased the cuffed artery diameter (Fig. 6). As before, removal of the CD11b+ cells from the SVF attenuated this prodilation response (Fig. 6). Similar to the normal arteries, no significant changes were found in the other artery morphometric parameters, indicating that SVF cell-dependent wall remodeling also did not occur (supplemental online Fig. 6). Additionally, injected cells populated the adventitia of the cuffed saphenous arteries and the inflammation-induced granulation tissue surrounding the artery segment within the cuff confines (Fig. 6).
Figure 6.
SVF cells relax vasomotor tone in inflamed saphenous arteries. (A): Schematic of the experimental plan involving the cell treatment of locally inflamed (cuffed) saphenous arteries of mice injected with syngeneic adipose SVF cells constitutively expressing luciferase and GFP reporter transgenes or SVF cells depleted of CD11b+ cells. Also shown is a gross view and a histological cross-section of a cuffed saphenous artery. (B): Hematoxylin and eosin-stained histological cross-sections of normal (noncuffed) and cuffed mouse saphenous arteries untreated or injected with SVF cells or SVF-11bΔ cells. Rightmost panels: Higher magnification images of the adjacent images. Scale bars = 25 μm in the left and right columns and 100 μm in the middle column. (C): Lumen diameters of untreated (n = 9) and cell-injected cuffed saphenous arteries measured from histological sections. Cell treatments included complete SVF cell isolates (C + SVF, n = 7) or SVF isolates depleted of CD11b+ cells (C + SVF-11bΔ, n = 7). Data are shown as the mean ± SEM; ∗, p < .05, determined by one-way analysis of variance. (D): Visualization of luciferase-positive SVF cells within histological paraffin sections of cuffed saphenous arteries from untreated, SVF-injected, and SVF-11bΔ-injected mice via immunostaining for luciferase. Brown stain indicates positive luciferase immune-staining and the presence of SVF cells. Tissues were harvested 1 week after cell delivery. Scale bars = 100 μm. Abbreviations: C, cuff; GFP, green fluorescent protein; PE, polyethylene; SVF, stromal vascular fraction; SVF-11bΔ, CD11b+ cell-depleted adipose SVF cells.
Discussion
One week after intravenous injection, freshly isolated, adipose SVF cells had populated the walls of peripheral arteries. One such artery harboring these cells, the saphenous artery, exhibited enhanced pressure-dependent, but not endothelium-dependent and -independent, relaxation, without a change to the arterial wall structure. Concomitantly, the injected SVF cells led to increased levels of the vasoactive mediator H2O2 within the artery wall, the scavenging of which attenuated the relaxation induced by the SVF cells. CD11b+ cells within the isolate were necessary for the change in artery tone and increase in H2O2. All of which indicates that a subset of CD11b+ cells within the i.v. delivered adipose SVF isolate spontaneously populate the walls of existing small arteries and modulate vessel tone, likely via a mechanism involving regulation of the reactive oxygen species balance within the vessel wall.
Although we were not practically able to remove the arteries from the cuff environment for vasoactivity analysis, the increase in the diameter of the SVF cell-treated cuffed saphenous arteries fixed at a constant pressure suggests that these cells also relax the artery in the context of chronic tissue inflammation, a condition known to increase vessel tone [9, 10]. Additionally, given the anti-inflammatory potential for adipose-derived cells [6, 36–39], it is reasonable to believe that they might have modulated the inflammation in the cuffed artery setting, thereby lessening the artery insult (i.e., oxidant stress). Regardless of the mechanism, it appears that the freshly isolated SVF cells are well suited to improving vascular function in inflamed tissue environments. Other vasoactive regulatory pathways, for example endothelial-dependent and -independent processes, were unaltered, suggesting that, overall, the vascular homeostatic mechanisms are still effective. This is important when considering the patient’s condition because different vascular beds could contain small arteries that are more or less dysfunctional. If the other vasoactive regulatory pathways are intact, we would expect to find increases in perfusion only in the dysfunctional beds after the SVF cell therapy, because these other regulatory pathways would compensate for any changes brought on by the cells in normally functioning arteries.
Most of the CD11b+ cells that were removed from the isolate expressed F4/80, a common marker of tissue macrophages (MΦs). Also, the F4/80+ cell population was the most depleted population after removal of the CD11b+ cells, which eliminated the relaxed tone effect. Moreover, MΦs derived from the SVF population were present within the saphenous artery wall (F4/80-GFP double-positive cells in our experiments) and, importantly, were reduced in numbers in the artery adventitia in the mice injected with SVF cells depleted of CD11b+ cells. Macrophages normally regulate vessel form and function [18, 40], actively express the hydrogen peroxide-producing enzyme super oxide dismutase-3 [41], and are capable of moving throughout tissue compartments [42]. Although the MΦ was not the only cell type in the CD11b+ population in the SVF isolate (subsets of Gr-1+ cells were also present), our findings strongly suggest that MΦs are the relevant, vasoregulatory cell type in the SVF isolate and necessary for the SVF-mediated change in vascular tone. A significant majority of the MΦs in the SVF isolate were positive for CD301, a commonly used marker for the M2 MΦ [43]. Simply based on this prevalence, it is likely that the MΦs delivered to the saphenous artery wall in our experiments were these prohealing, anti-inflammatory homeostatic, CD301+ macrophages.
Because the SVF cells were injected intravenously, those observed within the saphenous artery adventitia necessarily entered the artery wall via the circulation. In arteries, shear stress at the luminal surface is sufficiently high to impede cell attachment to the intimal endothelium [44]. Conversely, the adventitia of arteries contains the vaso vasorum, a microcirculatory bed perfusing the outer and medial layers of the vessel wall [18]. Because the SVF cells were localized to the adventitia and it is unlikely that the injected cells entered the vessel wall via the intima, it is most plausible that they entered the vessel wall via the vaso vasorum. Whether the SVF cells that entered the artery adventitia were directly derived from the injected isolate (i.e., after injection, circulated to the artery wall and entered the adventitia) or they were new, hematopoietic-derived cells (e.g., monocytes) from the SVF cells that populated the recipient’s bone marrow is unclear. Certainly, tissue-resident macrophages express a variety of cell adhesion and homing molecules that could mediate dissemination into the artery adventitia once removed from the adipose environment and injected into the blood stream [42, 45]. Consistent with the increase in bioluminescence over time, SVF-derived macrophages within the artery wall incorporated EdU, administered to the recipients for the 1 week after cell delivery (supplemental online Fig. 7), indicating cell proliferation. Thus, just as with the MΦs and MΦ precursors normally residing within the artery adventitia [46], the SVF-derived MΦs likely populated the saphenous artery adventitia independent of the hematopoietic origins, propagating within this new tissue compartment. Regardless of how the SVF-derived MΦs entered the artery wall, it appears that these cells, and their vasoactive activity, can persist in the artery wall for extended periods of time.
Conclusion
Many are exploring the therapeutic potential of adipose-derived cells, motivated by basic and preclinical studies supporting their anti-inflammatory and proangiogenesis activities [47]. We have provided evidence for an additional therapeutic mechanism, vasorelaxation of the peripheral arteries via reduced vasomotor tone without perturbing the other vasoactive homeostatic mechanisms. This clinically favorable activity depended on CD11b+ cells, likely tissue-resident macrophages. Given that macrophage phenotypes are often excluded in the cultured preparations of adipose mesenchymal or stromal cells, this activity might be unique to fresh adipose cell preparations. Because the injected SVF cells were widely disseminated throughout the animal and reset the vasomotor tone in the inflamed arteries, our findings foster an autologous cell therapeutic strategy targeting disease-related, diffuse vascular dysfunction.
Supplementary Material
Acknowledgments
We thank Dr. Jill Suttles for the useful discussion concerning macrophages and Darlene Burke for assistance with the statistical analyses. This work was supported by Department of Surgery, University of Louisville funds (to M.E.M.), a grant from the Kosair Children’s Hospital Foundation (to C.L.K. and J.B.H.). This project used the Kentucky Spinal Cord Injury Research Center/Cardiovascular Innovation Institute care facilities, supported by Grant P30 GM13507 from the NIH/National Institute of General Medical Sciences. M.E.M. is currently affiliated with the Department of Vascular Surgery, Baystate Medical Center, Springfield, MA.
Author Contributions
M.E.M.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.E.B., R.M.R., and J.R.D.: collection and/or assembly of data, manuscript writing; A.J.L.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation; C.L.K.: data analysis and interpretation; H.Z. and C.K.N.: collection and/or assembly of data; S.K.W.: conception and design, data analysis and interpretation, manuscript writing; J.B.H.: conception and design, financial support, provision of study material, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lolmède K, Duffaut C, Zakaroff-Girard A, et al. Immune cells in adipose tissue: Key players in metabolic disorders. Diabetes Metab. 2011;37:283–290. doi: 10.1016/j.diabet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Casteilla L, Planat-Benard V, Laharrague P, et al. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunes SS, Maijub JG, Krishnan L, et al. Generation of a functional liver tissue mimic using adipose stromal vascular fraction cell-derived vasculatures. Nat Sci Rep. 2013;3:2141–2148. doi: 10.1038/srep02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh YJ, Koh BI, Kim H, et al. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:1141–1150. doi: 10.1161/ATVBAHA.110.218206. [DOI] [PubMed] [Google Scholar]
- 6.Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr Opin Organ Transplant. 2010;15:86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 7.Leblanc AJ, Nguyen QT, Touroo JS, et al. Adipose-derived cell construct stabilizes heart function and increases microvascular perfusion in an established infarct. Stem Cells Translational Medicine. 2013;2:896–905. doi: 10.5966/sctm.2013-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzoni D, Porteri E, Boari GE, et al. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein AS, Khavandi K, Withers SB, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton PA, James ME, Goodwill AG, et al. Obesity and vascular dysfunction. Pathophysiology. 2008;15:79–89. doi: 10.1016/j.pathophys.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation. 2013;20:239–247. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palomares SM, Cipolla MJ. Myogenic tone as a therapeutic target for ischemic stroke. Curr Vasc Pharmacol. 2013;11:1–13. doi: 10.2174/15701611113116660155. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc AJ, Krishnan L, Sullivan CJ, et al. Microvascular repair: Post-angiogenesis vascular dynamics. Microcirculation. 2012;19:676–695. doi: 10.1111/j.1549-8719.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher JT, Goodwill AG, Stanley SC, et al. Blunted temporal activity of microvascular perfusion heterogeneity in metabolic syndrome: A new attractor for peripheral vascular disease? Am J Physiol Heart Circ Physiol. 2013;304:H547–H558. doi: 10.1152/ajpheart.00805.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonoyama K, Greenstein A, Price A, et al. Vascular remodeling: Implications for small artery function and target organ damage. Ther Adv Cardiovasc Dis. 2007;1:129–137. doi: 10.1177/1753944707086358. [DOI] [PubMed] [Google Scholar]
- 17.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: Phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14:341–346. doi: 10.1097/MCO.0b013e328347970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenmark KR, Yeager ME, El Kasmi KC, et al. The adventitia: Essential regulator of vascular wall structure and function. Annu Rev Physiol. 2013;75:23–47. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao YA, Wagers AJ, Beilhack A, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motoike T, Loughna S, Perens E, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28:75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Leblanc AJ, Chen B, Dougherty PJ, et al. Divergent effects of aging and sex on vasoconstriction to endothelin in coronary arterioles. Microcirculation. 2013;20:365–376. doi: 10.1111/micc.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang LS, Chen B, Reyes RA, et al. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol. 2011;300:H2105–H2115. doi: 10.1152/ajpheart.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol. 2007;292:H93–H100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 24.Moroi M, Zhang L, Yasuda T, et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen JE, Andreasen SO, Christensen JP, et al. CD11b expression as a marker to distinguish between recently activated effector CD8(+) T cells and memory cells. Int Immunol. 2001;13:593–600. doi: 10.1093/intimm/13.4.593. [DOI] [PubMed] [Google Scholar]
- 27.Lumeng CN, DelProposto JB, Westcott DJ, et al. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee PY, Wang JX, Parisini E, et al. Ly6 family proteins in neutrophil biology. J Leukoc Biol. 2013;94:585–594. doi: 10.1189/jlb.0113014. [DOI] [PubMed] [Google Scholar]
- 29.Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: The good, the bad, and the ugly. Immunol Res. 2013;57:172–184. doi: 10.1007/s12026-013-8455-2. [DOI] [PubMed] [Google Scholar]
- 30.Wagenfeld L, von Domarus F, Weiss S, et al. The effect of reactive oxygen species on the myogenic tone of rat ophthalmic arteries with and without endothelium. Graefes Arch Klin Exp Ophthalmol. 2013;251:2339–2344. doi: 10.1007/s00417-013-2387-3. [DOI] [PubMed] [Google Scholar]
- 31.Matoba T, Shimokawa H, Nakashima M, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: Role of NO and H(2)O(2) Am J Physiol Heart Circ Physiol. 2009;297:H1087–H1095. doi: 10.1152/ajpheart.00356.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 34.Maddox DE, Shibata S, Goldstein IJ. Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc Natl Acad Sci USA. 1982;79:166–170. doi: 10.1073/pnas.79.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimayuga PC, Li H, Chyu KY, et al. T cell modulation of intimal thickening after vascular injury: The bimodal role of IFN-gamma in immune deficiency. Arterioscler Thromb Vasc Biol. 2005;25:2528–2534. doi: 10.1161/01.ATV.0000190606.41121.00. [DOI] [PubMed] [Google Scholar]
- 36.Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35:213–221. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 37.Schweitzer KS, Johnstone BH, Garrison J, et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med. 2011;183:215–225. doi: 10.1164/rccm.201001-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo YR, Chen CC, Goto S, et al. Modulation of immune response and T-cell regulation by donor adipose-derived stem cells in a rodent hind-limb allotransplant model. Plast Reconstr Surg. 2011;128:661e–672e. doi: 10.1097/PRS.0b013e318230c60b. [DOI] [PubMed] [Google Scholar]
- 39.Premaratne GU, Ma LP, Fujita M, et al. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: Anti-inflammatory role. J Cardiothorac Surg. 2011;6:43. doi: 10.1186/1749-8090-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutertre CA, Clement M, Morvan M, et al. Deciphering the stromal and hematopoietic cell network of the adventitia from non-aneurysmal and aneurysmal human aorta. PLoS One. 2014;9:e89983. doi: 10.1371/journal.pone.0089983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukai T, Folz RJ, Landmesser U, et al. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res. 2002;55:239–249. doi: 10.1016/s0008-6363(02)00328-0. [DOI] [PubMed] [Google Scholar]
- 42.Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho KW, Morris DL, Lumeng CN. Flow cytometry analyses of adipose tissue macrophages. Methods Enzymol. 2014;537:297–314. doi: 10.1016/B978-0-12-411619-1.00016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popel AS, Johnson PC. Microcirculation and hemorheology. Annu Rev Fluid Mech. 2005;37:43–69. doi: 10.1146/annurev.fluid.37.042604.133933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Psaltis PJ, Puranik AS, Spoon DB, et al. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res. 2014;115:364–375. doi: 10.1161/CIRCRESAHA.115.303299. [DOI] [PubMed] [Google Scholar]
- 47.Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.