Abstract
CtBP1 and CtBP2 are closely related and evolutionarily conserved transcriptional corepressors. There is strong evidence linking CtBPs to tumorigenesis and tumor progression. CtBPs promote epithelial-mesenchymal transition and function as apoptosis antagonists. Also, CtBPs mediate repression of several tumor suppressor genes. Certain tumor suppressors also target CtBPs to restrain their tumor-promoting activity. Down-regulation of CtBPs mediated by some tumor suppressors results in p53-independent apoptosis and reduced tumor cell migration and invasion. The role of CtBPs in modulating the activities of different tumor suppressors is reviewed here. The results discussed here suggest that CtBPs may constitute a novel p53-independent anticancer target.
Background and Introduction
The CtBP family proteins are modulators of several essential cellular processes. Vertebrate genomes code for two related proteins, CtBP1 and CtBP2, whereas the invertebrate genomes code for a single protein. Vertebrate CtBP genes perform genetically related and unique functions during development. The major splice forms of CtBP1 and CtBP2 (collectively called CtBP here) function as transcriptional corepressors, whereas minor splice variants perform diverse cytosolic functions (1). A role of CtBP in oncogenesis was first inferred from studies with the adenovirus E1A oncogene (2–4). These studies identified the founding member of the CtBP family (CtBP1) and showed that E1A mutants in the CtBP-binding motif (PLDLS) enhanced transformation of primary rodent epithelial cells in cooperation with the activated Ras oncogene. Cells transformed by the mutant E1A and Ras were also highly tumorigenic and metastatic, suggesting that E1A-CtBP interaction restrains tumorigenesis by antagonizing the activity of CtBP. Studies by Frisch and colleagues (5, 6) showed that E1A expression in several cancer cell lines reverses the oncogenic properties as a result of “epithelialization” by activating expression of several epithelial genes and identified CtBP as an antagonist of the epithelial phenotype (7). Mutant E1A defective in interaction with CtBP did not activate expression of epithelial cell adhesion molecules such as E-cadherin, desmoglein-2, and plakoglobin. This study further identified the CtBP-interacting E-box repressor Zeb (8) as the negative regulator of E-cadherin, suggesting that E1A might activate E-cadherin expression by relieving transcriptional repression by disrupting Zeb-CtBP interaction (7).
CtBP in Epithelial-Mesenchymal Transition
CtBP-mediated repression of adhesion molecules such as E-cadherin suggests that CtBP is important in promoting epithelial-to-mesenchymal transition (EMT), a step that contributes to the malignant property of tumor cells due to the loss of intercellular adhesion in tumors, acquisition of motile and invasive phenotypes, and resistance to apoptosis (9). Zeb1 overexpression with concomitant E-cadherin repression has been reported in several human cancers. Studies with colon carcinomas revealed high levels of Zeb1 and CtBP were correlated with low levels of E-cadherin (10). Transcription factors such as Twist, Snail, Slug, and Zeb2 (SIP1) also repress E-cadherin expression in various cellular contexts. Among these factors, the activity of Zeb2, such as Zeb1, is also dependent on CtBP (11). The effect of Snail on E-cadherin may indirectly depend on CtBP via Zeb1. Overexpression of Snail increases expression of Zeb1 (12).
CtBP-mediated transcriptional repression of E-cadherin seems to be regulated by the hypoxic environment seen in solid tumors with poor vascularization and high metabolic activity. A hypoxic condition that increases free NADH levels (13) has been shown to enhance recruitment of CtBP to the E-cadherin promoter and motility of tumor cells (14). Cell motility was reduced by siRNA-mediated depletion of CtBP, suggesting that the effect was independent of HIF-1α or other E-cadherin repressors.
CtBP as an Apoptosis Antagonist
Work on E1A also revealed a potential apoptosis antagonist activity of CtBP (7). Tumor cell lines expressing E1A mutants defective in interaction with CtBP were less sensitive to “anoikis” (a form of apoptosis mediated by the loss of contact from the extracellular matrix). A comprehensive gene expression profiling study (15) using CtBP-null mouse embryo fibroblasts (MEF) and CtBP1-rescued MEF revealed corepression of several epithelial genes and proapoptotic genes such as PERP (p53-effector related to pmp-22), p21, Bax, and Noxa by CtBP. Additionally, PTEN, a phosphatase and a negative regulator of the survival kinase Akt was also identified as a CtBP repression target. Consistent with these results, the CtBP-null MEFs were found to be hypersensitive to anoikis and apoptotic stimuli such as FasL and UV. Thus, CtBP seems to contribute to EMT by repressing expression of epithelial cell adhesion molecules and proapoptotic genes. The role of CtBP in EMT may be particularly critical in cancers (e.g., breast, colon, and endometrial) that overexpress Zeb.
CtBP in Repression of Tumor Suppressors
As discussed above, CtBP plays a prominent role in repression of E-cadherin, which suppresses tumorigenesis by restricting tumor cell motility and invasion. CtBP may also play a role in repression of the PTEN tumor suppressor gene. It is well-established that the tumor suppressor activity of PTEN is linked to its control of cell migration through the PI3K/Akt pathway. In addition to the microarray data (15), transient overexpression of CtBP2 was shown to reduce PTEN expression with a concomitant increase in phospho-Akt (16). A role for Snail in repression of the PTEN promoter has been reported (17). As pointed out earlier, the mammalian Snail may also function through Zeb1 and CtBP.
Recent results suggest that CtBP may modulate the expression and activities of the Ink4 family tumor suppressors. The Ink4 region codes for three different cell cycle inhibitors, p16Ink4a, Ink4a/Arf, and p15Ink4b. Although p16Ink4a and p15Ink4b function in the Rb pathway by inhibiting CDK4 and CDK6, Ink4a/Arf mediates its tumor suppressive function by stabilizing p53 and by p53-independent mechanisms. Rocco and colleagues (18) have reported that expression of E1A in primary human fibroblasts and keratinocytes resulted in a substantial increase in p16Ink4a expression. The effect of E1A on p16Ink4a expression was lost in cells that expressed a mutant of E1A lacking the CtBP-binding motif, suggesting that interaction of E1A with CtBP was responsible for this activity. The specific involvement of CtBP was ascertained by shRNA-mediated knockdown of CtBP1/2 and by determining the effect of CtBP overexpression on p16 promoter activity in reporter assays. Depletion of CtBP1/2 also resulted in reduction of an epigenetic histone mark associated with transcriptional repression (trimethylation of lysine-27 of H3, H3K27me3) of the p16 promoter, without significant change in the histone mark associated with transcriptional activation (H3K4me3). A hypoxic experimental condition was also shown to enhance the occupancy of CtBP at the p16 promoter with a corresponding decrease in mRNA levels.
In addition to the involvement of CtBP in repression of p16Ink4a, CtBP may also be important in regulating p15Ink4b expression. A dramatic increase in expression of p15Ink4b has been reported in Zeb1 null MEF, linking Zeb1 to p15Ink4b repression (19). Considering the dependence of Zeb1 on CtBP in transcriptional repression, it has been suggested that transforming growth factor β, an activator of p15Ink4b expression via activated SMAD, may mediate its effect by forming an activation complex consisting of Zeb1-SMAD-p300 and acetylation of the CtBP-binding domain resulting in displacement of CtBP (11, 19). CtBP may play a role in repression of the p15Ink4b gene through other transcriptional repressors as well. A recent genome wide expression profiling study identified p15Ink4b as the target gene for the oncoprotein Znf217 (20). It is well established that Znf217 forms a repression complex with corepressors CoREST and CtBP (21). Although the precise role of CtBP in Znf217-mediated repression of the p15Ink4b promoter remains to be investigated, it is possible that CtBP may contribute to the overall repressive function of Znf217.
CtBP as a Target for Tumor Suppressors
Although CtBP represses expression of several tumor suppressors, others in the tumor suppressor clan take their revenge by targeting CtBP for destruction. The homeodomain interacting protein kinase 2 (HIPK2) is a tumor suppressor that functions primarily by phosphorylation and stabilization of p53. A p53-independent mechanism of action of HIPK2 may include phosphorylation and proteasomal degradation of CtBP. In cells exposed to UV, CtBP was shown to be phosphorylated by HIPK2 (at Ser-422 of CtBP1; ref. 22) or c-Jun-NH2-kinase (JNK)1 (23) resulting in proteasome-mediated clearance of CtBP and apoptosis. Apoptosis induced by HIPK2 and JNK1 through degradation of CtBP seems to be independent of p53 because siRNA-mediated depletion of CtBP in p53-null tumor cells resulted in apoptosis. Interestingly, a sustained activation of JNK1 by treatment with the common chemotherapeutic drug cisplatin resulted in degradation of CtBP and apoptosis of H1299 tumor cells that lack p53. Because cisplatin treatment also activates HIPK2 (24), it is possible that HIPK2 may also contribute to cisplatin-induced apoptosis in a p53-independent manner. These results suggest that CtBP may be an attractive target for chemotherapy in human cancers that lack functional p53.
Recent work from the laboratories of Grossman and Lewis (16, 25, 26) indicate that CtBP is a direct target for the Ink4a/Arf tumor suppressor. Attempts to elucidate the p53-independent mode of action of Arf identified CtBP2 as an Arf-interacting protein in yeast two hybrid screenings (25). Further studies confirmed interaction of both CtBP2 and CtBP1 with mArf and hArf via a conserved domain. They showed that exogenous introduction of Arf in human colon cancer cells lacking both p53 and Arf inhibited cell migration under hypoxic conditions via down-regulation of CtBP2 (16). These studies also linked the effect of Arf overexpression and down-regulation of CtBP2 with the regulation of cell migration by the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Overexpression of CtBP2 in tumor cells resulted in reduction of PTEN levels accompanied by an increase in the levels of PI3K and pAkt under hypoxia. A similar effect was not observed with a CtBP2 mutant in the NAD(H) binding motif, suggesting that the effect of CtBP2 on PTEN expression and activation of PI3K and Akt was a result of increase in the ratio of NADH/NAD in cells exposed to hypoxia.
Ectopic expression of p19Arf in mouse hepatocellular carcinoma cell lines lacking p53 and p19Arf was also shown to inhibit the invasive potential of the tumor cells, without significant alterations in cell proliferation, anchorage-independent growth, and expression of EMT markers, including E-cadherin (26). The effect of p19Arf was linked to CtBP using Arf mutants defective in interaction with CtBP and knockdown of CtBP1/2. These results suggest that CtBP may control tumor cell migration and invasion by EMT-independent mechanisms in these cells. Some of these activities may be linked to the activation of PTEN expression as a result of Arf-CtBP interaction. The possibilities such as cytoskeletal remodeling during cell migration and invasion influenced by Arf-CtBP interaction remains to be elucidated. Also, the issue of how Arf down-regulates the activity of CtBP needs to be addressed. The nucleolar localization of CtBP mediated by Arf in transfected cells (25) could at least partially contribute to the loss of CtBP transcriptional repression activity. The interesting results obtained from these in vitro studies emphasize the need to carry out a detailed analysis of CtBP status along with Arf and PTEN in human cancers.
Three different groups have discovered a strong interaction between CtBP and another tumor suppressor, adenomatous polyposis coli (APC; refs. 27–29). One of these reports (29) provided compelling evidence that APC may function as a platform for proteasome-dependent degradation of CtBP1, in addition to regulating the stability of β-catenin. This view was supported by the observation that adenomas from patients with familial adenomatous polyposis contained high levels of CtBP1 compared with matched healthy samples. Furthermore, reintroduction of APC in colon carcinoma cell lines also resulted in degradation of CtBP1. These results received strong support from a zebrafish model harboring a mutant form of APC and expressing high levels of CtBP1 (whereas CtBP1 mRNA level remained unchanged). These results are consistent with a model in which APC targets both h-catenin and CtBP1 simultaneously to inhibit expression of Wnt target genes and relieve repression of CtBP-target genes (e.g., retinol dehydrogenase) that are involved in intestinal cell differentiation. It is known that HIPK2 is also a negative regulator of Wnt signaling via repression of the transcriptional activity of β-catenin (30). This activity of HIPK2 has been linked to interaction with CtBP. Based on the results discussed above, it would be interesting to determine whether the HIPK2-CtBP complex also contains APC and β-catenin. Thus, it seems that APC may exert one of its tumor suppressive effects via CtBP, in addition to its well-known role in inhibiting the canonical Wnt-signaling pathway.
Concluding Remarks
The results reviewed above suggest a strong role for CtBP as a negative regulator of important tumor suppressors such as E-cadherin, p16Ink4a, p15Ink4b, and PTEN. Furthermore, tumor suppressors such as HIPK2, Ink4a/Arf, and APC target CtBP for down-regulation to induce apoptosis. These attributes suggest that CtBP plays a critical positive role in tumorigenic conversion of normal cells and in tumor progression (Fig. 1). The availability of knockout mouse models for CtBP1 and CtBP2 offers an excellent opportunity to investigate the relationship among different tumor suppressors and CtBP in oncogenesis in the mouse model. Because CtBP depletion induces apoptosis in p53-null tumor cells, CtBP may constitute an important new target for anticancer therapies. The notion may have a more general appeal considering that CtBP may contribute to the activities of oncogenes such as Evi (31), Hdm2 (32), and Znf217 (33) and that CtBP1 may contribute multiple drug resistance through activation of the MDR1 gene (34). Because the structures of CtBP1 and CtBP2 and the mode of interaction of critical CtBP cofactors have been elucidated, it may be possible to formulate structure-based anti-CtBP strategies. Although some recent genomic studies have identified CtBP as a biomarker in prostate (35) and colon cancers (10), more large-scale studies with multiple human cancers are warranted considering the exciting results obtained from cell culture studies.
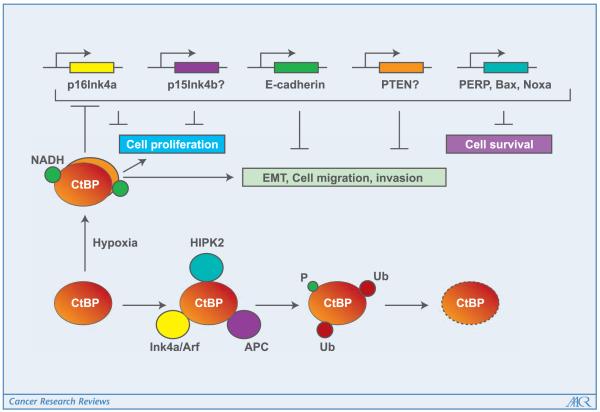
Figure 1.
Role of CtBP in tumorigenesis and tumor progression. The transcriptional corepressor activity of CtBP is depicted to be stimulated under hypoxic tumor environment as a result of elevated NADH levels and CtBP dimerization. CtBP is shown to enhance cell proliferation via transcriptional repression of cell cycle inhibitors p16Ink4a and possibly p15Ink4b. The transcriptional repression activity of CtBP is shown to repress E-cadherin and possibly PTEN to promote EMT, tumor cell migration, and invasion. As a result of repression of the proapoptotic genes (PERP, Bax, and Noxa), CtBP is shown to promote cell survival. These activities of CtBP are depicted to be down-regulated by physical association with tumor suppressors HIPK2 or Ink4a/Arf or APC, which promote CtBP degradation as a result of phosphorylation and ubiquitination.
Acknowledgments
We thank Bill Wold, Maurice Green, Ling Zhao, T. Subramanian, and Carolyn Mulhall for their comments on the manuscript and S. Vijayalingam for the figure. The author received support from a grant by the National Cancer Institute (CA84941).
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Chinnadurai G. CtBP, an Unconventional transcriptional corepressor in development and oncogenesis. Mol Cell. 2002;9:213–24. doi: 10.1016/s1097-2765(02)00443-4. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian T, La Regina M, Chinnadurai G. Enhanced ras oncogene mediated cell transformation and tumorigenesis by adenovirus 2 mutants lacking the C-terminal region of E1a protein. Oncogene. 1989;4:415–20. [PubMed] [Google Scholar]
- 3.Boyd JM, Subramanian T, Schaeper U, et al. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. EMBO J. 1993;12:469–78. doi: 10.1002/j.1460-2075.1993.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaeper U, Boyd JM, Verma S, et al. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc Natl Acad Sci U S A. 1995;92:10467–71. doi: 10.1073/pnas.92.23.10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisch SM. Antioncogenic effect of adenovirus E1A in human tumor cells. Proc Natl Acad Sci U S A. 1991;88:9077–81. doi: 10.1073/pnas.88.20.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisch SM. E1a induces the expression of epithelial characteristics. J Cell Biol. 1994;127:1085–96. doi: 10.1083/jcb.127.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grooteclaes ML, Frisch SM. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene. 2000;19:3823–8. doi: 10.1038/sj.onc.1203721. [DOI] [PubMed] [Google Scholar]
- 8.Postigo AA, Dean DC. ZEB represses transcription through interaction with the corepressor CtBP. Proc Natl Acad Sci U S A. 1999;96:6683–8. doi: 10.1073/pnas.96.12.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 10.Pena C, Garcia JM, Garcia V, et al. The expression levels of the transcriptional regulators p300 and CtBP modulate the correlations between SNAIL, ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas. Int J Cancer. 2006;119:2098–104. doi: 10.1002/ijc.22083. [DOI] [PubMed] [Google Scholar]
- 11.Postigo AA, Depp JL, Taylor JJ, et al. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–62. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guaita S, Puig I, Franci C, et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem. 2002;277:39209–16. doi: 10.1074/jbc.M206400200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Piston DW, Goodman RH. Regulation of corepressor function by nuclear nadH. Science. 2002;295:1895–7. doi: 10.1126/science.1069300. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Wang SY, Nottke AC, et al. Redox sensor CtBP mediates hypoxia-induced tumor cell migration. Proc Natl Acad Sci U S A. 2006;103:9029–33. doi: 10.1073/pnas.0603269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grooteclaes M, Deveraux Q, Hildebrand J, et al. C-terminal-binding protein corepresses epithelial and proapoptotic gene expression programs. Proc Natl Acad Sci U S A. 2003;100:4568–73. doi: 10.1073/pnas.0830998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paliwal S, Kovi RC, Nath B, et al. The alternative reading frame tumor suppressor antagonizes hypoxia-induced cancer cell migration via interaction with the COOH-terminal binding protein corepressor. Cancer Res. 2007;67:9322–9. doi: 10.1158/0008-5472.CAN-07-1743. [DOI] [PubMed] [Google Scholar]
- 17.Escriva M, Peiro S, Herranz N, et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during γ radiation-induced apoptosis. Mol Cell Biol. 2008;28:1528–40. doi: 10.1128/MCB.02061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mroz EA, Baird AH, Michaud WA, et al. COOH-terminal binding protein regulates expression of the p16INK4A tumor suppressor and senescence in primary human cells. Cancer Res. 2008;68:6049–53. doi: 10.1158/0008-5472.CAN-08-1279. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, El-Naggar S, Darling DS, et al. Zeb1 links epithelial-mesenchymal transition and cellular senescence. Development. 2008;135:579–88. doi: 10.1242/dev.007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thillainadesan G, Isovic M, Loney E, et al. Genome analysis identifies the p15ink4b tumour suppressor as a direct target of the ZNF217/Corest complex. Mol Cell Biol. 2008;28:6066–77. doi: 10.1128/MCB.00246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowger JJ, Zhao Q, Isovic M, et al. Biochemical characterization of the zinc-finger protein 217 transcriptional repressor complex: identification of a ZNF217 consensus recognition sequence. Oncogene. 2007;26:3378–86. doi: 10.1038/sj.onc.1210126. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Yoshimatsu Y, Hildebrand J, et al. Homeodomain interacting protein kinase 2 promotes apoptosis by downregulating the transcriptional corepressor CtBP. Cell. 2003;115:177–86. doi: 10.1016/s0092-8674(03)00802-x. [DOI] [PubMed] [Google Scholar]
- 23.Wang SY, Iordanov M, Zhang Q. c-Jun NH2-terminal kinase promotes apoptosis by down-regulating the transcriptional co-repressor CtBP. J Biol Chem. 2006;281:34810–5. doi: 10.1074/jbc.M607484200. [DOI] [PubMed] [Google Scholar]
- 24.Di Stefano V, Soddu S, Sacchi A, et al. HIPK2 contributes to PCAF-mediated p53 acetylation and selective transactivation of p21Waf1 after nonapoptotic DNA damage. Oncogene. 2005;24:5431–42. doi: 10.1038/sj.onc.1208717. [DOI] [PubMed] [Google Scholar]
- 25.Paliwal S, Pande S, Kovi RC, et al. Targeting of C-terminal binding protein (CtBP) by ARF results in p53-independent apoptosis. Mol Cell Biol. 2006;26:2360–72. doi: 10.1128/MCB.26.6.2360-2372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YW, Paliwal S, Draheim K, et al. p19Arf inhibits the invasion of hepatocellular carcinoma cells by binding to C-terminal binding protein. Cancer Res. 2008;68:476–82. doi: 10.1158/0008-5472.CAN-07-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamada F, Bienz M. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear β-catenin from TCF. Dev Cell. 2004;7:677–85. doi: 10.1016/j.devcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Sierra J, Yoshida T, Joazeiro CA, et al. The APC tumor suppressor counteracts β-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20:586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadauld LD, Phelps R, Moore BC, et al. Adenomatous polyposis coli control of C-terminal binding protein-1 stability regulates expression of intestinal retinol dehydrogenases. J Biol Chem. 2006;281:37828–35. doi: 10.1074/jbc.M602119200. [DOI] [PubMed] [Google Scholar]
- 30.Wei G, Ku S, Ma GK, et al. HIPK2 represses β-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:13040–5. doi: 10.1073/pnas.0703213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izutsu K, Kurokawa M, Imai Y, et al. The corepressor CtBP interacts with Evi-1 to repress transforming growth factor β signaling. Blood. 2001;97:2815–22. doi: 10.1182/blood.v97.9.2815. [DOI] [PubMed] [Google Scholar]
- 32.Mirnezami AH, Campbell SJ, Darley M, et al. Hdm2 recruits a hypoxia-sensitive corepressor to negatively regulate p53-dependent transcription. Curr Biol. 2003;13:1234–9. doi: 10.1016/s0960-9822(03)00454-8. [DOI] [PubMed] [Google Scholar]
- 33.Quinlan KG, Verger A, Yaswen P, et al. Amplification of zinc finger gene 217 (ZNF217) and cancer: when good fingers go bad. Biochim Biophys Acta. 2007;1775:333–40. doi: 10.1016/j.bbcan.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Jin W, Scotto KW, Hait WN, Yang JM. Involvement of CtBP1 in the transcriptional activation of the MDR1 gene in human multidrug resistant cancer cells. Biochem Pharmacol. 2007;74:851–9. doi: 10.1016/j.bcp.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas G, Jacobs KB, Yeager M, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310–5. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]