Abstract
The G protein–coupled receptor allosteric modulator SCH-202676 (N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene)methanamine), which affects a wide range of structurally unrelated G protein–coupled receptors, has highly divergent effects on purine receptors. SCH-202676 inhibited radioligand binding to human adenosine A1, A2A, and A3 receptors (IC50 = 0.5–0.8 µM) and affected dissociation kinetics, but at the human P2Y1 nucleotide receptor it had no effect. SCH-202676 (10 µM) selectively accelerated agonist dissociation at adenosine A3 receptors and either slowed (adenosine A1 receptors) or accelerated (adenosine A2A receptors) antagonist dissociation. Thus, SCH-202676 differentially modulated A1, A2A, and A3 receptors as well as agonist- and antagonist-occupied receptors.
Keywords: Adenosine receptor, P2Y receptor, Allosteric modulator, 7TM receptor, SCH-202676
Introduction
Allosteric sites on cell surface receptors are emerging as important targets for drug development (Christopoulos, 2002; Christopoulos and Kenakin, 2002). Both adenosine receptors and P2Y receptors are of increasing therapeutic interest (Fredholm et al., 2001; Jacobson et al., 2002). Allosteric modulators for adenosine and P2Y receptors are of potential clinical use (Linden, 1997; Christopoulos and Kenakin, 2002).
SCH-202676 (N-(2,3-diphenyl-1,2,4-thiadiazol-5-(2H)-ylidene)methanamine) was recently reported to be a universal allosteric modulator of a wide variety of unrelated G protein–coupled receptors, including the human μ-, δ-, and κ-opioid, α- and β-adrenergic, muscarinic M1 and M2, and dopaminergic D1 and D2 receptors (Fawzi et al., 2001). It appeared to affect binding non-competitively, yet reversibly, to a range of structurally unrelated G protein–coupled receptors, including those coupled through Gi/Go, Gs and Gq proteins. Moreover, at 6 µM it completely blocked the ability of an agonist to activate the α2A-adrenergic receptor. However, it is not known whether SCH-202676 has any effect on G protein–coupled receptors that respond to adenosine or adenine nucleotides.
In this study we examined the ability of SCH-202676 to compete for ligand binding to human adenosine A1, A2A, and A3 receptors and P2Y1 nucleotide receptors stably expressed in CHO, HEK-293 or 1321N1 astrocytoma cells. We also studied the effects on rates of ligand dissociation from those receptors. We found that SCH-202676 interacts with adenosine receptors but not with P2Y1 receptors. SCH-202676 differentially modulated adenosine A1, A2A, and A3 receptors as well as agonist- and antagonist-occupied receptors.
Methods
Materials
CPA (N6-cyclopentyladenosine), NECA (5′-N-ethylcarboxamidoadenosine), Cl-IB-MECA (2-chloro-N6-(3-iodobenzyl)-5′-N-methylcarbamoyladenosine), and MRS2179 (N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate) were purchased from Sigma (St. Louis, MO, USA). [125I]I-AB-MECA (N6-(4-amino-3-iodobenzyl)-5′-N-methylcarboxamidoadenosine; 2000 Ci/mmol), [3H]PSB-11 (8-ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2.1-i]purin-5-one; 53 Ci/mmol), [3H]R-PIA (N6-[(R)-phenylisopropyl]adenosine; 34 Ci/mmol), [3H]DPCPX (8-cyclopentyl-1,3-dipropylxanthine; 120 Ci/mmol), and [3H]CGS21680 (47 Ci/mmol) were from Amersham Pharmacia Biotech (Buckinghamshire, UK). [3H]ZM241385 (4-2-[7-amino-2-(2-furyl)-1,2,4-triazolo[1,5-a][1,3,5]triazin-5-ylamino] ethylphenol; 17 Ci/mmol) and SCH-202676 were from Tocris (Ballwin, MO, USA). [3H]MRS2279 (2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate) was prepared as described (Waldo et al., 2002).
Cell culture and membrane preparation
CHO cells stably expressing recombinant human adenosine A1 receptors and human and rat adenosine A3 receptors, as well as HEK293 cells stably expressing human adenosine A2A receptors, and human astrocytoma cells expressing human P2Y1 receptors were cultured in Dulbecco’s modified Eagle’s medium (JRH Biosciences, Inc., Lenexa, KS, USA) and F12 (1:1) supplemented with 10% fetal bovine serum, 100 units penicillin/ml, 100 µg streptomycin/ml, 2 µmol glutamine/ml, and 500 µg geneticin/ml. After harvesting, the cells were homogenized and suspended and then centrifuged at 100 g for 5 min at room temperature. The pellet was resuspended in 50 mM tris(hydroxymethyl)aminomethane (Tris)·HCl buffer (pH 7.4). The suspension was homogenized with a Polytron electric homogenizer (Brinkmann, NY, USA) for 10 s and was then recentrifuged at 20,000 g for 20 min at 4 °C. The resultant pellets were resuspended in buffer in the presence of 3 units adenosine deaminase/ml, and the suspension was stored at −80 °C until the binding experiments. The protein concentration was measured with the Bradford assay (Bradford, 1976).
Radioligand binding assays
Adenosine and P2Y1 receptor binding experiments were performed as previously described (Gao et al., 2002; Waldo et al., 2002). Briefly, the binding of [3H]R-PIA (2.0 nM) or [3H]DPCPX (1.0 nM) to human adenosine A1 receptors from CHO cell membranes (40 µg protein/tube), [3H]CGS21680 (15 nM) and [3H]ZM241385 (2.0 nM) to adenosine A2A receptors from HEK293 cell membranes (20 µg protein/tube) and [125I]I-AB-MECA (0.5 nM) and [3H]PSB-11 (5 nM) (Müller et al., 2002) to adenosine A3 receptors was measured after incubation at 25 °C for 60 min in 200 µl of 50 mM TrisµHCl (pH 7.4) containing 10 mM MgCl2. The dissociation was initiated by the addition of 1 µM CPA, 10 µM CGS21680, and 1 µM Cl-IB-MECA for the adenosine A1, A2A, and A3 receptors, respectively. The time course of dissociation of total binding was measured by rapid filtration at appropriate time intervals. Binding reactions were terminated by filtration through Whatman GF/B glass-fiber filters under reduced pressure with a MT-24 cell harvester (Brandel, Gaithersburg, MD, USA), and radioactivity was determined with a liquid scintillation counter (Parkard, IL, USA) or a Beckman 5500B γ-counter (Beckman, USA). For P2Y1 receptor binding experiments, membranes (40 µg protein) from astrocytoma cells stably expressing human P2Y1 receptors were incubated with [3H]MRS2279 (8 nM) for 30 min at 4 °C in a total assay volume of 100 µl. Radiolabeled ligand concentrations used in all assays approximated the Kd value of the receptor.
Binding parameters were analyzed by GraphPAD Prism software (GraphPAD, San Diego, CA, USA). Data were expressed as mean ± standard error.
Results
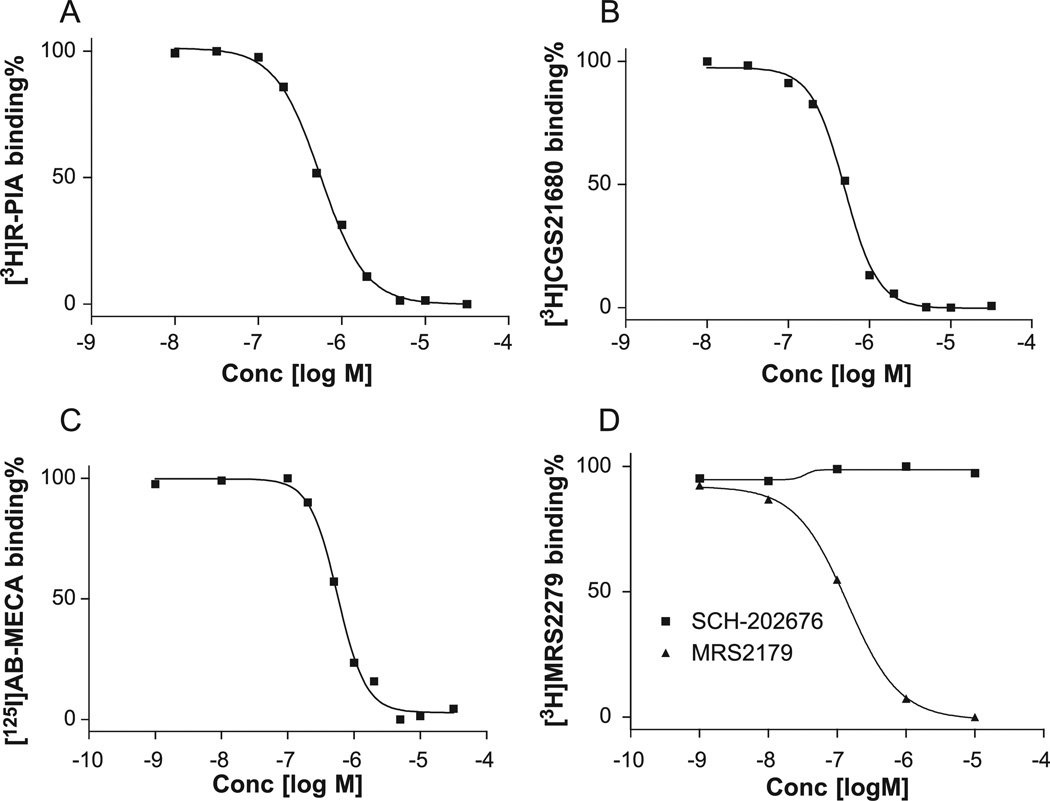
It was recently shown that SCH-202676 allosterically modulated several structurally diverse G protein–coupled receptors but had no effect on G proteins (Fawzi et al., 2001). In this study, we examined the possible interaction of SCH-202676 with adenosine and P2Y1 receptors, first by examining inhibition of agonist binding to human adenosine A1, A2A, and A3 receptors and to the human P2Y1 receptor. As shown in Fig. 1, SCH-202676 inhibited ligand binding to adenosine A1, A2A, and A3 receptors with similar potencies (IC50 = 0.77 ± 0.10, 0.55 ± 0.19, and 0.49 ± 0.18 µM, respectively) but with slopes significantly greater than unity (Hill slopes = 1.8 ± 0.2, 1.9 ± 0.1, and 1.7 ± 0.2, respectively). Unlike its effect on adenosine receptors, SCH-202676 did not inhibit the binding of the newly available P2Y1 receptor antagonist [3H]MRS2279 (Waldo et al., 2002) to P2Y1 receptors. In contrast, the selective P2Y1 receptor antagonist MRS2179 displaced the binding of [3H]MRS2279 corresponding to an IC50 value of 171 ± 36 nM and a slope of 1.1 ± 0.1. Because there is a pronounced species difference between human and rat adenosine A3 receptors, we further examined the ability of SCH-202676 to displace [125I]I-AB-MECA binding to the rat adenosine A3 receptor. The IC50 and slope values were determined to be 0.82 ± 0.11 µM and 1.7 ± 0.1, respectively, which are similar to those of human adenosine A3 receptors.
Fig. 1.
Ability of SCH-202676 to inhibit the binding of radioligands [3H]R-PIA (2.0 nM), [3H]CGS21680 (15 nM), [125I]I-AB-MECA (0.5 nM), and [3H]MRS2279 (8.0 nM), respectively, to human adenosine A1 (A), A2A (B), and A3 (C) receptors and to P2Y1 (D) receptors. Data shown are from one experiment representative of at least three independent experiments performed in duplicate. The IC50 values listed in the text are from at least three independent experiments.
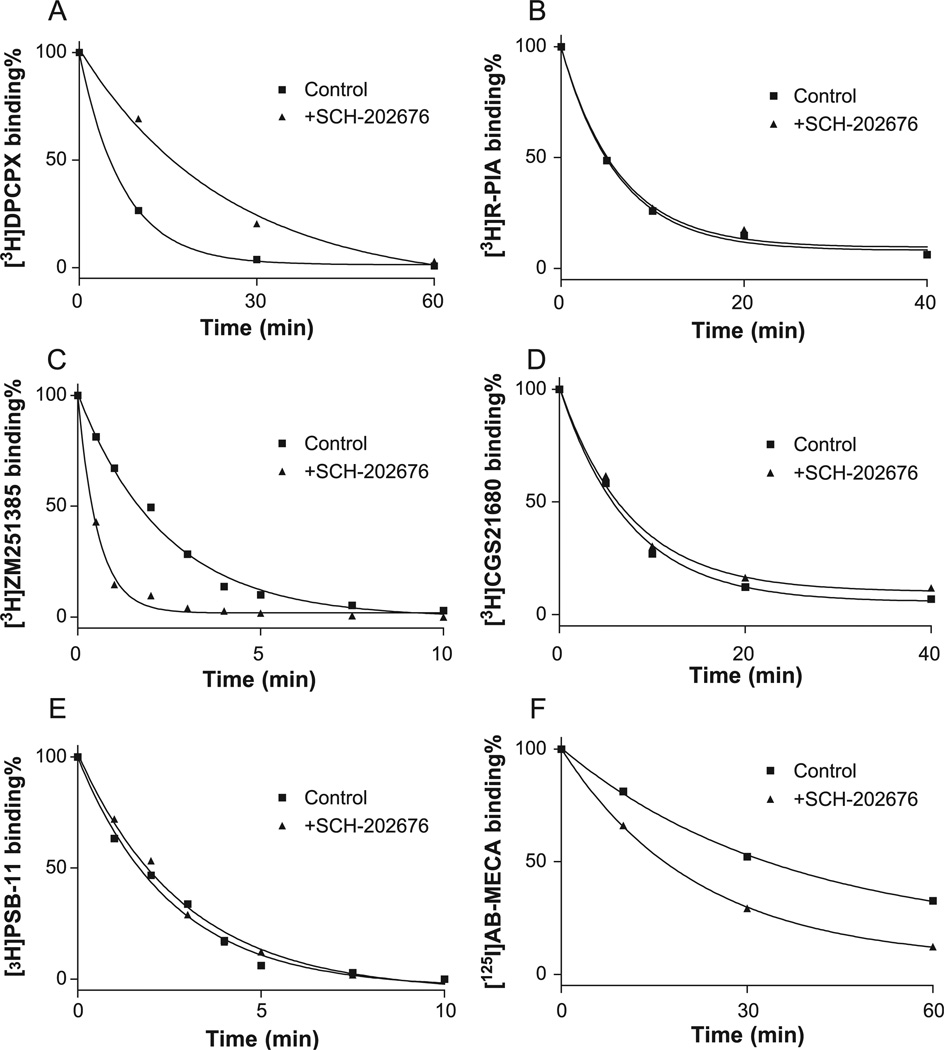
We next examined the effect of SCH-202676 on the dissociation of antagonists from adenosine receptors. Fig. 2 shows that 10 µM SCH-202676 dramatically decreased the dissociation rate of [3H]DPCPX while increasing the [3H]ZM241385 dissociation rate from A1 and A2A receptors, respectively. In contrast, the dissociation rate of [3H]PSB-11 from adenosine A3 receptors was not significantly influenced. The dissociation rate of [3H]MRS2279 from the hP2Y1 receptor, like the dissociation rate from adenosine A3 receptors, was not influenced by 10 µM SCH-202676 (data not shown).
Fig. 2.
Dissociation of agonists and antagonists from adenosine receptors in the absence and presence of 10 µM SCH-202676. The procedures are described in “Methods.” Data shown are from one experiment representative of at least three independent experiments performed in duplicate. Adenosine A1 receptor antagonist (A) and agonist (B), adenosine A2A receptor antagonist (C) and agonist (D), adenosine A3 receptor antagonist (E) and agonist (F).
In contrast to the effects of SCH-202676 on the antagonist dissociation rates, the dissociation of agonists [3H]R-PIA from adenosine A1 or [3H]CGS21680 from A2A receptors was not significantly influenced, but the [125I]I-AB-MECA dissociation rates from both human (Fig. 2) and rat (data not shown) adenosine A3 receptors were significantly increased. The dissociation rates in the absence and presence of 10 µM SCH-202676 were summarized in Table 1.
Table 1.
Dissociation rates (k−1, min−1) in the absence and presence of 10 µM SCH-202676
| Radioligand | Control | + SCH-202676 (10 µM) |
|---|---|---|
| [3H]R-PIA | 0.160 ± 0.013 | 0.158 ± 0.015 |
| [3H]DPCPX | 0.136 ± 0.016 | 0.040 ± 0.008* |
| [3H]CGS21680 | 0.133 ± 0.010 | 0.132 ± 0.012 |
| [3H]ZM241385 | 0.419 ± 0.031 | 1.80 ± 0.11* |
| [125I]I-AB-MECA | 0.028 ± 0.005 | 0.046 ± 0.004* |
| [3H]PSB-11 | 0.390 ± 0.082 | 0.366 ± 0.056 |
| [3H]MRS2279 | 0.368 ± 0.045 | 0.357 ± 0.036 |
n = 3.
p < 0.05 compared with control, student’s test.
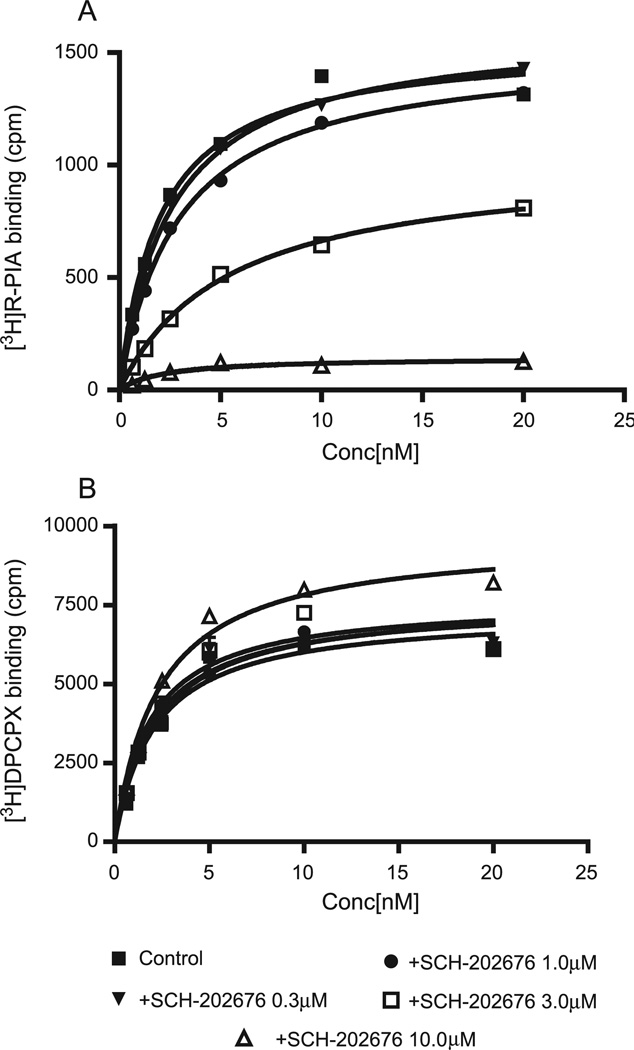
In order to explore further the effects of SCH-202676 on adenosine receptors, saturation experiments were performed on A1 receptors in the absence and presence of a range of concentrations of SCH-202676 (Fig. 3). SCH-202676 significantly increased the Kd (5.44 ± 0.39 nM at 3 µM versus 2.04 ± 0.36 nM for control) while decreasing the Bmax values of the agonist radioligand [3H]R-PIA (p < 0.05). For binding of the antagonist radioligand [3H]DPCPX, 10 µM SCH-202676 significantly increased the Bmax but not the Kd value.
Fig. 3.
Effects of SCH-202676 on the saturation binding of [3H]R-PIA and [3H]DPCPX to membranes of CHO cells stably expressing human A1 receptors.
Discussion
The present study demonstrated that SCH-202676 is an allosteric modulator for human adenosine A1, A2A, and A3 receptors but not for P2Y1 receptors, which respond to adenosine 5′-diphosphate. SCH-202676 allosterically modulated adenosine receptors in a manner that was both unique and selective. The structural basis for these differences remains to be elucidated. Fawzi et al. (2001) suggested that the allosteric effects of SCH-202676 may occur through a structural motif common to many G protein–coupled receptors or conceivably by activation or inhibition of an unidentified accessory protein that regulates the G protein–coupled receptors. We envision following these initial observations with receptor mutagenesis experiments.
Allosteric modulation of adenosine receptors has been extensively studied in adenosine A1 receptors (Bruns and Fergus, 1990; Linden, 1997; van der Klein et al., 1999; Tranberg et al., 2002) and to a lesser extent in adenosine A2A receptors (Gao and IJzerman, 2000). We recently identified and characterized several chemical classes of allosteric modulators for adenosine A3 receptors, including the imidazoquinoline DU124183, the pyridinylisoquinoline VUF5455, and amiloride analogues (Gao et al., 2001, 2002, 2003a,b). In contrast to the effect of the classical allosteric modulator PD81723 on A1 receptors, which decreased agonist dissociation rate but did not affect antagonist dissociation (Bruns and Fergus, 1990), SCH-202676 dramatically decreased the adenosine A1 receptor antagonist dissociation rate but did not affect adenosine A1 receptor agonist dissociation. Based on the available data, SCH-202676 appears to have a different mechanism of action compared to the prototypic adenosine receptor allosteric modulator, PD81723. SCH-202676 is different from DU124183, and VUF5455 in its effect on the A3 receptors; it increased adenosine A3 receptor agonist dissociation without affecting A3 receptor antagonist dissociation (Gao et al., 2001, 2002). The effects of SCH-202676 are also different from those of amiloride analogues. Amiloride analogues increased antagonist dissociation rates from all three subtypes of adenosine receptors but only decreased the agonist dissociation rate from adenosine A3 receptors (Gao and IJzerman, 2000; Gao et al., 2003a,b).
SCH-202676 has potent antagonist activity for several G protein-coupled receptors (Fawzi et al., 2001), however, it is not yet known if SCH-202676 is an antagonist for adenosine receptors or not as functional assays were performed in the present study. It can be seen from Fig. 3 that SCH-202676 does not affect agonist and antagonist binding equally, which suggests that its effects on adenosine receptors and other G protein-coupled receptors are different (Fawzi et al., 2001). SCH-202676 decreased agonist binding but also increased antagonist binding at a higher concentration (10 µM), which is similar to that of sodium ions in this respect (Gao and IJzerman, 2000). SCH-202676 apparently decreased the dissociation rate of the antagonist radioligand [3H]DPCPX, however, the binding affinity of [3H]DPCPX was not increased as demonstrated in the saturation binding, suggesting that SCH-202676 may also decrease the association rate.
In summary, SCH-202676 inhibited the binding of radiolabeled ligands to human adenosine A1, A2A, and A3 receptors, with IC50 values in the range of 0.5–0.8 µM, but failed to displace binding to human P2Y1 nucleotide receptors. The rate of antagonist dissociation was dramatically slowed at the adenosine A1 receptor, accelerated at the adenosine A2A receptor, and not affected at the adenosine A3 receptor by SCH-202676. In contrast, the dissociation rates of adenosine A1 and A2A receptor agonists were not significantly affected, and the dissociation rate of an adenosine A3 receptor agonist increased. Thus, SCH-202676 differentially modulated adenosine A1, A2A, and A3 receptors as well as agonist- and antagonist-occupied receptors.
Acknowledgements
We thank Dr. Gary Stiles (Duke University, Durham, NC, USA) for gifts of CHO cells expressing human and rat adenosine A3 receptors, Dr. Joel Linden (University of Virginia, Charlottesville, VA, USA) for the gift of HEK293 cells expressing human adenosine A2A receptors, Dr. T. Kendall Harden (University of North Carolina, Durham, NC, USA) for the gift of astrocytoma cells expressing human P2Y1 receptors, and Dr. Christa Müller (Pharmaceutical Institute, University of Bonn, Germany) for providing [3H]PSB-11.
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Fergus JH. Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Molecular Pharmacology. 1990;38:939–949. [PubMed] [Google Scholar]
- Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nature Review Drug Discovery. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacological Review. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Fawzi AB, Macdonald D, Benbow LL, Smith-Torhan A, Zhang H, Weig BC, Ho G, Tulshian D, Linder ME, Graziano MP. SCH-202676: an allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Molecular Pharmacology. 2001;59:30–37. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology: XXV. Nomenclature and classification of adenosine receptors. Pharmacological Review. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, IJzerman AP. Allosteric modulation of A2A adenosine receptors by amiloride analogues and sodium ions. Biochemical Pharmacology. 2000;60:669–676. doi: 10.1016/s0006-2952(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Van Muijlwijk-Koezen JE, Chen A, Muller CE, IJzerman AP, Jacobson KA. Allosteric modulation of A3 adenosine receptors by a series of 3-(2-pyridinyl)isoquinoline derivatives. Molecular Pharmacology. 2001;60:1057–1063. [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Kim SG, Soltysiak KA, Melman N, IJzerman AP, Jacobson KA. Selective allosteric enhancement of agonist binding and function at human A3 adenosine receptors by a series of imidazoquinoline derivatives. Molecular Pharmacology. 2002;62:81–89. doi: 10.1124/mol.62.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Melman N, Erdmann A, Kim SG, Müller CE, IJzerman AP, Jacobson KA. Differential allosteric modulation by amiloride analogues of agonist and antagonist binding at A1 and A3 adenosine receptors. Biochemical Pharmacology. 2003a;65:525–534. doi: 10.1016/s0006-2952(02)01556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Kim SK, Gross AS, Chen A, Blaustein JB, Jacobson KA. Identification of essential residues involved in the allosteric modulation of the human A3 adenosine receptor. Molecular Pharmacology. 2003b;63:1021–1031. doi: 10.1124/mol.63.5.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. Journal of Medicinal Chemistry. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Allosteric enhancement of adenosine receptors. In: Jacobson KA, Jarvis MF, editors. Purinergic Approaches in Experimental Therapeutics. 1997. pp. 85–97. [Google Scholar]
- Müller CE, Diekmann M, Thorand M, Ozola V. [3H]8-Ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]-purin-5-one [3H]PSB-11), a novel high-affinity antagonist radioligand for human A3 adenosine receptors. Bioorganic and Medicinal Chemistry Letters. 2002;12:501–503. doi: 10.1016/s0960-894x(01)00785-5. [DOI] [PubMed] [Google Scholar]
- Tranberg CE, Zickgraf A, Giunta BN, Luetjens H, Figler H, Murphree LJ, Falke R, Fleischer H, Linden J, Scammells PJ, Olsson RA. 2-Amino-3-aroyl-4,5-alkylthiophenes: agonist allosteric enhancers at human A1 adenosine receptors. Journal of Medicinal Chemistry. 2002;45:382–389. doi: 10.1021/jm010081p. [DOI] [PubMed] [Google Scholar]
- van der Klein PA, Kourounakis AP, IJzerman AP. Allosteric modulation of the adenosine A1 receptor. Synthesis and biological evaluation of novel 2-amino-3-benzoylthiophenes as allosteric enhancers of agonist binding. Journal of Medicinal Chemistry. 1999;42:3629–3635. doi: 10.1021/jm991051d. [DOI] [PubMed] [Google Scholar]
- Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, Lacy J, Jacobson KA, Harden TK. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Molecular Pharmacology. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]