Abstract
γδ T cells are attractive effector cells for cancer immunotherapy as they can secrete cytokines abundantly and exert potent cytotoxicity against a wide range of cancer cells. They comprise 1-5% of peripheral blood T cells, the majority expressing the Vγ9Vδ2 T cell receptor that recognizes phosphoantigens. Direct in vivo activation of Vγ9Vδ2 T cells in cancer patients as well as adoptive transfer of ex vivo expanded Vγ9Vδ2 T cells has been investigated in several clinical trials. We previously established a large-scale in vitro expansion method for Vγ9Vδ2 T cells using zoledronate and interleukin-2 (IL-2). We found that Vγ9Vδ2 T cells from patients with advanced cancer as well as from healthy donors underwent extensive proliferation under these conditions. Such cultured Vγ9Vδ2 T cells retained cytokine secretion capacity and mediated cytotoxicity against a variety of cancer cell lines. Recently, we conducted a phase I clinical study to evaluate safety and potential anti-tumor effects of re-infusing ex vivo expanded γδ T cells in patients with advanced or recurrent non-small-cell lung cancer (NSCLC) refractory to or intolerant of current conventional treatments. There were no severe adverse events related to the therapy. All patients remained alive during the study period with a median survival of 589 days and median progression-free survival of 126 days. Six patients had stable disease (SD), whereas the remaining six evaluable patients experienced progressive disease (PD) four weeks after the sixth transfer. We conclude that adoptive transfer of zoledronate-expanded γδ T cells is safe and feasible in patients with NSCLC, refractory to other treatments.
Keywords: Non-small-cell lung cancer (NSCLC), cell therapy, γδ T cells, adoptive transfer
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide; more than one million people die every year (1). Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all cases and most patients with NSCLC are diagnosed at an advanced stage and have a poor prognosis, with a 5-year survival rate of <5%. Conventional treatment for this disease consists of surgery, radiotherapy, chemotherapy, and multimodality therapies. The patient’s cancer staging, histology, and tolerance including performance status and comorbidities used to determine the indication for the treatment. Recently, treatment decisions for NSCLC are driven by their tumour genotype or phenotype, such as mutations in epidermal growth factor receptor (EGFR) and the fusion oncogene EML4-ALK (2). Bevacizumab, a monoclonal antibody that binds to vascular endothelial growth factor-A, erlotinib and gefitinib, small molecule tyrosine kinase inhibitors (TKIs) that inhibit EGFR, and crizotinib, a TKI that inhibits EML4-ALK are widely used for the treatment. So-called “immune checkpoint blockade” T-cell modulating agents, such as antibodies against cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), programmed death 1 (PD-1) and PD-L1, are currently being investigated. Despite the introduction of these new treatment modalities, outcomes remain poor, requiring for new treatment approaches. Active immunotherapy such as adoptive T cell-transfer represents one promising approach for lung cancer therapy (3). Growing body of evidence suggests that γδ T cells are attractive candidates for anticancer immunotherapy. This review discusses recent advances in basic γδ T cell research and data from clinical trials on the use of γδ Τ cells in the treatment of lung cancers.
γδ T cell
While most human peripheral blood T lymphocytes express αβ T cell receptor (TCR) (αβ T cell), 1-5% of peripheral blood T cells express γδ TCR (γδ T cell) (4) and <1% express invariant TCR (Vα24, Vβ11) (NKT cell). Major differences between these three lymphocytes are summarized in Table 1. γδ T cells like αβ T cell are derived from bone marrow derived precursor cells; αβ/γδ lineage commitment occurs during thymocyte development. The transcriptome analysis of γδ T cells demonstrated that the gene signature of γδ T cells is a hybrid of those from αβ T and NK cells, with more ‘NK cell’ genes than αβ T cells have and more ‘T cell’ genes than NK cells (5).
Table 1. Lymphocytes for immunotherapy.
| αβ T cell | γδ T cell | NKT cell | |
|---|---|---|---|
| PBMC (%) | 65-75 | 1-5 | <1 |
| Distribution | Blood, lymphoid organ | Blood, epithelium, lymphoid organ | Blood, bone marrow, liver, lung |
| Cell surface molecules | αβ TCR | γδ TCR | Invariant TCR (Vα24, Vβ11) |
| CD3 | CD3 | CD3 | |
| CD4/CD8 | CD4-CD8-/CD8αα+ | CD4/CD8 | |
| NKG2D | NK receptors | ||
| Antigen | MHC/peptide complex | IPP, ApppI, MICA/B | CD1d/glycolipid |
| MHC restriction | Yes | No | No |
| TCR diversity | Very diverse | Relatively restricted, expression variance dictated by tissue localization | Restricted |
| Cytotoxicity | Yes | Yes | Yes |
| Function | Adaptive immunity | Immune regulation, surveillance, homeostasis | Immune regulation |
Abbreviations: TCR, T cell receptor; MICA/B, MHC class I-related molecules A and B; IPP, isopentenyl pyrophosphate; PMBC, peripheral blood mononuclear cell.
γδ T cells fulfill a role of rapid lymphoid stress-surveillance system to stress-induced tissue perturbation (Figure 1A). The lymphoid stress-surveillance response is initiated by many forms of surface expression of stress-induced ligands for γδ TCR or NKG2D and provides an immediate source of cytokines, chemokines, and other factors that can substantially affect adaptive immunity (6). There are two main subsets of γδ T cells in humans: one expressing the TCR variable regions Vγ9 and Vδ2, represents the majority of peripheral-blood γδ T lymphocytes; the second subset of Vδ1 T cells is resident mainly within epithelia, where these cells serve as a first line of defense against infections or malignancies (7). During the intrathymic development, γδ T cell precursors express transcription factors, such as PLZF1, T-bet, Eomes, RORγt and SOX13. Distinct expression of transcription factors in γδ T cell subsets that bear particular TCR V regions determine the acquisition of discrete sets of homing receptors for particular peripheral tissues and effector functions with restricted expression patterns for cytokines (Figure 1B) (8).
Figure 1.
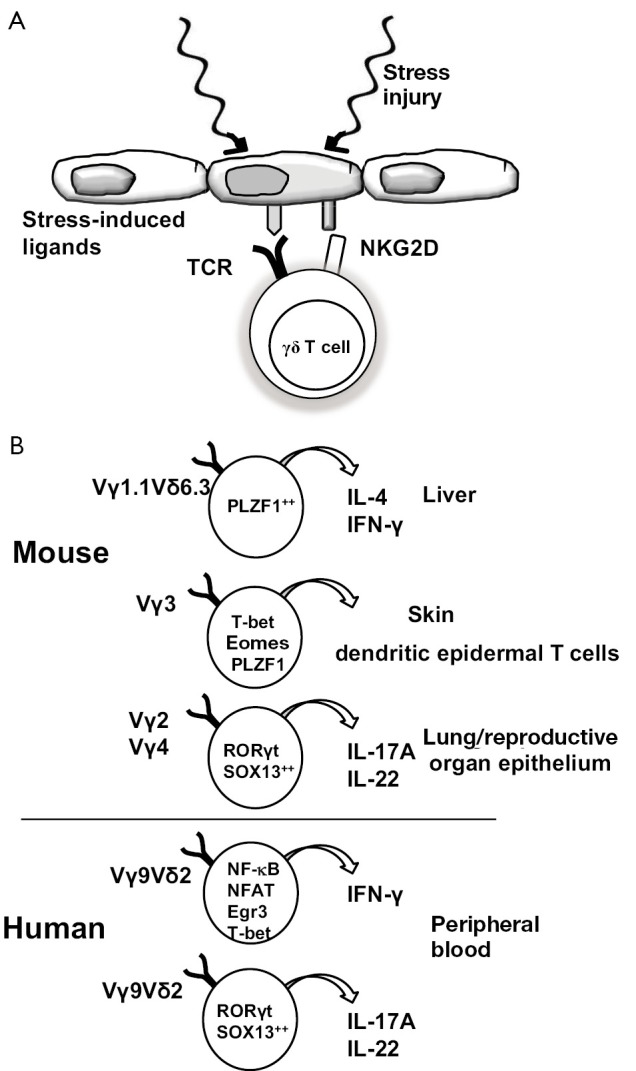
Lymphoid stress surveillance (A) and Tissue resident γδ T cells (B). (A) Injury or stress that upregulate surface expression of ligands for γδ TCR or for NKG2D initiate the lymphoid stress-surveillance response; (B) The intrathymic developmental programming determines the tissue distribution of γδ T cells. The emerging γδ thymocyte subsets with distinct transcriptional modules bear particular TCR V regions, which control the acquisition of discrete sets of HRs and effector functions. Abbreviations: TCR, T cell receptor; HR, homing receptor.
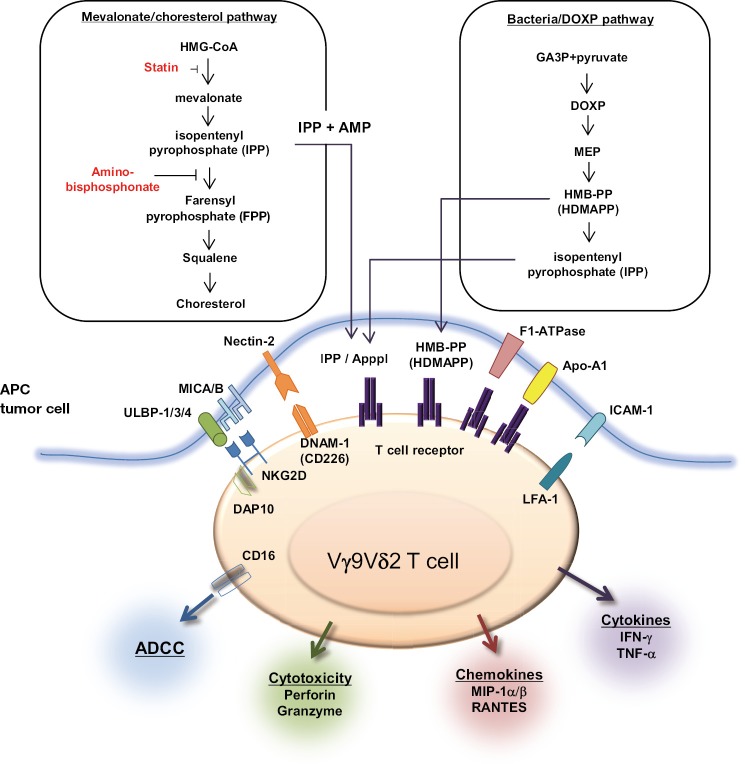
For lymphocytes to be suitable for stress surveillance, they express various receptors and recognize a spectrum of molecules signifying cellular dysregulation (Figure 2) (6,9). Historically, γδ T cells were shown to display a unique reactivity to mycobacteria rather than major histocompatibility complex (MHC) class I/peptide complex (10). It is now well known that low molecular weight phosphoantigens, such as isopentenyl pyrophosphate (IPP), are the ligands for Vγ9Vδ2 TCR (11). IPP is an intermediate metabolite of mevalonate/cholesterol pathway in mammalian cells. Though microbial IPP level may not reach the minimum required for γδ T cell activation and thus do not explain Vγ9Vδ2 T cell responses to infection, an intermediate of the alternative, nonmevalonate pathway of isoprenoid biosynthesis, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), has 10,000 times more effective bioactivity to Vγ9Vδ2 T cells than IPP (12). Stress-induced MHC class I-related molecules A and B (MICA/B) as well as UL16-binding proteins (ULBP) 1-4 and Retinoic acid early transcript 1 (RAET1) are recognized by NKG2D and unique products of common pathogens are recognized by TLRs. The cells can then display several types of function appropriate to different types of stress, directed against microbial infection or tumor cells. As immediate effector cells, γδ T cells secrete cytokines (IFN-γ, IL-17, IL-5, IL-13, IL-10, IL-4, LT-β) and chemokines (MIP-1α/β, RANTES) and exert cytotoxicity and antibody dependent cellular cytotoxicity (ADCC) upon pleiotropic antigen recognition. Therefore, γδ T cells are implicated in the first line of the defense against pathogens and have anti-tumor activity against cancers. Especially, robust cytotoxicity and IFN-γ and TNF-α secretion contribute to anti-tumor immunity.
Figure 2.
Tumor cell ligands recognized by human γδ T cells. Left panel, IPP is an intermediate metabolite produced through the mevalonate/cholesterol production pathway in mammalian cells. Pharmacological agents that can block upstream (statins) or downstream (aminobisphosphonate) this pathway lead to decreased or increased intracellular IPP levels, respectively. Endogenous IPP accumulation is observed in diverse tumor cells; IPP metabolites can be converted into ApppI, which could then be presented at the cell surface with much higher affinity to γδ TCR than IPP. Right panel, in pathogen-infected cells (e.g., mycobacterial infection), bacterial HMB-PP, or HDMAPP produced through the DOXP pathway could be presented. Abbreviations: IPP, isopentenyl pyrophosphate; TCR, T cell receptor; HMB-PP, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate; HDMAPP, 4-hydroxy-3-dimethylallyl pyrophosphate.
Tumor cell recognition by Vγ9Vδ2 T cells
Tumor surveillance is a key component of lymphoid stress surveillance; Transformation-induced changes are efficiently recognized by γδ T cells that may result in the enhance tumor immunogenicity. It has been reported that mutant p53, which is present in more than half of all human cancers, can significantly upregulate mevalonate pathway activity in cancer cells, which contributes to the γδ T cell recognition of tumor cells (13). Therefore, IPP and its isomer dimethylallyl pyrophosphate (DMAPP) accumulate in the tumor cells and are recognized by γδ TCR (14). Aminobisphosphonates, which are used to treat osteoporosis and metastatic bone disease of malignant tumors such as multiple myeloma, breast and prostate cancer, inhibit the farensyl pyrophospate (FPP) synthase, that mediates the conversion of IPP and DMAPP to FPP, leading to intracellular accumulation of upstream metabolites, including IPP and DMAPP (Figure 2) (9). Therefore, bisphosphonate sensitize tumor cells for the efficient recognition by γδ T cells. Recently, another metabolite, triphosphoric acid 1-adenosin-5'-yl ester 3-(3-methylbu-3-enyl) ester (ApppI) that is synthesized from IPP and ATP is considered as a natural activator of Vγ9Vδ2 T cells (15). Of note, ApppI is readily detectable in tumor cells such as Daudi cells without aminobisphosphonate treatment and may thus represent a natural ligand of γδ T cells. Moreover, Vγ9Vδ2 TCRs interact with F1-ATPase expressed at the tumor cell surface; MICA and MICB as well as ULBP 1-4 expressed by different types of epithelial tumor cells are recognized by γδ T cells through NKG2D receptors in a MHC unrestricted manner (16). Thus, γδ T cells can directly recognize molecules that are expressed on cancer cells without need of antigen processing and presentation, suggesting that they may exert anti-tumor effects even on target cells with reduced or absent expression of MHC class I molecules. These results imply the potential application of γδ T cell-mediated immunotherapy for the treatment of advanced cancers.
Immunotherapy with in vivo activation of γδ T cells
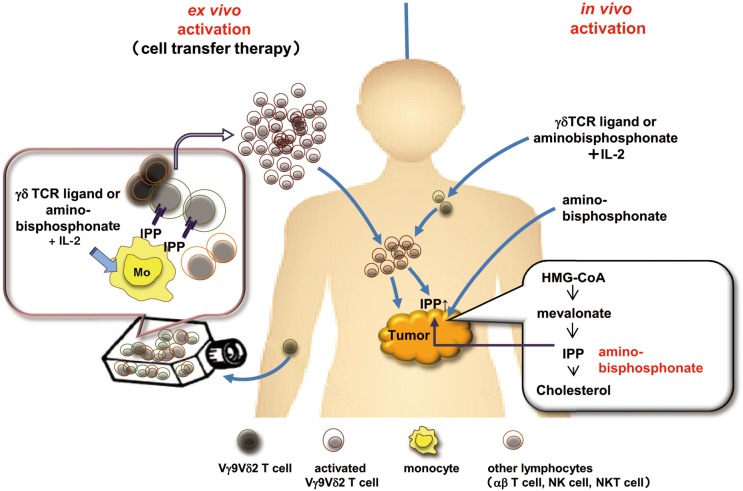
As shown in Figure 3, two strategies could be developed to apply the anti-tumor activity of γδ T cells to cancer immunotherapy: in vivo administration of compounds that activate γδ T cells or adoptive transfer of ex vivo expanded γδ T cells (17). Fever observed in patients under bisphosphonate treatment make us aware that bisphosphonate activated γδ T cells in peripheral blood mononuclear cells (PBMCs). Kunzmann et al. reported four of ten patients given pamidronate for increased bone resorption had a substantial increase in the percentage of γδ T cells in their PBMCs (18). Since then, immunotherapy seeking to exploit γδ T cells to destroy malignant cells was developed by administering aminobisphosphonate and interleukin-2 (IL-2), to activate and expand γδ T cells in vivo. A pilot study on the intravenous infusion of low-dose IL-2 in combination with pamidronate in patients with relapsed and/or refractory low-grade non-Hodgkin lymphoma or multiple myeloma demonstrated that activation and proliferation of γδ T cells in vivo was observed in five patients (55%) and partial responses were seen in three of the nine for which expansion of γδ T cells was observed in vitro (19). Dieli et al. treated hormone-refractory prostate cancer with either zoledronate in combination with IL-2 (n=9) or zoledronate alone (n=9) (20). Neither group of patients experienced any severe adverse events. The response rate was 67% in the first group and 22% in the second group, with actual responses dependent on the expansion, number, and phenotype of γδ T cells. While these aminobisphosphonates indirectly activate γδ T lymphocytes as a consequence of the inhibition of FPP (a key enzyme of the mevalonate pathway) that leads to intracellular accumulation of endogenous phosphoantigens, direct activation of γδ T cells by synthetic stimulators have also been described. In phase I trial, synthetic stimulators, phosphorylated bromohydrin (BrHPP) that mimics the biological properties of natural phosphoantigens, was administered to the patients with IL-2 (21). While BrHPP administration induces a potent γδ T cell expansion in patients, anti-tumor activity was not clear. One of the disadvantage of in vivo activation of γδ T cells is that the proliferative response is transient, probably because repeated injection of BrHPP and IL-2 induced activation induced cell death of Vγ9Vδ2 T cell and an exhaustion of the response (22).
Figure 3.
Strategies for γδ T cell based immunotherapy. Left panel, the adoptive cell transfer of in vitro expanded γδ T cells. Right panel, the in vivo activation of γδ T cells by phosphoantigens (e.g., BrHPP) or aminobisphosphonates and low-dose IL-2. The concomitant injection of aminobisphosphonate leads to intracellular accumulation of IPP/ApppI in tumor cells by blocking the mevalonate pathway, resulting in the sensitization of tumor cells to γδ T cells. Abbreviations: BrHPP, phosphorylated bromohydrin; IL-2, interleukin-2; IPP, isopentenyl pyrophosphate.
Immunotherapy with ex vivo activated γδ T cells
Adoptive transfer of γδ T cells following ex vivo expansion by using IL-2 and phosphoantigen or aminobisphosphonate could represent an alternative to their in vivo activation. Efficient Vγ9Vδ2 T cell expansion can be obtained by co-culturing PBMCs with γδ TCR ligands, such as 2-methyl-3-butenyl-1-pyrophosphate (2M3B1-PP) and BrHPP. More than 1×109 ex vivo expanded γδ T cells were administered to the patients with metastatic renal cell carcinoma, multiple myeloma, non-small cell lung cancer (23-28). Adoptive transfer of γδ T cells was well tolerated and objective clinical responses in some of the patients were reported. The injection of zoledronate might precede the infusion of expanded γδ T cells in patients with cancer. By blocking the mevalonate pathway, zoledronate leads to intracellular accumulation of IPP/ApppI in tumor cells that are then recognized by Vγ9Vδ2 T cells (Figure 3).
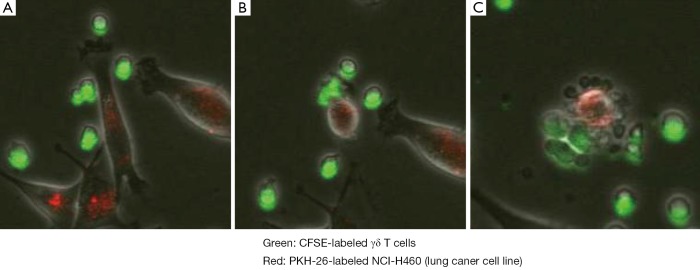
We have established a large-scale in vitro expansion method for Vγ9Vδ2 T cells using zoledronate and IL-2 (29,30). PBMC were stimulated with 5 μM zoledronate and IL-2. Fourteen days after in vitro stimulation, ex vivo expanded γδ T cells were harvested and scrutinized for their sterility and purity. As shown in Figure 4, the percentage of CD3+TCRVγ9+ T cells in PBMC in this example was 2.1% on day 0. The dominant populations were CD27+CD45RA+ naive or CD27+CD45RA- central memory phenotypes. When γδ T cells were efficiently stimulated, the frequency of γδ T cells increased to more than 92.0% of the cultured cells in successful γδ T cell cultures on day 14. The cultured γδ T cells upregulated NKG2D and CD69 expression and displayed CD27-CD45RA- effector memory phenotype. We found that Vγ9Vδ2 T cells from patients with advanced cancer as well as from healthy donors underwent extensive proliferation under these conditions. Such cultured Vγ9Vδ2 T cells retained cytokine secretion capacity and mediated cytotoxicity against a variety of cancer cell lines, including lung cancer cell lines. As shown in Figure 5, γδ T cells attached to the tumor cells, resulting in collapse of the tumor cell membranes and apoptosis.
Figure 4.
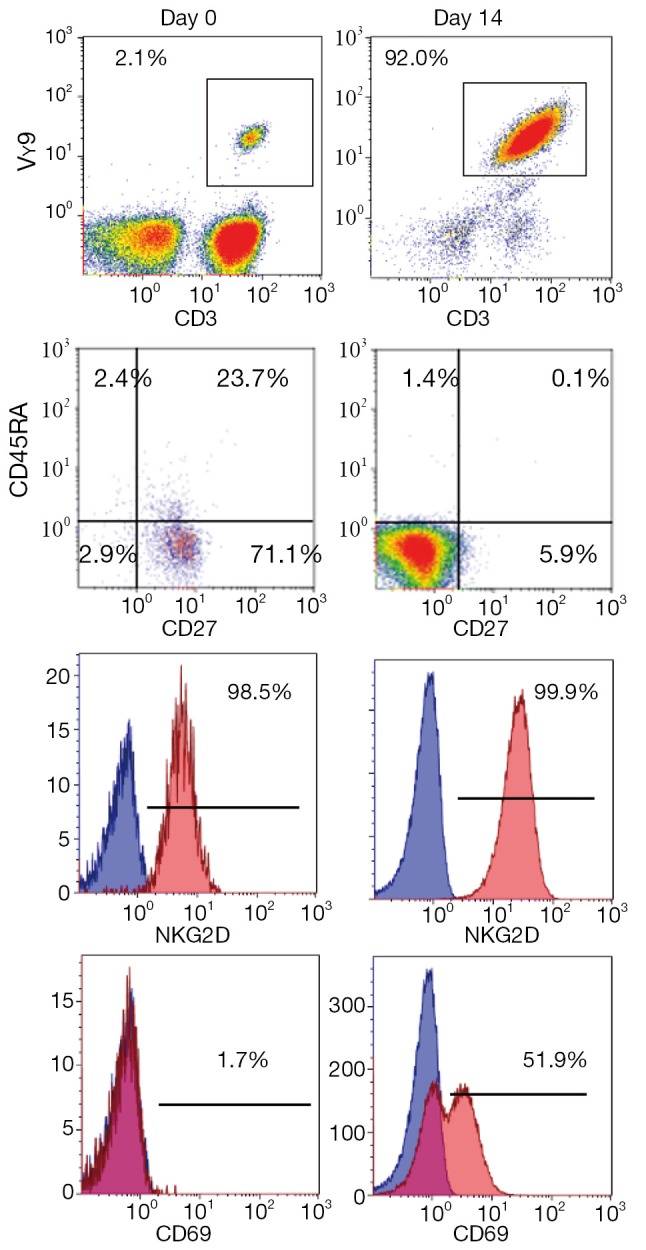
Typical phenotype of zoledronate-expanded γδ T cells by flow cytometric analysis. Fresh peripheral blood mononuclear cells or cells cultured for 14 days were stained with anti-CD3 and TCRVγ9 mAb. The CD3+Vγ9+ population was further analyzed with anti-CD27, CD45RA, NKG2D or CD69 mAbs.
Figure 5.
γδ T cell cytotoxicity. γδ T cells (green staining with CFSE) recognized and killed the lung cancer cell line, NCI H460 (red staining with PKH-26), by direct contact. In tumor cells attacked by the γδ T cells, collapse of the cell membranes led to apoptosis. It took approximately 2 h to progress from A to C.
γδ T cells and lung cancer
There are several reports that γδ T cells can recognize and kill lung cancer cells. By analysis of tumor infiltrating lymphocytes (TIL), γδ T cells were detected at human lung cancer; both Vδ1+ and Vδ2+ TILs killed the N592 lung cancer cell line (31). It has been reported that Vγ9Vδ2 T cells stimulated by synthetic phosphoantigen BrHPP lysed tumor cell lines, including a primary NSCLC cell line (lung-ca 459) (32). We examined whether zoledronate-activated Vγ9Vδ2 T cells displayed cytotoxic activity against a lung cancer cell lines with different histology, mutated genes, and sensitivity to chemotherapeutic drugs, using a panel of lung cancer cell lines described in Table 2. Zoledronate-activated γδ T cells recognized and killed these lung cancer cell lines irrelevant to their histology, mutated genes, and sensitivity to chemotherapeutic drugs. These results support the idea that γδ T cells can be used in immunotherapy for lung cancer.
Table 2. Lung cancer cell lines.
| Cell line | NCI-H1299 | A549 | NCI-H460 | NCI-H358 |
|---|---|---|---|---|
| Histology | NSCLC | Ad | La | BAC |
| Mutation | ||||
| EGFR | wt | wt | wt | wt |
| KRAS | wt | mut | mut | mut |
| p53 | del | wt | wt | del |
| Sensitivity to drug IC50 (μM) | ||||
| CDDP | 16.60 | 33.90 | 0.800 | 21.50 |
| Paclitaxel | 0.02 | 0.03 | 0.008 | 0.05 |
| 5FU | 0.20 | 1.90 | 0.600 | 4.70 |
| Gemcitabine | 0.04 | 0.01 | 0.008 | >0.10 |
| Gefitinib | 29.60 | 2.90 | 14.100 | 5.40 |
| Surface marker | ||||
| HLA | ++ | + | ++ | ++ |
| CD54 | + | – | + | |
| CD166 | + | + | ++ | ++ |
| MICA | ++ | + | ++ | – |
| Sensitivity to γδ T cell | + | + | +++ | ++ |
Abbreviations: NSCLC, non-small-cell lung cancer; Ad, adenocarcinoma; La, large cell carcinoma; BAC, bronchioloalveolar carcinoma; wt, wild type; mut, mutation; del, deletion.
γδ T cell therapy for the treatment of NSCLC
We conducted a phase I clinical study to evaluate safety and potential antitumor effects of re-infusing ex vivo expanded γδ T cells in patients with recurrent or advanced NSCLC (33,34). The research protocol was approved by the Ethics Committee of the University of Tokyo Hospital, and it was registered at the University Hospital Medical Information Network Clinical Trials Registry (Unique trial number: C000000336) on March 1, 2006. Written informed consent was obtained from each patient before they entered the study. The study was performed in accordance with the Declaration of Helsinki. Patients aged ≥20 years with advanced or recurrent NSCLC refractory to or intolerant of current conventional treatments were eligible for the study. Fifteen patients underwent adoptive immunotherapy with these γδ T cells. Patient’s PBMCs were stimulated with zoledronate (5 μM) and IL-2 (1,000 IU/mL) for 14 days. Harvested cells, mostly γδ T cells, were given intravenously every two weeks without additional IL-2, a total of six times. If there were some clinical benefit, the treatment was repeated until the disease progressed. Though adverse events were observed in five patients, such as elevated liver enzymes, Flu-like symptoms, bacterial pneumonia, radiation pneumonitis, tumor pain, dyspnea, and weight loss, there were no severe adverse events related to the therapy (Table 3).
Table 3. Adverse events.
| Adverse events | CTCAE grade |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Flu-like symptoms | 2 | |||
| Bacterial pneumonia | 1 | |||
| Radiation pneumonitis | 1 | |||
| Dyspnea | 1 | |||
| Weight loss | 1 | |||
| Tumor pain | 1 | |||
| Increased GGT | 1 | |||
| Increased AST | 1 | |||
| Increased ALT | 1 | |||
The results of phase I study of γδ T cell therapy was summarized in Table 4. The number of intravenous γδ T cell infusions ranged from 3 to 12. Twelve patients completed a course of six injections, three of whom received additional infusions. All patients remained alive during the study period with a median survival of 589 days and median progression-free survival (PFS) of 126 days. Maruyama et al. reported that median PFS was two months with gefitinib (250 mg/d) or docetaxel (60 mg/m2) in patients with advanced/metastatic NSCLC who had failed one or two chemotherapy regimens (35). Considering that the study population of ours was also quite similar to that study, the clinical responses achieved here are promising, though the number of study was small. According to the Response Evaluation Criteria In Solid Tumors, six patients had stable disease (SD), whereas the remaining six evaluable patients experienced progressive disease (PD) four weeks after the sixth transfer. We conclude that adoptive transfer of zoledronate-expanded γδ T cells is safe and feasible in patients with NSCLC, refractory to other treatment.
Table 4. Clinical results of adoptive γδ T cell transfer therapy for the treatment of NSCLC.
| Patient ID | No. γδT infusions | Plasma IFN-γ | Plasma MICA | Clinical response | PFS (d) | OS (d) |
|---|---|---|---|---|---|---|
| 1 | 6 | – | + | PD | 62 | 640 |
| 2 | 6 | – | – | SD | 188 | 1,505 |
| 3 | 6 | + | + | PD | 105 | 295 |
| 4 | 12 | + | – | SD | 126 | 738 |
| 5 | 6 | + | – | SD | 285 | 861 |
| 6 | 3 | – | + | Withdrawn (pneumonitis) | 276 | 360 |
| 7 | 6 | + | – | SD | 244 | 244 |
| 8 | 4 | – | – | Withdrawn (bacterial pneumonia) | 200 | 937 |
| 9 | 4 | – | – | PD | 34 | 589 |
| 10 | 12 | + | – | SD | 139 | 965 |
| 11 | 6 | – | – | Excluded (prostate cancer) | ||
| 12 | 8 | – | + | PD | 100 | 567 |
| 13 | 6 | – | – | SD | 237 | 616 |
| 14 | 6 | + | – | PD | 120 | 269 |
| 15 | 6 | + | – | PD | 72 | 202 |
| Median | 126 | 589 |
Abbreviations: NSCLC, non-small-cell lung cancer; MICA, MHC class I-related molecules A; PFS, progression-free survival; OS, overall survival; PD, progressive disease; SD, stable disease.
The number of peripheral γδ T cells gradually increased with increasing numbers of infusion. However, the increases of γδ T cells in PBMC after transfer therapy had no association with their clinical responses. It remains to be elucidated whether transferred γδ T cells actually infiltrated in the tumor. Though the association failed to achieve statistical significance, plasma IFN-γ elevation is a potential indicator of better prognosis in the small patient group reported here (Table 4). In contrast, soluble MICA in patients’ plasma was associated with poor prognosis; even though the presence of MICA in the plasma did not impair the ex vivo expansion of γδ T cells under our culture conditions. It has been reported that MICA is expressed by many cancers including primary lung cancers, and its recognition by NKG2D contributes to immunosurveillance against cancer. However, tumor cells shed MICA molecules into the serum to escape from recognition by immune cells. Soluble MICA downregulates NKG2D expression by CD8 T cells, NK cells, and γδ T cells (36). We are currently conducting phase II study to examine the efficacy and safety of adoptive γδ T cell therapy for the treatment of NSCLC. We will examine whether these molecules, IFN-γ and soluble MICA, can be applied as biomarkers; detection of IFN-γ or soluble MICA might be a marker for better prognosis or resistance to γδ T cell therapy, respectively, helping to determine in advance which patients would be likely to benefit from this treatment.
Future directions
Recent advances in tumor immunology have identified crucial roles of immune suppressive cells and immune checkpoint systems in inhibiting anti-tumor immune responses in cancer patients. Immune suppressive cells, such as regulatory T cells (Treg) and myeloid derived suppressor cells (MDSC), increase in cancer-bearing hosts and suppress anti-cancer immune responses. Despite general concerns that chemotherapy would inhibit the efficacy of immunotherapy because of bone marrow suppression and immunosuppressive effects, anticancer therapies can enhance anti-tumor immune response by depleting immunosuppressive cells (37). Because persistent presentation of tumor antigens makes T cell tolerant or unresponsive to the antigens, the physical removal of tumor burden by surgery can potentiate an immune response. Monoclonal antibodies that block CTLA-4, PD-1 and PD-L1 molecules inhibited tumour-induced immune suppression, thereby allowing T cells to continue to survive, proliferate, infiltrate into the tumor site, produce cytokines and promoting tumour rejection (38). These results suggest that there is great potential for surgery, chemotherapies and molecularly targeted agents, to work synergistically with γδ T cell based immunotherapy, making combinatorial strategies a key area of future clinical research. The clinical efficacy of γδ T cell transfer therapy should be evaluated further in prospective clinical trials, and combinations of this newly emerging therapy with established treatments are expected to improve the survival of lung cancer patients in the future.
Acknowledgements
We thank Makoto Kondo, Takamichi Izumi, and Takuya Takahashi for performing γδ T cell cultures; Nao Fujieda, Atsushi Kondo, Kaori Kanbara, and Ryuji Maekawa for immunological monitoring and laboratory assistance; Takashige Kondo, Yoko Yamashita, Tomoko Ishida, Haruka Matsushita, Yuki Nagasawa, Hiroki Yoshihara and Akiko Fukuzawa for wonderful technical assistance. This study was supported in part by a Grant-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology (Kazuhiro Kakimi and Jun Nakajima).
Disclosure: Dr. Kazuhiro Kakimi received research support from Medient Co. Ltd. (Yokohama, Japan). The costs of the entire γδ T cell culture production and part of the immunological assays were covered by Medinet Co. Ltd. The study sponsors had no involvement in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. All other authors have declared there are no financial conflicts of interest related to this work.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 2.Bonanno L, Favaretto A, Rugge M, et al. Role of genotyping in non-small cell lung cancer treatment: current status. Drugs 2011;71:2231-46. [DOI] [PubMed] [Google Scholar]
- 3.Kakimi K, Nakajima J, Wada H.Active specific immunotherapy and cell-transfer therapy for the treatment of non-small cell lung cancer. Lung Cancer 2009;65:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 2000;18:975-1026. [DOI] [PubMed] [Google Scholar]
- 5.Pont F, Familiades J, Déjean S, et al. The gene expression profile of phosphoantigen-specific human γδ T lymphocytes is a blend of αβ T-cell and NK-cell signatures. Eur J Immunol 2012;42:228-40. [DOI] [PubMed] [Google Scholar]
- 6.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 2009;31:184-96. [DOI] [PubMed] [Google Scholar]
- 7.Ferrarini M, Ferrero E, Dagna L, et al. Human gammadelta T cells: a nonredundant system in the immune-surveillance against cancer. Trends Immunol 2002;23:14-8. [DOI] [PubMed] [Google Scholar]
- 8.Narayan K, Sylvia KE, Malhotra N, et al. Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat Immunol 2012;13:511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riganti C, Massaia M, Davey MS, et al. Human γδ T-cell responses in infection and immunotherapy: common mechanisms, common mediators? Eur J Immunol 2012;42:1668-76. [DOI] [PubMed] [Google Scholar]
- 10.Modlin RL, Pirmez C, Hofman FM, et al. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature 1989;339:544-8. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Morita CT, Tanaka Y, et al. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 1995;375:155-8. [DOI] [PubMed] [Google Scholar]
- 12.Eberl M, Hintz M, Reichenberg A, et al. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett 2003;544:4-10. [DOI] [PubMed] [Google Scholar]
- 13.Freed-Pastor WA, Mizuno H, Zhao X, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012;148:244-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurnher M, Nussbaumer O, Gruenbacher G.Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res 2012;18:3524-31. [DOI] [PubMed] [Google Scholar]
- 15.Champagne E.γδ T cell receptor ligands and modes of antigen recognition. Arch Immunol Ther Exp (Warsz) 2011;59:117-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groh V, Steinle A, Bauer S, et al. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998;279:1737-40. [DOI] [PubMed] [Google Scholar]
- 17.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res 2010;70:10024-7. [DOI] [PubMed] [Google Scholar]
- 18.Kunzmann V, Bauer E, Wilhelm M.Gamma/delta T-cell stimulation by pamidronate. N Engl J Med 1999;340:737-8. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm M, Kunzmann V, Eckstein S, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 2003;102:200-6. [DOI] [PubMed] [Google Scholar]
- 20.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 2007;67:7450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennouna J, Levy V, Sicard H, et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vgamma9Vdelta2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol Immunother 2010;59:1521-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicard H, Ingoure S, Luciani B, et al. In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol 2005;175:5471-80. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H, Tanaka Y, Yagi J, et al. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 2007;56:469-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennouna J, Bompas E, Neidhardt EM, et al. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 2008;57:1599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi H, Tanaka Y, Yagi J, et al. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother 2011;60:1075-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicol AJ, Tokuyama H, Mattarollo SR, et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer 2011;105:778-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe Y, Muto M, Nieda M, et al. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol 2009;37:956-68. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi A, Kaneko T, Kamigaki T, et al. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy 2011;13:92-7. [DOI] [PubMed] [Google Scholar]
- 29.Kondo M, Sakuta K, Noguchi A, et al. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 2008;10:842-56. [DOI] [PubMed] [Google Scholar]
- 30.Kondo M, Izumi T, Fujieda N, et al. Expansion of human peripheral blood γδ T cells using zoledronate. J Vis Exp 2011;(55). pii: 3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrarini M, Heltai S, Pupa SM, et al. Killing of laminin receptor-positive human lung cancers by tumor infiltrating lymphocytes bearing gammadelta(+) t-cell receptors. J Natl Cancer Inst 1996;88:436-41. [DOI] [PubMed] [Google Scholar]
- 32.Shojaei H, Oberg HH, Juricke M, et al. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human gammadelta T cells. Cancer Res 2009;69:8710-7. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima J, Murakawa T, Fukami T, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg 2010;37:1191-7. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto M, Nakajima J, Murakawa T, et al. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδTcells: a phase I clinical study. J Immunother 2011;34:202-11. [DOI] [PubMed] [Google Scholar]
- 35.Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244-52. [DOI] [PubMed] [Google Scholar]
- 36.Groh V, Wu J, Yee C, et al. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002;419:734-8. [DOI] [PubMed] [Google Scholar]
- 37.Ménard C, Martin F, Apetoh L, et al. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother 2008;57:1579-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275-80. [DOI] [PMC free article] [PubMed] [Google Scholar]