Summary
Background
Laminin is the most abundant non-collagenous protein in the basement membrane. Recent studies have shown that laminin supports platelet adhesion, activation and aggregation under flow conditions, highlighting a possible role for laminin in hemostasis.
Objective
To investigate the ability of laminin to promote coagulation and support thrombus formation under shear.
Results and methods
Soluble laminin accelerated factor (F) XII activation in a purified system, and shortened the clotting time of recalcified plasma in a FXI- and FXII-dependent manner. Laminin promoted phosphatidylserine exposure on platelets and supported platelet adhesion and fibrin formation in recalcified blood under shear flow conditions. Fibrin formation in laminin-coated capillaries was abrogated by an antibody that interferes with FXI activation by activated FXII, or an antibody that blocks activated FXI activation of FIX.
Conclusion
This study identifies a role for laminin in the initiation of coagulation and the formation of platelet-rich thrombi under shear conditions in a FXII-dependent manner.
Keywords: factor XI, factor XII, fibrin, laminin, platelets
Introduction
When vessel walls are damaged, exposed extracellular matrix (ECM) proteins trigger a series of events that lead to the formation of a hemostatic plug [1,2]. Hemostasis requires an orchestrated series of receptor-mediated events to facilitate platelet recruitment to the ECM in the presence of shear, leading to platelet adhesion, rapid cellular activation, and accumulation of additional platelets (see review [3]). ECM-bound von Willebrand factor (vWF) plays a critical role in initial platelet deposition at high shear due to the rapid on-rate of binding between the platelet receptor glycoprotein Ibα (GPIbα) and vWF [4–6]. The platelet receptors GPVI and α2β1 mediate platelet activation and subsequent adhesion to the exposed matrix proteins, while integrin αIIbβ3-mediated binding of additional platelets is required for the formation of a stable hemostatic plug [7].
Concomitant with platelet recruitment and activation are the first steps of blood coagulation (secondary hemostasis), namely the exposure of blood to tissue factor [8]. Initiation of the coagulation cascade leads to the sequential conversion of coagulation factors into their corresponding active serine protease forms, and culminates in the generation of thrombin. Thrombin not only activates platelets and cleaves fibrinogen, which leads to fibrin production and clot formation, but also mediates the activation of coagulation factors (F) XIII and XI and cofactors V and VIII, which in concert contribute to the rapid and efficient arrest of bleeding at sites of vascular injury.
Recent work has highlighted a dual role of exposed ECM proteins in contributing to thrombus formation. van der Meijden [9] and colleagues demonstrated that the ECM protein collagen triggers the activation of coagulation via FXII, and thus subsequent FXI activation, in addition to the well-defined role for collagen in mediating platelet recruitment and activation. It is currently unknown whether additional ECM proteins, such as laminin, can contribute to the initiation of coagulation and thrombus formation in a similar fashion.
The members of the laminin family of heterotrimers are major constituents of all basement membranes, the 50- to 100-nm thick layer of specialized ECM protein complexes found basolateral to all cell monolayers (epithelium and endothelium) in tissues. We recently demonstrated that laminin activates platelets through the collagen receptor GPVI, and it is established that laminin supports platelet adhesion through the integrin α6β1 [10,11]. Further studies by Inoue and colleagues revealed that immobilized laminin supports platelet recruitment under shear flow in a GPIbα-vWF dependent manner [12]. Thus, the process of platelet recruitment, activation and adhesion on laminin under shear is mechanistically analogous to the interactions of platelets with collagen under shear, prompting the hypothesis that laminin and collagen may act together to promote hemostasis. In the present study, we aimed to determine if the similarity in function between laminin and collagen extends to the initiation of coagulation. Here, we present the first evidence that laminin can contribute to the activation of FXII and that surface-associated laminin alone can trigger the formation of fibrin- and platelet-rich clots under shear.
Experimental procedures
Reagents
Plasma-derived FXII was purchased from Haematologic Technologies, Inc. (Essex Junction, VT, USA). Plasmin and corn trypsin inhibitor (CTI) were from Enzyme Research Laboratories, Inc. (South Bend, IN, USA). Pefachrome FXIIa was obtained from Centerchem, Inc. (Norwalk, CT, USA). Tissue factor was purchased from Siemens Healthcare Diagnostics (Deerfield, IL, USA). Purified D-dimer was obtained from Cell Sciences (Canton, MA, USA). Equine Type I collagen was from Chrono-log Corporation (Havertown, PA, USA). Murine monoclonal anti-factor XI antibodies 1A6 and 14E11 were cloned, expressed and purified as described [13,14]. Laminin from human placenta was purchased from Sigma-Aldrich (St Louis, MO, USA). A silver stain of laminin subjected to SDS–PAGE under reducing conditions showed bands at molecular weights consistent with those listed in the Sigma product sheet (Supplemental Fig. S1). The predominant laminin isoform present in this preparation has been shown to be α5β1γ1, as characterized elsewhere [15]. All other reagents were from Sigma-Aldrich, Inc. (St Louis, MO, USA) or previously named sources [16–18].
Collection of human blood and preparation of plasmas and washed platelets
Human venous blood was drawn by venipuncture from healthy adult volunteers into sodium citrate. In order to reduce potential tissue factor contamination, the first 2 mL of each blood draw were taken into a separate syringe and discarded. Platelet-rich plasma (PRP) was prepared by centrifugation of citrated whole blood (in 0.38% w/v sodium citrate) at 200 ×g for 20 min. Fresh PRP was prepared prior to each experiment. For washed platelet preparation, platelets were pelleted by centrifugation of PRP (in 0.38% sodium citrate and 1:10 v/v acid/citrate/dextrose) at 1000 × g in the presence of prostacyclin (0.1 μg mL−1). The pellet was washed once by centrifugation and resuspended in modified Tyrode buffer (in mmol L−1: 129 NaCl, 0.34 Na2HPO4, 2.9 KCl, 12 NaHCO3, 20 HEPES, 5 glucose, 1 MgCl2), pH 7.3 as previously described [18].
Platelet-poor plasma (PPP) was prepared by centrifugation of citrated whole blood (in 0.32% w/v sodium citrate) from three separate donors at 2150 × g for 10 min. Further centrifugation of the plasma fractions at 2150 × g for 10 min yielded PPP, which was then pooled and stored at −80 °C until use.
Clotting times and activation of FXII
Clotting times of human PRP or PPP were measured with a KC4 Coagulation Analyzer (Trinity Biotech, Bray, Co. Wicklow, Ireland). Samples were pretreated at room temperature (RT) for 3 min with 1A6, 14E11 or CTI, followed by incubation with vehicle, collagen or laminin in the absence or presence of tissue factor for 3 min at 37 °C. Coagulation was then initiated by the addition of Ca2+(16.6 mmol L−1 final), and clotting time was recorded.
Cleavage of the activated factor XII (FXIIa) substrate, Pefachrome FXIIa, was monitored in the presence of vehicle or laminin. Reactions contained combinations of purified FXII (95 nmol L−1), laminin (5–50 μg mL−1), CTI (4 μmol L−1), prekallikrein (30 nmol L−1), and high-molecular-weight kininogen (30 nmol L−1) in HEPES buffer (136 mmol L−1 NaCl, 5 mmol L−1 HEPES, 2.7 mmol L−1 KCl, 2 mmol L−1 MgCl2, 0.42 mmol L−1 NaH2PO4, pH 7.45). After addition of 0.8 mmol L−1 Pefachrome FXIIa substrate, the rate of increase in absorption at 405 nm was determined for a period of 10 min.
Detection of phosphatidylserine exposure
Washed platelets were incubated over laminin- or collagen-coated coverslips for 30 min at 37 °C. Coverslips were gently washed with modified Tyrode buffer to remove non-adherent cells before incubation with Oregon Green® 488 conjugated Annexin V (Invitrogen Corp., Carlsbad, CA, USA) in a binding buffer (in mmol L−1: 140 NaCl, 10 HEPES, 2 CaCl2, pH 7.4). Coverslips were assembled onto a Quick change Chamber (Warner Instruments) and imaged using differential interference contrast (DIC) optics and fluorescence microscopy.
Recalcified blood flow
Glass capillary tubes (0.2 × 2 mm; VitroCom, Mountain Lakes, NJ, USA) were incubated for 1 h at RT with a solution of laminin (50 μg mL−1), collagen (100 μg mL−1), or denatured BSA (5 mg mL−1). Surfaces were blocked with 5 mg mL−1 denatured BSA for 1 h prior to assembly into a flow system on the stage of a Zeiss Axiovert 200 M microscope (Carl Zeiss, Thornwood, NY, USA). Sodium citrate (0.38% w/v) anticoagulated whole blood was perfused through the chamber for 12 min at an initial wall shear rate of 250 s−1. A solution of 37.5 mmol L−1 Ca2+ and 18.75 mmol L−1 Mg2+ was perfused at 1:5 vol/vol of the blood using a second, infusion syringe pump. The Ca2+/Mg2+ solution entered the perfusion system via a Y-connection just prior to the capillary. Blood flow was followed by washing with modified Tyrode buffer and imaging the capillaries using DIC optics. Washed capillaries were treated for 5 min with 1x lysis buffer (10 mmol L−1 Tris, 150 mmol L−1 NaCl, 1 mmol L−1 EGTA, 1 mmol L−1 EDTA, 1% NP-40 and 5 mmol L−1 PMSF), followed by treatment with 1 μmol L−1 plasmin for 40 min at RT, and the capillary eluate was collected for analysis.
Fibrin deposition during flow experiments was evaluated by separating the eluate samples by 6% SDS–PAGE under non-reducing conditions. Gels were evaluated by immunoblotting with anti-fibrinogen antiserum (MP Biomedicals, Irvine, CA, USA) to detect the 220 kDa fibrin degradation product, D-dimer. Sample D-dimer levels were compared with known amounts of purified D-dimer on the same blot (1–100 ng).
Capillary occlusion assay
Capillary tubes were prepared as described above, aligned vertically and connected to a reservoir. The capillary exit was immersed in PBS. Sodium citrate-(0.38% w/v) anticoagulated whole blood was sequentially supplemented with 7.5 mmol L−1 Ca2+ and 3.75 mmol L−1 Mg2+ in 1 mL aliquots to reduce the residence time of recalcified blood during each experiment. Flow through the capillary was driven by the force of gravity, and the height of the sample reservoir was regulated in order to produce an initial shear rate of 300 s−1 according to the following equation [19]:
where γwall is wall shear rate, ρb is the density of the blood, ρpbs is the density of the PBS, hc is the height of the capillary tube, hb is the height of the blood in the reservoir, hpbs is the height that the capillary is submerged in PBS, g is acceleration due to gravity, μ is viscosity of blood, and 2a is the width of the capillary. The time to capillary occlusion was recorded, within a maximum observation period of 40 min.
Data analysis
Data are shown as means ± SEM. Statistical significance of differences between means was determined by ANOVA. If means were shown to be significantly different, multiple comparisons were performed by the Tukey test. Probability values of P < 0.05 were selected to be statistically significant.
Results
Laminin shortens clotting times in recalcified plasma in a FXII-dependent manner
The extracellular matrix protein laminin has recently been shown to support platelet recruitment and activation [10,12]. Our study was designed to determine the role of laminin in the initiation of coagulation. The presence of 40 μg mL−1 laminin shortened the clotting time of recalcified platelet-rich plasma (PRP) compared with vehicle (Fig. 1A). Pretreating PRP with the FXIIa inhibitor, CTI (4 lmol L−1), 14E11 (20 μg mL−1), which binds to the second apple (A2) domain of FXI and inhibits FXI activation by FXIIa [13], or the anti-FXI mAb 1A6 (20 μg mL−1), which binds to the third apple (A3) domain of FXI and blocks FIX binding to FXIa [13], prior to incubation with laminin prolonged clotting times compared with the presence of laminin alone (Fig. 1A). The presence of CTI, 14E11 or 1A6 did not affect clotting times in the presence of laminin plus 1 pmol L−1 tissue factor (Fig. 1A). Similar trends were observed in platelet-poor plasma (PPP, Fig. 1B), suggesting that the presence of platelets plays a minimal role in the mechanism by which laminin shortens clotting times.
Fig. 1.
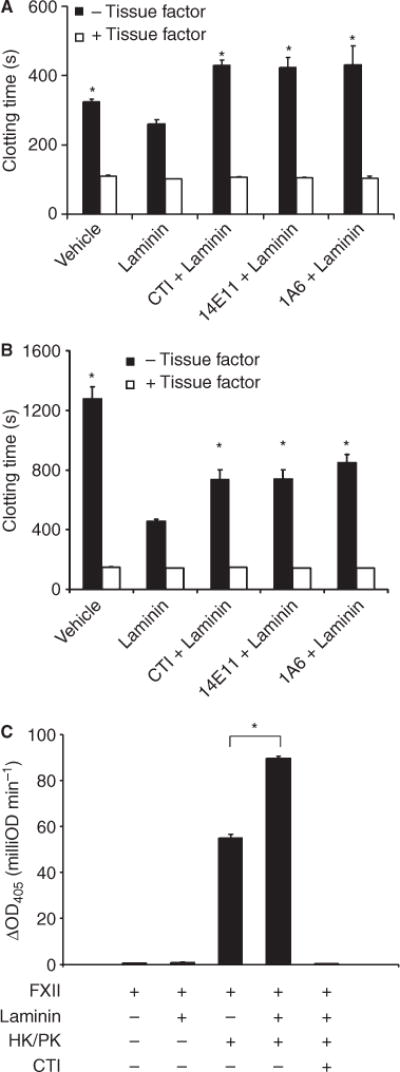
Laminin enhances coagulation. Human sodium citrate-anticoagulated (A) platelet-rich plasma (PRP) or (B) platelet-poor plasma (PPP) was pretreated with 4 lmol L−1 CTI, 20 μg mL−1 14E11 or 20 μg mL−1 1A6. Laminin was then added to the plasma (40 μg mL−1 in PRP, 10 μg mL−1 in PPP) in the absence (black bars) or presence (white bars) of 1 pmol L−1 tissue factor. Following a 3-min incubation, coagulation was initiated by the addition of 16.6 mmol L−1 CaCl2, and clotting times were recorded. Data reported are mean ± SEM of at least three experiments; *P < 0.05 compared with clotting times in the presence of laminin alone. (C) FXII activation was monitored in the presence of 50 μg mL−1 laminin. Experiments were performed in the presence of high molecular weight kininogen/prekallikrein (HK/PK, 30 nmol L−1 each) or CTI (4 μmol L−1) as indicated. FXIIa was detected by measuring cleavage of its chromogenic substrate, Pefachrome FXIIa. Data are mean ± SEM of three experiments. (*P < 0.05).
Collagen has been shown to activate FXII and promote coagulation in recalcified PRP [9]. In experiments performed here, PRP was incubated with either collagen (40 μg mL−1) or vehicle, followed by the initiation of coagulation by adding CaCl2. In accord with van der Meijden et al. [9], the presence of collagen significantly shortened clotting time compared with vehicle (Supplemental Fig. S2A). Clotting times in the presence of collagen, but not collagen plus 1 pmol L−1 tissue factor, were prolonged by the addition of 1A6, 14E11 or CTI (Supplemental Fig. S2A).
In order to address the possibility that reduced clotting times in the presence of laminin may be attributed to a residual amount of contaminating collagen, we determined the minimum concentration of collagen required to generate equivalent functional responses. Our results show that a collagen concentration of at least 0.625 μg mL−1 was required to reduce clotting times in PRP to a similar degree as observed for 40 μg mL−1 laminin (Supplemental Fig. S3). It is noteworthy that the addition of 0.3125 μg mL−1 collagen had no effect on PRP clotting times, yet concentrations of collagen as low as 0.3125 μg mL−1 retained the ability to induce platelet shape change in PRP aggregations (Supplemental Fig. S4A–B). In contrast, the addition of 40 μg mL−1 laminin to PRP failed to initiate either platelet shape change or aggregation (Supplemental Fig. S4B). A similar set of results was observed with washed human platelets (data not shown). These studies provide evidence against the suggestion that contamination by collagen plays a role in mediating the functional response we observed with laminin.
Activation of FXII in the presence of laminin
We used a plasma-free system to measure the effects of laminin on FXII activation. The incubation of laminin with FXII in the presence of high-molecular-weight kininogen (HK) and prekallikrein (PK) significantly accelerated the cleavage of a FXIIa chromogenic substrate compared with the presence of HK and PK alone (Fig. 1C). Chromogenic activity was negligible in the presence of CTI (Fig. 1C).
Laminin-bound platelets expose phosphatidylserine
A population of activated platelets expose procoagulant phosphatidylserine (PS) on their outer membrane surface, which serves as a site for assembly and activation of the tenase and prothrominase complexes [20,21]. To study the ability of laminin to support PS exposure on platelets, we layered purified platelets on laminin for 30 min before staining the adherent cells with fluorescently-labeled annexin V, which binds to PS with high affinity. Our data showed that approximately 23% of laminin-bound platelets bound annexin V (Fig. 2). The portion of collagen-bound platelets that supported annexin V binding was 49% (Supplemental Fig. S2B). A minimal degree of platelet adhesion and PS exposure was observed on BSA-coated surfaces (data not shown).
Fig. 2.
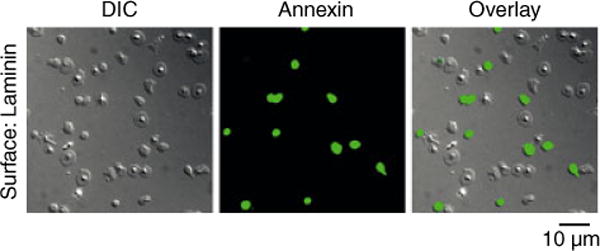
Laminin-bound platelets expose phosphatidylserine (PS). Washed human platelets were exposed to a surface of immobilized laminin. Bound platelets were incubated with a solution of fluorescently-labeled annexin V to visualize PS exposure. Images were recorded using DIC and fluorescence microscopy. Corresponding brightfield and fluorescent images are shown alone and in the overlay.
Laminin supports thrombus formation in the presence of flow
In order to characterize the ability of laminin to support thrombus formation, recalcified blood was perfused over immobilized laminin at a shear rate of 250 s−1 for 12 min at 37 °C. Thrombus formation was visually recorded using DIC microscopy and the degree of fibrin formation was analyzed by Western blotting for the fibrin degradation product, D-dimer, following clot lysis with plasmin. Our results demonstrate that laminin supported platelet adhesion and aggregation and fibrin deposition in recalcified blood under shear (Fig. 3A). Fibrin strands were visible throughout the laminin-coated capillary (Fig. 3A), and D-dimer was readily detected in clot lysates (Fig. 3A). The degree of fibrin deposition, platelet aggregation and D-dimer detection was visibly higher for laminin as compared with BSA-coated surfaces (Fig. 3C). In order to rule out effects of collagen contamination in the laminin preparations, flow experiments were repeated on collagenase-treated laminin surfaces as previously described [10]. Collagenase-treated laminin surfaces supported equivalent levels of platelet- and fibrin-rich thrombus formation (data not shown). In contrast, fibrin formation was abrogated in the presence of the direct thrombin inhibitor, hirudin (20 μg mL−1). The treatment of blood with the anti-FXI antibody 1A6, which blocks activation of FIX by FXIa, or 14E11, which interferes with FXI activation by FXIIa, or the FXIIa inhibitor, CTI, prior to perfusion over laminin surfaces drastically reduced both visible fibrin formation and D-dimer detection compared with vehicle (Fig. 3A).
Fig. 3.
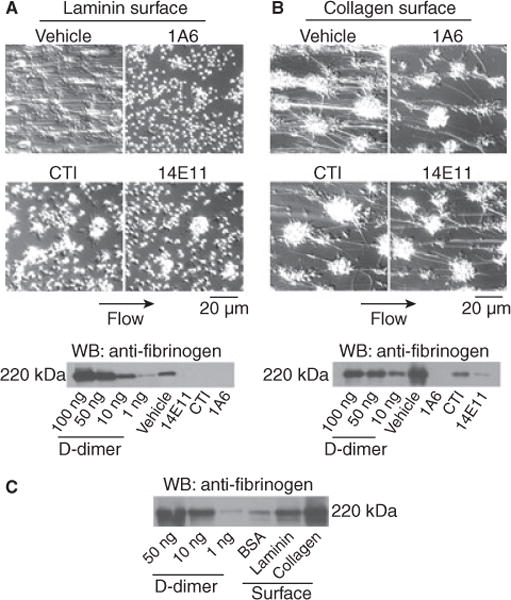
Laminin supports thrombus formation under shear. Sodium citrate-anticoagulated whole human blood was co-perfused with a solution of Ca2+ /Mg2+(7.5 mmol L−1/3.75mmol L−1 final, respectively) over (A) laminin, (B) collagen or (C) BSA surfaces for 12 min at 250 s−1, followed by perfusion of modified Tyrode buffer to remove non-adherent cells. In separate experiments, blood was pretreated with 20 μg mL−1 1A6, 40 μg mL−1 CTI or 20 μg mL−1 14E11 for 10 min at 37 °C prior to perfusion. Images were recorded using DIC microscopy prior to lysis and immunoblotting for the fibrin degradation product, D-dimer. Images and blots are representative of at least three experiments.
We have previously demonstrated that inhibition of FXI with the anti-FXI mAb, 1A6, reduces fibrin deposition on collagen in both in vitro and in vivo models [14]. Our current study extends these findings to characterize the role of FXII in fibrin formation on collagen. Our results demonstrate that platelet aggregates and fibrin strands form on collagen following the perfusion of recalcified blood. The presence of 1A6 abrogated both visible fibrin formation and D-dimer detection, while the presence of either 14E11 or CTI reduced, but did not eliminate, fibrin formation and D-dimer detection (Fig. 3B).
Laminin supports occlusive thrombus formation under a constant pressure gradient
We next investigated whether laminin was capable of supporting occlusive thrombus formation. We have recently developed an ex vivo model of occlusive thrombosis formation that is driven by a constant pressure rather than constant volumetric flow rate [19]. In this ex vivo model, recalcified blood is driven by a constant pressure gradient at an initial shear rate through capillaries coated with laminin, collagen or tissue factor. The initial shear rate was adjusted to 300 s−1, and flow through the capillary was monitored until occlusion. Our data demonstrate that an occlusive thrombus developed on laminin after 22.0 ± 3.2 min. Similar occlusion times were observed in collagen- or tissue factor-coated capillaries (Fig. 4), while occlusion in BSA-coated control capillaries occurred after 30.6 ± 1.7 min. Occlusive thrombus formation on laminin, collagen or tissue factor was dependent upon the activity of thrombin, as recalcified blood pretreated with the direct thrombin inhibitor, hirudin (20 μg mL−1), failed to occlude in the capillary over the 40 min observation time.
Fig. 4.
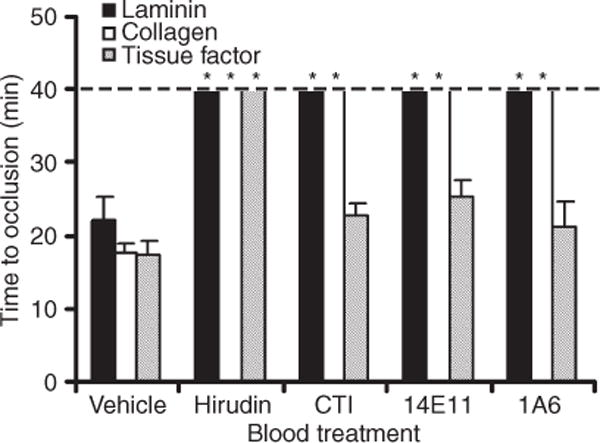
Laminin supports occlusive thrombus formation under a constant pressure gradient. Whole human blood in 0.38% sodium citrate was recalcified and perfused through a laminin-, collagen- or tissue factor-coated glass capillary until occlusion. Blood flow was driven by a constant pressure difference. In selected experiments, blood was pretreated with either 40 μg mL−1 CTI, 20 μg mL−1 14E11 or 20 μg mL−1 1A6. Data are reported as mean ± SEM of at least three experiments. *P < 0.05 compared with occlusion time in the presence of vehicle on each respective surface.
FXI has been shown to play a critical role in thrombus growth and stability in vivo, and its inhibition prolongs occlusion times in animal models of thrombosis [14,22–25]. Our data demonstrate that pretreatment of blood with the anti-FXI mAb 1A6 or 14E11 or the FXIIa inhibitor CTI prolonged the time to occlusion on laminin past the 40 min observation period (Fig. 4). Similar results were observed in collagen-coated capillaries. In contrast, occlusion times in tissue factor-coated capillaries were equivalent in the presence of vehicle, CTI, 14E11 or 1A6 (Fig. 4).
Discussion
Despite the fact that laminin is a predominant member of the basement membrane, the contribution of laminin to hemostasis and thrombosis remains unclear. While expression of ECM proteins such as collagen types I, III, V and VI, as well as the adhesive proteins fibronectin and vitronectin, is enhanced in atherosclerotic plaques, laminin expression levels remain surprisingly constant [26–28]. Perhaps this is indicative of a physiological role for laminin in hemostasis. The current study confirms and extends the recent studies by Inoue and colleagues demonstrating that laminin supports platelet recruitment, adhesion and activation under static and flow conditions in a manner analogous to collagen [10,12]. As collagen surfaces have been shown to support the initiation of coagulation in the presence of flow [9,14], our aim was to characterize (i) the ability of laminin to initiate coagulation by augmenting FXII activation and (ii) the ability of laminin surfaces to support platelet procoagulant activity in the presence of shear. Data presented here demonstrate that the presence of laminin accelerated clotting in recalcified plasma in a FXII-dependent manner. In a purified system, laminin accelerated the activation of FXII in the presence of HK and PK. Furthermore, immobilized laminin was able to stimulate platelet procoagulant activity and support thrombus formation and occlusion in the presence of flow.
The contribution of FXII to hemostasis is unclear as FXII deficiency in humans is not associated with a bleeding diathesis. Conversely, recent thrombosis studies in FXII-deficient mice have demonstrated an important role for FXII in thrombus formation and stability [24,29]. FXII has been shown to be activated by native materials that are not normally exposed to circulating blood, or that are exposed in the event of a vascular injury, such as collagen [9,29], RNA [30] and polyphosphates that are contained in platelet-dense granules [31]. The current study builds on previous work demonstrating that FXII binds to laminin surfaces [32], and adds laminin to the ever-increasing list of physiological surfaces that accelerate FXII activation. As FXIIa is a potent activator of FXI during contact activation, exposure of blood to one or more of these substances would result in the activation of FXI.
Epidemiological data indicate that men in the upper quintile of the normal distribution for plasma FXI levels have a ~ 2-fold increased risk of myocardial infarction compared with those in the lowest quintile, while severe deficiency of FXI is protective against ischemic stroke [33–36]. The mild bleeding risk in the absence of FXI, along with evidence that FXI and FXII each support thrombus formation, makes the intrinsic pathway of coagulation an attractive target for antithrombotic therapy. We have recently demonstrated that the administration of the FXI-blocking antibody, 1A6, which blocks FIX binding to FXIa, to non-human primates dramatically reduced both platelet and fibrin deposition on collagen [18]. Moreover, pharmacological inhibition of FXI with 1A6 prevented the occlusion of 2-mm diameter collagen-coated grafts without affecting template bleeding times [18]. The current study provides evidence that the blockade of FXI inhibits thrombus formation and occlusion on laminin-coated surfaces, identifying FXI as a common regulator of thrombus formation downstream of the basement membrane proteins collagen and laminin. Future studies using the anti-FXI mAb 14E11, which interferes with the ability of FXIIa to activate FXI, in these non-human primate models of thrombus formation and template bleeding should provide essential insights into the physiological role of FXII and FXI in hemostasis and thrombosis.
In conclusion, this study provides the first evidence that the ECM protein, laminin, is able to contribute to the initiation of coagulation and support thrombus formation in the presence of shear. Laminin joins fellow extracellular matrix protein, collagen, and a growing list of physiological materials that are exposed to circulating blood in the event of an injury, and have been shown to contribute to FXII activation [9,30,31]. Further studies are warranted to more fully describe the role that extracellular matrix proteins may play in other coagulation reactions.
Supplementary Material
Fig. S1. Biochemical characterization of laminin.
Fig. S2. Collagen supports procoagulant processes.
Fig. S3. Effects of decreasing doses of collagen on PRP clotting times.
Fig. S4. Laminin does not induce platelet shape change or aggregation.
Acknowledgments
We thank J. Pang, B. Fuchs, T. Eshel-Green and P. Simonson for experimental assistance. This work was supported in part by American Heart Association Grants 0910025G (T. C. White-Adams), 09PRE2230117 (M. A. Berny), 0850056Z (A. Gruber) and 09GRNT2150003 (O. J. T. McCarty), and National Institutes of Health Grant HL58837 (D. Gailani and A. Gruber). T. C. White-Adams is a Vertex Scholar, I. A. Patel is a Johnson Scholar, and T. C. White-Adams and M. A. Berny are ARCS Scholars.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–95. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003;1:1602–12. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 3.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIbbeta3 signaling in platelets. J Thromb Haemost. 2005;3:1752–62. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 4.McCarty OJ, Calaminus SD, Berndt MC, Machesky LM, Watson SP. von Willebrand factor mediates platelet spreading through glycoprotein Ib and alpha(IIb)beta3 in the presence of botrocetin and ristocetin, respectively. J Thromb Haemost. 2006;4:1367–78. doi: 10.1111/j.1538-7836.2006.01966.x. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki Y, Asazuma N, Suzuki-Inoue K, Berndt MC. Platelet GPIb-IX-V-dependent signaling. J Thromb Haemost. 2005;3:1745–51. doi: 10.1111/j.1538-7836.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–34. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 7.Jurk K, Kehrel BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. 2005;31:381–92. doi: 10.1055/s-2005-916671. [DOI] [PubMed] [Google Scholar]
- 8.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–22. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 9.van der Meijden PE, Munnix IC, Auger JM, Govers-Riemslag JW, Cosemans JM, Kuijpers MJ, Spronk HM, Watson SP, Renne T, Heemskerk JW. Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114:881–90. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
- 10.Inoue O, Suzuki-Inoue K, McCarty OJ, Moroi M, Ruggeri ZM, Kunicki TJ, Ozaki Y, Watson SP. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of GPVI. Blood. 2006;107:1405–12. doi: 10.1182/blood-2005-06-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hindriks G, Ijsseldijk MJ, Sonnenberg A, Sixma JJ, de Groot PG. Platelet adhesion to laminin: role of Ca2+ and Mg2+ ions, shear rate, and platelet membrane glycoproteins. Blood. 1992;79:928–35. [PubMed] [Google Scholar]
- 12.Inoue O, Suzuki-Inoue K, Ozaki Y. Redundant mechanism of platelet adhesion to laminin and collagen under flow: involvement of von Willebrand factor and glycoprotein Ib-IX-V. J Biol Chem. 2008;283:16279–82. doi: 10.1074/jbc.C700241200. [DOI] [PubMed] [Google Scholar]
- 13.Kravtsov DV, Matafonov A, Tucker EI, Sun MF, Walsh PN, Gruber A, Gailani D. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114:452–8. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–44. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wondimu Z, Gorfu G, Kawataki T, Smirnov S, Yurchenco P, Tryggvason K, Patarroyo M. Characterization of commercial laminin preparations from human placenta in comparison to recombinant laminins 2 (alpha2beta1gamma1), 8 (alpha4beta1gamma1), 10 (alpha5beta1gamma1) Matrix Biol. 2006;25:89–93. doi: 10.1016/j.matbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Berny MA, White TC, Tucker EI, Bush-Pelc LA, Di Cera E, Gruber A, McCarty OJ. Thrombin mutant W215A/E217A acts as a platelet GPIb antagonist. Arterioscler Thromb Vasc Biol. 2008;28:329–34. doi: 10.1161/ATVBAHA.107.156273. [DOI] [PubMed] [Google Scholar]
- 17.White TC, Berny MA, Tucker EI, Urbanus RT, De Groot PG, Fernandez JA, Griffin JH, Gruber A, McCarty OJ. Protein C supports platelet binding and activation under flow: role of glycoprotein Ib and apolipoprotein E receptor 2. J Thromb Haemost. 2008;6:995–1002. doi: 10.1111/j.1538-7836.2008.02979.x. [DOI] [PubMed] [Google Scholar]
- 18.White-Adams TC, Berny MA, Tucker EI, Gertz JM, Gailani D, Urbanus RT, de Groot PG, Gruber A, McCarty OJ. Identification of coagulation factor XI as a ligand for platelet apolipoprotein E receptor 2 (ApoER2) Arterioscler Thromb Vasc Biol. 2009;29:1602–7. doi: 10.1161/ATVBAHA.109.187393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berny MA, Patel IA, White-Adams TC, Simonson P, Gruber A, Rugonyi S, McCarty OJ. Rational design of an ex vivo model of thrombosis. Cel Mol Bioeng. 2010 doi: 10.1007/s12195-010-0103-5. [DOI] [Google Scholar]
- 20.Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186–93. [PubMed] [Google Scholar]
- 21.Heemskerk JW, Siljander PR, Bevers EM, Farndale RW, Lindhout T. Receptors and signalling mechanisms in the procoagulant response of platelets. Platelets. 2000;11:301–6. doi: 10.1080/09537100050144704. [DOI] [PubMed] [Google Scholar]
- 22.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–5. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 23.Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–6. [PubMed] [Google Scholar]
- 24.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita A, Nishihira K, Kitazawa T, Yoshihashi K, Soeda T, Esaki K, Imamura T, Hattori K, Asada Y. Factor XI contributes to thrombus propagation on injured neointima of the rabbit iliac artery. J Thromb Haemost. 2006;4:1496–501. doi: 10.1111/j.1538-7836.2006.01973.x. [DOI] [PubMed] [Google Scholar]
- 26.Stenman S, von Smitten K, Vaheri A. Fibronectin and atherosclerosis. Acta Med Scand Suppl. 1980;642:165–70. doi: 10.1111/j.0954-6820.1980.tb10949.x. [DOI] [PubMed] [Google Scholar]
- 27.van Zanten GH, de Graaf S, Slootweg PJ, Heijnen HF, Connolly TM, de Groot PG, Sixma JJ. Increased platelet deposition on atherosclerotic coronary arteries. J Clin Invest. 1994;93:615–32. doi: 10.1172/JCI117014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoop AA, Lupu F, Pannekoek H. Colocalization of thrombin, PAI-1, and vitronectin in the atherosclerotic vessel wall: A potential regulatory mechanism of thrombin activity by PAI-1/vitronectin complexes. Arterioscler Thromb Vasc Biol. 2000;20:1143–9. doi: 10.1161/01.atv.20.4.1143. [DOI] [PubMed] [Google Scholar]
- 29.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–81. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, von Bruehl ML, Sedding D, Massberg S, Gunther A, Engelmann B, Preissner KT. Extracellular RNA constitutes a natural procoagulant co-factor in blood coagulation. Proc Natl Acad Sci USA. 2007;104:6388–93. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci USA. 2006;103:903–8. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schousboe I. Endothelial cells express a matrix protein which binds activated factor XII in a zinc-independent manner. Thromb Haemost. 2006;95:312–9. doi: 10.1160/TH05-06-0458. [DOI] [PubMed] [Google Scholar]
- 33.Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: opposite and synergistic effects of factors XI and XII. Blood. 2006;108:4045–51. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 34.Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 35.van Hylckama Vlieg A, van der Linden IK, Bertina RM, Rosendaal FR. High levels of factor IX increase the risk of venous thrombosis. Blood. 2000;95:3678–82. [PubMed] [Google Scholar]
- 36.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–7. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Biochemical characterization of laminin.
Fig. S2. Collagen supports procoagulant processes.
Fig. S3. Effects of decreasing doses of collagen on PRP clotting times.
Fig. S4. Laminin does not induce platelet shape change or aggregation.


