Abstract
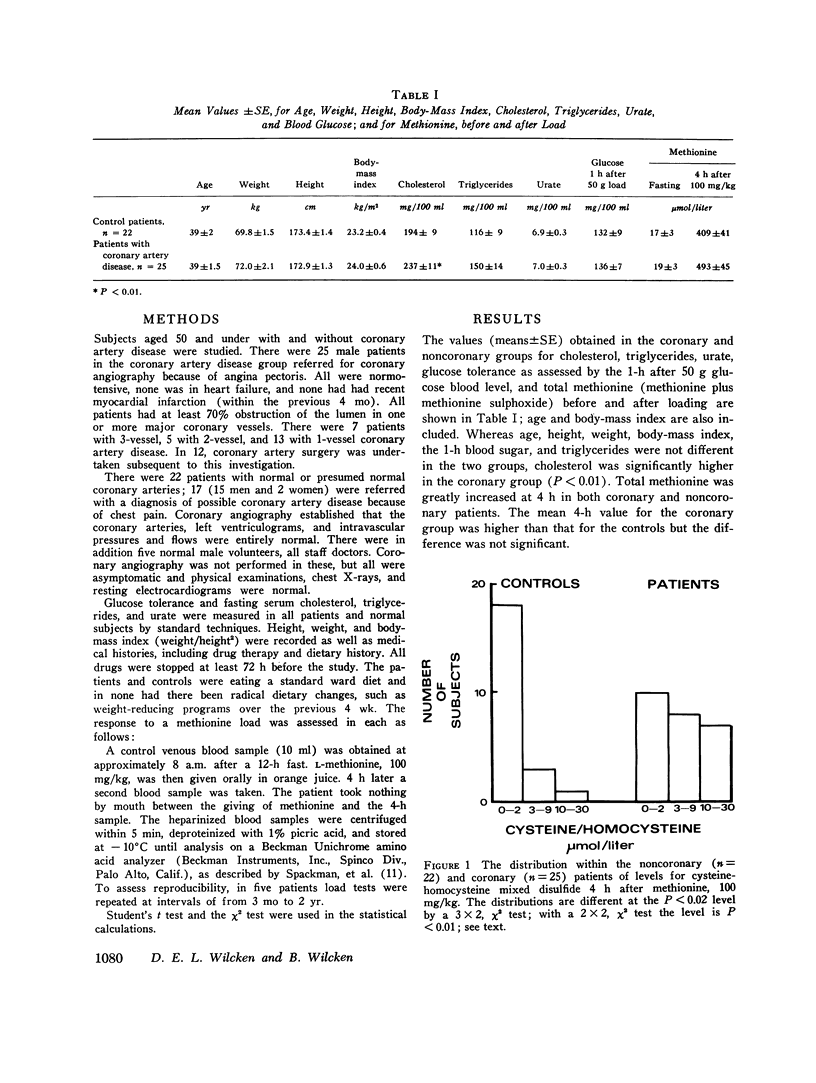
Homocystinuria, an abnormality of methionine metabolism is associated with severe vascular disease in infancy and childhood. Homocysteine is formed during the metabolism of methionine and accumulations of this and of cysteine-homocysteine mixed disulfide in the plasma indicate a partial block in the methionine degradation pathway. Methionine metabolism was investigated in 25 patients aged under 50 with angiographically proved coronary artery disease and in 22 control patients, of whom 17 had normal coronary arteries at angiography and 5 were healthy volunteers. After an overnight fast, venous blood was drawn before and 4 h after oral L-methionine, 100 mg/kg. Plasma methionine levels at 4 h were not different in the two groups, but there were significant differences in the levels of cysteine-homocysteine mixed disulfide. This was detected in 5 of 22 in the noncoronary group and in higher concentration in 17 of 25 coronary patients (P less than 0-01). Age, weight, height, body-mass index, glucose tolerance, fasting serum urate, and triglycerides were not different, but serum cholesterol was higher in the coronary patients (P lessthan 0.01). These results suggest a reduced ability to metabolise homocysteine in some patients with premature coronary artery disease when this pathway is stressed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DICKINSON J. C., ROSENBLUM H., HAMILTON P. B. ION EXCHANGE CHROMATOGRAPHY OF THE FREE AMINO ACIDS IN THE PLASMA OF THE NEWBORN INFANT. Pediatrics. 1965 Jul;36:2–13. [PubMed] [Google Scholar]
- HAUST M. D., MORE R. H., MOVAT H. Z. The role of smooth muscle cells in the fibrogenesis of arteriosclerosis. Am J Pathol. 1960 Oct;37:377–389. [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J., Scott C. R., Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974 Sep 12;291(11):537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- Keys A., Aravanis C., Blackburn H., Van Buchem F. S., Buzina R., Djordjevic B. S., Fidanza F., Karvonen M. J., Menotti A., Puddu V. Probability of middle-aged men developing coronary heart disease in five years. Circulation. 1972 Apr;45(4):815–828. doi: 10.1161/01.cir.45.4.815. [DOI] [PubMed] [Google Scholar]
- Levy H. L., Mudd S. H., Schulman J. D., Dreyfus P. M., Abeles R. H. A derangement in B12 metabolism associated with homocystinemia, cystathioninemia, hypomethioninemia and methylmalonic aciduria. Am J Med. 1970 Mar;48(3):390–397. doi: 10.1016/0002-9343(70)90070-7. [DOI] [PubMed] [Google Scholar]
- McCully K. S., Ragsdale B. D. Production of arteriosclerosis by homocysteinemia. Am J Pathol. 1970 Oct;61(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- McCully K. S. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969 Jul;56(1):111–128. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. II. Effect of repeatedly injected foreign protein in rabbits fed a lipid-rich, cholesterol-poor diet. Am J Pathol. 1973 Nov;73(2):265–300. [PMC free article] [PubMed] [Google Scholar]
- Moore S. Thromboatherosclerosis in normolipemic rabbits. A result of continued endothelial damage. Lab Invest. 1973 Nov;29(5):478–487. [PubMed] [Google Scholar]
- Perry T. L., Hansen S. Technical pitfalls leading to errors in the quantitation of plasma amino acids. Clin Chim Acta. 1969 Jul;25(1):53–58. doi: 10.1016/0009-8981(69)90226-5. [DOI] [PubMed] [Google Scholar]
- Sardharwalla I. B., Fowler B., Robins A. J., Komrower G. M. Detection of heterozygotes for homocystinuria. Study of sulphur-containing amino acids in plasma and urine after L-methionine loading. Arch Dis Child. 1974 Jul;49(7):553–559. doi: 10.1136/adc.49.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaet T. H. Editorial: Optimism in the control of atherosclerosis. N Engl J Med. 1974 Sep 12;291(11):576–577. doi: 10.1056/NEJM197409122911109. [DOI] [PubMed] [Google Scholar]
- Steele P. P., Weily H. S., Davies H., Genton E. Platelet function studies in coronary artery disease. Circulation. 1973 Dec;48(6):1194–1200. doi: 10.1161/01.cir.48.6.1194. [DOI] [PubMed] [Google Scholar]
- Stehbens W. E. Haemodynamic production of lipid deposition, intimal teats, mural dissection and thrombosis in the blood vessel wall. Proc R Soc Lond B Biol Sci. 1974 Feb 12;185(1080):357–373. doi: 10.1098/rspb.1974.0024. [DOI] [PubMed] [Google Scholar]
- Wilcken B. Letter: Incidence of homocystinuria. Lancet. 1975 Feb 1;1(7901):273–274. doi: 10.1016/s0140-6736(75)91168-x. [DOI] [PubMed] [Google Scholar]


