Abstract
A very recent finding is the role of immune activation in cancer. The assumption that stimulating the patient’s immune system to attack tumors is a valuable treatment option in malignant diseases has gained more acceptance. However the high immunosuppressive effects caused by the tumor limits this beneficial effect. There is a delicate balance between immunoactivation and immunosuppression in a patient. Especially in non small cell lung cancer (NSCLC), the role of immunosuppressive cells hampering immune activation is high. But also in small cell lung cancer (SCLC) and mesothelioma immunosuppressive activity is high. This is suggested to be related to the type of tumor, advanced stage of the disease, and the tumor load. In this review, we provide an overview of the progress and challenges in the immunotherapeutic approaches in lung cancer. We conclude with the concept that immunotherapy in thoracic malignancies must be tailored made to the balance of the immune system.
Keywords: Non small cell lung cancer (NSCLC), immunotherapy, immune checkpoint antibodies
Introduction
Lung cancer
Around 85% of lung cancers are non-small cell lung cancer (NSCLC), which includes three principal histological subtypes: squamous cell carcinoma, adenocarcinoma and large cell carcinoma (1). The treatment of NSCLC is based on the patient’s clinical signs and symptoms, tumor stage and subtype, medical and family history, and data from imaging and laboratory evaluation.
The majority of patients (70%) is diagnosed with advanced (stage IV) disease, and until recently, palliative chemotherapy with platinum doublet therapy was the optimal treatment for these patients (2-4). However, chemotherapy has a limited impact on long-term survival of NSCLC and the five-year survival rate is extremely poor (5).
Recently the concept of driving mutations in NSCLC has dramatically changed the field of lung cancer treatment. Identification of these genotypic anomalies including activating mutations and fusion genes has set the stage for personalized medicine for distinct subsets of genetically defined NSCLC (6). It involves tailoring treatment according to the genetic profile and molecular makeup of each patient, depending on the availability of targeted drugs. To date, several prognostic and predictive mutations have been identified in NSCLC; including oncogenic activation of epidermal growth factor receptor (EGFR) (HER1/ErbB-1); translocation of EML4-ALK or CD74-ROS; point mutations in BRAF, PIK3CA, and MEK1; amplification of MET (7-10). Patients with mutations have benefited from the development of target-specific therapy; e.g., gefitinib or erlotinib are effective EGFR tyrosine kinase inhibitors; crizotinib is used for ALK activation and sunitinib can be used when PDGFR is amplified (11,12). Objective response rates of 55% to 90% percent are observed when patients were selected based upon molecular criteria (8).
One of the most disappointing findings is the fact that tumors develop resistance to these agents (13). The development of this resistance can either be mutation dependent, for instance genetic alteration of the drug target, or mutation independent, for instance via transformation of histology.
Small cell lung cancer (SCLC) accounts for approximately 15% of the lung cancer cases (14,15). In general, SCLC is initially sensitive to chemotherapy and radiotherapy (16). However, responses are often short-term and recurrence rates are high (16,17). Unfortunately, approximately 70% of patients diagnosed with SCLC have extended disease at presentation (18). These patients are treated with platinum-doublet chemotherapy and have a median survival of 10-12 months (19,20). The development of targeted therapies for SCLC has proven to be challenging, mainly due to the complex and not fully uncovered biology of SCLC (20,21).
Malignant mesothelioma
Malignant mesothelioma is a highly aggressive cancer caused by neoplastic transformation of mesothelial cells that line the body’s serous cavities and the internal organs. In the majority of mesothelioma patients the tumor is localized within the pleural cavity. With a median survival of 9-12 months after first signs of illness, the prognosis is poor. Chemotherapy has shown to moderately prolong survival in these patients, while combined modality approaches, such as extrapleural pneumonectomy followed by radiochemotherapy, is only of benefit in a highly selective patient population (22,23).
The limited treatment options and poor prognoses of lung cancer and mesothelioma emphasize the need for novel treatments. Therefore, immunotherapeutic approaches are being investigated in these thoracic malignancies and an overview of these efforts is included in this review.
Complex relationship between the immune system and cancer
In 2007, Koebel et al. demonstrated that tumors induced in mice by the chemical carcinogen methylcholanthrene can be controlled by the host immune system (24). Suppressing the activity of the immune system allowed dormant tumors to wrest themselves from immune control and start dividing, disseminating and ultimately causing death of the host. Assumptions that the immune system plays a role in eradicating cancerous lesions or maintains them in a state of dormancy have a history back to 1909 by Paul Ehrlich and by Lewis Thomas and MacFarland Burnet in 1957. The immunosurveillance concept is now accepted by the scientific community and “avoiding immune destruction” is included as the latest hallmark of cancer (25). Outgrowth of a tumor is divided in three phases often referred to as the three E’s (Elimination, Equilibrium, Escape) of immunoediting. In the first phase, tumor cells are recognized by the immune system and eliminated or controlled in their growth. In the equilibrium phase the immune system iteratively selects and/or promotes the generation of tumor cell variants with increasing capacities to survive immune attack. In the escape phase the immunologically sculpted tumor expands in an excessive manner leading to physical symptoms of cancer by the host (26). It is important to note that at this third stage, the tumor and the immune system have been causing distorted immune system’s cytotoxic activity, either by immunosuppressive activity or shedding of tumor antigens.
Setting the stage for immunotherapy
Developments of therapeutic antibodies, cancer vaccines, and cell-based immunotherapeutic approaches reveal both the promise and relative infancy of these agents to extend the life of patients with cancer. In 2010, sipuleucel-T (Provenge; Dendreon Corporation) received the first FDA approval of a cancer vaccine for the treatment of metastatic castration-resistant prostate cancer (27). It employs an adjuvant component to enhance the function of antigen presenting cells and immune effectors such as T cells. This was followed with the FDA approval in 2011 of the drug ipilimumab (Yelvoy, Bristol-Meyers Squibb) for the treatment of metastatic melanoma through potentiating T cell activity (28). Both agents, whose activity is discussed in more detail below, demonstrate improved survival in randomized phase 3 trails and reignited enthusiasm for the field of active immunotherapy. With the many clinical programs currently underway, new approvals for therapeutic cancer vaccines by FDA and other ruling authorities as EMA are expected in the coming years. Immunotherapy is now considered as the third wave in cancer therapy after conventional treatments and targeted agents.
Types of immunotherapeutic approaches
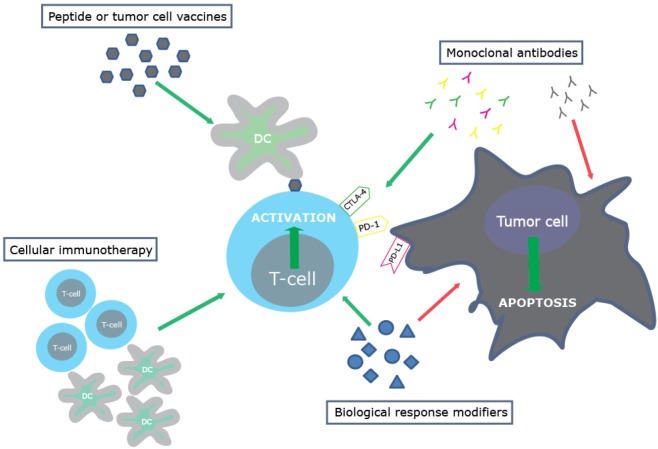
Immunotherapy attempts to stimulate or restore the body’s natural ability of the immune system to fight cancer. There are various strategies to activate the immune system and these are classified here into the following categories: biological response modifiers, monoclonal antibodies, peptide or tumor cell vaccines, and cellular immunotherapy (Figure 1 and Table 1). There is no consensus regarding which of the four categories is the optimal approach for thoracic malignancies, this will probably be highly dependent on the tumor characteristics of each individual patient.
Figure 1.
Immunotherapeutic approaches. Biological response modifiers are compounds which can specifically enhance the immune response, either by directly stimulating the immune system and/or by the direct induction of tumor cell apoptosis. These compounds can activate the anti-tumor immune response via the direct stimulation of pro-inflammatory immune cells or via the inhibition of detrimental suppressive immune cells like Tregs or myeloid-derived suppressor cells. Monoclonal antibodies bind specifically to one epitope and their application as potential immunotherapeutic agents has received a lot of attention recently. The development of antibodies which bind to co-inhibitory molecules activated during T-cell activation has led to the possibility to prevent T-cell inhibitory mechanisms and therefore enhance the anti-tumor immune response (29). Monoclonal antibodies targeting tumor growth related antigens (like EGFR) may diminish tumor growth by blockade of this receptor. On the other hand the IgG side of the antibody may cause immune-activation and tumor cell destruction in an immune related way. Monoclonal antibodies can also scavenge immunomodulatory proteins like VEGF. Peptide or tumor cell vaccines are designed to deliver tumor antigens to APCs, which can subsequently induce a tumor specific immune response by the adaptive immune system. These vaccines can consist of tumor specific antigens or can be composed of manipulated tumor cells. Cellular immunotherapy includes the adoptive transfer of autologous or allogeneic activated immune cells. The goal of this approach is to induce a tumor-specific immune response via the infusion of, e.g., tumor-antigen loaded dendritic cells or specifically activated T-cells.
Table 1. Immunotherapeutic approaches that have been tested or are currently under investigation for lung cancer and mesothelioma.
| Approach types | Examples of clinical studies in lung cancer and mesothelioma | Mode of action | References |
|---|---|---|---|
| Biological response modifiers | |||
| Triggering inflammation | PF-3512676 (CpG 7909) | Toll-like receptor 9 agonist | (30-33) |
| CpG-ODN 2006 | Downregulation of Tregs | (34) | |
| Bacillus Calmette-Guerin (BCG), Mycobacterium vaccae (SRL172) | Nonspecific immune stimulants now often tested as adjuvants | (35) | |
| Cytokine therapy | IL-2 + tumor necrosis factor alpha (TNF-α) or interferon alpha (IFN-α) | Induces T-cell proliferation | (36,37) |
| Interferon gamma (IFN-γ) | Induces tumor cell apoptosis | (38,39) | |
| Mda-7 (IL-24) | Mda-7/IL-24 induces tumor cell apoptosis and inhibits tumor angiogenesis | (40) | |
| Colony-stimulating factors | Granulocyte colony-stimulating factor (G-CSF) | Treatment of chemotherapy-induced neutropenia | (41,42) |
| Multi-modal effectors | Multi-target VEGFR: thalidomide and analogues such as lenalidomide and pomalidomide | Besides anti-metastatic, anti-angiogenic also immunomodulatory drugs (43,44) | (45,46) |
| Cyclophosphamide | Targets regulatory T-cells (47,48) | (47) | |
| Cyclosporine | Targets regulatory T-cells (49) | (50) | |
| Denileukin Diftitox | Targets regulatory T-cells (51) and cancer cells which express the IL-2 receptor | (52) | |
| Talactoferrin | Recombinant human lactoferrin, promotes innate and adaptive immunity against tumor cells in the gut-associated lymphoid tissue (53,54) | (55-57) | |
| Trabectedin (Yondelis) | Targets tumor-associated macrophages (58) and tumor cells | (59,60) | |
| All-trans-retinoic acid (ATRA) | Targets myeloid-derived suppressor cells (MDSCs) | (61) | |
| Monoclonal antibodies | |||
| Directed against tumor cells | Cetuximab, Panitumumab, Matuzumab, Necitumunab | Chimeric or fully humanized antibodies which target the epidermal growth factor (EGF) receptor on tumor cells | (62-64) |
| Trastuzumab (Herceptin) | Anti-HER2, targets tumor cells which overexpress the human epidermal growth factor 2 (HER2) protein | (65-67) | |
| CAT-5001 (SS1P) | Anti-mesothelin immunotoxin, targets mesothelin expressed in malignant mesothelioma and lung adenocarcinoma | (68) | |
| Amatuximab (MORab-009) | Chimeric anti-mesothelin monoclonal antibody | (69) | |
| Directed against tumor products | Bevacizumab | Slows the growth of tumor blood vessels by targeting the VEGF protein. Blockade of VEGF is also immunomodulatory | (70,71) |
| Immune checkpoint inhibitors | Anti-CTLA-4 (Ipilimumab/tremelimumab) |
Prevents T cell inhibitory mechanisms and allows T cells to continue cancer cell destruction | (72,73) |
| Anti-PD-L1 [(BMS-936559/MPDL-3280A)/PD-1 (BMS-936558 (nivolumab)/MK3475 (lambrolizumab)] | (74,75) | ||
| Peptide or tumor cell vaccines | |||
| Vaccines | GVAX | GM-CSF gene-transfected tumor cell vaccine | (76,77) |
| Belagenpumatucel-L (Lucanix) | Allogeneic tumor cell vaccine made with four irradiated NSCLC cell lines modified with TGF-β2 antisense plasmid | (78,79) | |
| MAGE-A3 vaccine | Vaccine composed of MAGE-A3 protein and adjuvant AS15 | (80) | |
| (L)-BLP-25 anti-MUC-1 (Stimuvax) | Vaccine which targets MUC-1 expressed on tumor cells | (81,82) | |
| TG4010 | Vaccinia vector coding MUC1 and IL-2 | (83,84) | |
| CimaVax EGF | Vaccine composed of human recombinant Epidermal Growth Factor (EGF) conjugated to a carrier protein | (85) | |
| WT1 peptide vaccine | Vaccine composed of four WT1 (Wilms’ tumor suppressor gene) analogue peptides | (86) | |
| CRS-207 | Live-attenuated Listeria monocytogenes vector encoding human mesothelin (87) | (88) | |
| Bec2/BCG | Induces anti-GD3 antibodies (overexpressed on 60% of SCLC patients) (89) | (90,91) | |
| GV1001 | Vaccine which targets the telomerase peptide GV1001 | (92) | |
| Racotumomab (Vaxira) | Anti-idiotypic antibody which mimicks the NGcGM3 ganglioside that is expressed on multiple human cancers (93) | (94), ClinicalTrials.gov: NCT01460472 | |
| Tergenpumatucel-L | Vaccine composed of irradiated and gene-transfected lung cancer cell lines | ClinicalTrials.gov: NCT01774578 | |
| Cellular immunotherapy | |||
| Dendritic cells (DCs) | Ex vivo generated DC-vaccines | Dendritic cells loaded with tumor antigens | (95-98) |
| T-cells | Ex vivo generated lymphokine-activated killer cells (LAK) | Autologous lymphokine-activated killer cells (99) | (100,101) |
| Cytokine-induced killer cells (CIK) | Autologous cytokine-activated T-cells and NK cells (102) | (103) | |
| Activated T-cells | Adoptive transfer of activated T-lymphocytes | (104) | |
| Gamma delta T cells | Adoptive transfer of zoledronate expanded gamma delta T-cells | (105) | |
| Natural Killer (NK) cells | NK cells | Adoptive transfer of allogeneic Natural Killer (NK) cells (106) | (107) |
Within this research field, there is much attention for activating effector and memory T-lymphocytes because the release of their cytotoxic granules containing perforin and granzymes upon stimulation can lead to death of tumor cells by apoptosis. Indeed, the infiltration of lung cancer with effector T-cells (CD3+CD8+) and memory T cells (CD45RO+) is associated with longer disease-free survival and/or a better overall survival in NSCLC (108-113). However, many other leukocyte types infiltrate the tumor environment: natural killer (T) cells, neutrophils, B- and T-lymphocyte subsets, myeloid derived suppressor cells, macrophages and dendritic cells. Based on their functions, these cells can be divided into cells with a potentially positive impact on the antitumor response and cells with a detrimental effect. The net effect of the interactions between these various cell types and their secreted products within the environment of an established tumor participates in determining anti-tumor immunity, angiogenesis, metastasis, overall cancer cell survival and proliferation (114).
The generation of a tumor specific cytotoxic T-cell response is dependent on the recognition of tumor antigens by the T-cell receptor (TCR). The TCR can only recognize tumor antigen peptides presented by the MHC molecule on the membrane of an antigen-presenting cell (APC) like the dendritic cell. In addition to specific antigen recognition, co-stimulation is required for activation of the T-cell (115). This second signal is antigen nonspecific and is provided through the interaction between co-stimulatory molecules on the membrane of the APC and the T-cell. Both the TCR engagement and the co-stimulatory signal are necessary to stimulate optimal T-cell differentiation and proliferation. Antigen recognition by the TCR in the absence of the second co-stimulatory signal results in T cell anergy or apoptosis (116).
Therefore, in addition to the activation of the anti-tumor T-cell response, there is increasing interest to modify this immunological balance, e.g., by targeting immune suppressive cell types or factors.
Different approaches are currently studied to overcome the earlier described immunosuppressive environment and to enhance the cytotoxic T-cell response. We developed dendritic-cell based therapy with the intention to potentiate the anti-tumor immune response and ultimately improve outcome in mesothelioma patients. It was demonstrated that this approach was safe and effective in mesothelioma patients (95,117).
Immunotherapy in lung cancer and mesothelioma: the hurdles. Data of our own research
Our institute has experience in immunological research in mesothelioma and lung cancer. We study the role of the immune system both in a preclinical and clinical model with focus on therapeutic interventions in patients.
We observed both in an animal model and in patients that the immune activity is prohibited via the upregulation of the number of immunosuppressive cells, which will be discussed below. We did found that the immune activity in patients with lung cancer or mesothelioma is often profoundly inhibited by a large array of immunosuppressive mechanisms (118-120). Regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs) were found to be increased in the peripheral blood of lung cancer and mesothelioma patients (30). In addition, within the tumor anti-tumor T-cell responses were additionally inhibited by tumor-associated macrophages (121). In order to improve T-cell activity we have studied in our animal model whether modulating the suppressive function of Tregs (122), MDSCs (123) and macrophages (121,124) could improve immunotherapeutic approaches. In general we found that targeting these immunosuppressive cells has beneficial effects on the capacity of the immune system to be activated.
The plethora of immunosuppressive mechanisms is tumor-stage dependent. In our murine mesothelioma model, the immune activity correlated with tumor load (117). Also in patients with lung cancer this seems present (32). In advanced-stage mesothelioma patients only very limited tumor specific cytotoxic T-cell responses could be detected (95). This hampered anti-tumor immune response is likely to be caused by the presence of immunosuppressive cells (118).
This may explain, as we reviewed recently, the low number of responders to immune checkpoint antibodies in these patients (29), as for the efficacy of these antibodies, cytotoxic T cell activity is crucial. However, as we did found a large variation in the number of MDSC and also CD8 positive cells in patients with advanced disease NSCLC (32), this also clarifies why responders do occur. In individual patients with lung cancer and mesothelioma, also in the same stage of the disease, a different profile of immunosuppression is present (data submitted for publication).
But still in most patients with advanced disease an ex-vivo immune activation is obligatory. As described above, our department has experience with dendritic cell based therapy as cellular immunotherapy (95,117). Combining this therapy with one of the other forms was found synergistic in our animal model (35-37). The results of the patient study on regulatory T cell depletion in combination with dendritic cell immunotherapy are pending.
Immunotherapy in lung cancer and mesothelioma: the way to go
The complex interplay between the tumor and the immune system which differs per patient and per time point causes the need for personalized immunotherapy. Depending on the state of the immune system, different forms of immunotherapy will have to be deployed. Future research will have to take this complex interplay into account.
Another key issue is the definition of effectiveness in clinical trials. Immunotherapeutic approaches show clinical success that may take months to develop, even after an initial period of presumed tumor growth (125,126). Therefore new criteria for development of these drugs in early phase clinical trials are proposed to take these unique tumor response kinetics into account (127). Redefining appropriate clinical trial end points in the coming years is essential to determine the efficacy of the different classes of immunotherapy in patients with cancer at an early phase of drug development.
It is desirable that before the start of any immunotherapy trial proper biomarkers are selected. To make bigger steps these must also be incorporated in early phase trials. Unfortunately large phase III studies have been performed without taking these provisos into account. The recent negative trials on talactoferrin and anti-MUC-1 are probably examples of this absent patient selection beforehand. Unfortunately also at present large phase III trials are enrolling patients without proper patient selection or selection done on unclear conditions. The ongoing phase III trials on immune checkpoint inhibitors purely in squamous cell lung cancer are examples of patient selection without a clear theoretical background but driven on the (unexpected and unexplained) very preliminary data on efficacy.
Conclusions
Immunotherapy is one of the most exciting and promising developments in recent years in the treatment of cancer. Facing the difficulty of its working mechanism and the read-out of the efficacy, investigators have the responsibility to search for biomarkers and outcome parameters, which then have to be embraced by the scientific community.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [DOI] [PubMed] [Google Scholar]
- 2.Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23Suppl 7:vii56-64. [DOI] [PubMed] [Google Scholar]
- 3.Underwood JM, Townsend JS, Tai E, et al. Racial and regional disparities in lung cancer incidence. Cancer 2012;118:1910-8. [DOI] [PubMed] [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-36. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol 2011;8:661-8. [DOI] [PubMed] [Google Scholar]
- 7.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [DOI] [PubMed] [Google Scholar]
- 8.Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 2011;29:2121-7. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res 2011;17:2081-6. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol 2010;17:889-97. [DOI] [PubMed] [Google Scholar]
- 11.Cataldo VD, Gibbons DL, Pérez-Soler R, et al. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med 2011;364:947-55. [DOI] [PubMed] [Google Scholar]
- 12.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tartarone A, Lazzari C, Lerose R, et al. Mechanisms of resistance to EGFR tyrosine kinase inhibitors gefitinib/erlotinib and to ALK inhibitor crizotinib. Lung Cancer 2013;81:328-36. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 15.Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [DOI] [PubMed] [Google Scholar]
- 16.Rosti G, Bevilacqua G, Bidoli P, et al. Small cell lung cancer. Ann Oncol 2006;17Suppl 2:ii5-10. [DOI] [PubMed] [Google Scholar]
- 17.Chua YJ, Steer C, Yip D. Recent advances in management of small-cell lung cancer. Cancer Treat Rev 2004;30:521-43. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen M, Pijls-Johannesma M, Felip E, et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21Suppl 5:v120-5. [DOI] [PubMed] [Google Scholar]
- 19.Stupp R, Monnerat C, Turrisi AT, 3rd, et al. Small cell lung cancer: state of the art and future perspectives. Lung Cancer 2004;45:105-17. [DOI] [PubMed] [Google Scholar]
- 20.Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: beyond traditional treatment approaches. J Thorac Oncol 2013;8:587-98. [DOI] [PubMed] [Google Scholar]
- 21.Rüegg C, Peters S.Thalidomide in small cell lung cancer: wrong drug or wrong disease? J Natl Cancer Inst 2009;101:1034-5. [DOI] [PubMed] [Google Scholar]
- 22.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [DOI] [PubMed] [Google Scholar]
- 23.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet 2005;366:397-408. [DOI] [PubMed] [Google Scholar]
- 24.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007;450:903-7. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [DOI] [PubMed] [Google Scholar]
- 26.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [DOI] [PubMed] [Google Scholar]
- 27.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [DOI] [PubMed] [Google Scholar]
- 28.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 2013;73:2381-8. [DOI] [PubMed] [Google Scholar]
- 30.Belani CP, Nemunaitis JJ, Chachoua A, et al. Phase 2 trial of erlotinib with or without PF-3512676 (CPG 7909, a Toll-like receptor 9 agonist) in patients with advanced recurrent EGFR-positive non-small cell lung cancer. Cancer Biol Ther 2013;14:557-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsh V, Paz-Ares L, Boyer M, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2667-74. [DOI] [PubMed] [Google Scholar]
- 32.Manegold C, van Zandwijk N, Szczesna A, et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol 2012;23:72-7. [DOI] [PubMed] [Google Scholar]
- 33.Yamada K, Nakao M, Fukuyama C, et al. Phase I study of TLR9 agonist PF-3512676 in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Sci 2010;101:188-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YY, He XY, Cai YY, et al. The variation of CD4+CD25+ regulatory T cells in the periphery blood and tumor microenvironment of non-small cell lung cancer patients and the downregulation effects induced by CpG ODN. Target Oncol 2011;6:147-54. [DOI] [PubMed] [Google Scholar]
- 35.Bottomley A, Debruyne C, Felip E, et al. Symptom and quality of life results of an international randomised phase III study of adjuvant vaccination with Bec2/BCG in responding patients with limited disease small-cell lung cancer. Eur J Cancer 2008;44:2178-84. [DOI] [PubMed] [Google Scholar]
- 36.Schiller JH, Morgan-Ihrig C, Levitt ML. Concomitant administration of interleukin-2 plus tumor necrosis factor in advanced non-small cell lung cancer. Am J Clin Oncol 1995;18:47-51. [DOI] [PubMed] [Google Scholar]
- 37.Jansen RL, Slingerland R, Goey SH, et al. Interleukin-2 and interferon-alpha in the treatment of patients with advanced non-small-cell lung cancer. J Immunother (1991) 1992;12:70-3. [DOI] [PubMed] [Google Scholar]
- 38.Mattson K, Niiranen A, Pyrhönen S, et al. Recombinant interferon gamma treatment in non-small cell lung cancer. Antitumour effect and cardiotoxicity. Acta Oncol 1991;30:607-10. [DOI] [PubMed] [Google Scholar]
- 39.Jett JR, Maksymiuk AW, Su JQ, et al. Phase III trial of recombinant interferon gamma in complete responders with small-cell lung cancer. J Clin Oncol 1994;12:2321-6. [DOI] [PubMed] [Google Scholar]
- 40.Gupta P, Emdad L, Lebedeva IV, et al. Targeted combinatorial therapy of non-small cell lung carcinoma using a GST-fusion protein of full-length or truncated MDA-7/IL-24 with Tarceva. J Cell Physiol 2008;215:827-36. [DOI] [PubMed] [Google Scholar]
- 41.Timmer-Bonte JN, de Boo TM, Smit HJ, et al. Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony-stimulating factor in small-cell lung cancer: a Dutch Randomized Phase III Study. J Clin Oncol 2005;23:7974-84. [DOI] [PubMed] [Google Scholar]
- 42.Timmer-Bonte JN, Punt CJ, vd Heijden HF, et al. Prophylactic G-CSF and antibiotics enable a significant dose-escalation of triplet-chemotherapy in non-small cell lung cancer. Lung Cancer 2008;60:222-30. [DOI] [PubMed] [Google Scholar]
- 43.Galustian C, Dalgleish A.Lenalidomide: a novel anticancer drug with multiple modalities. Expert Opin Pharmacother 2009;10:125-33. [DOI] [PubMed] [Google Scholar]
- 44.Elkinson S, McCormack PL. Pomalidomide: first global approval. Drugs 2013;73:595-604. [DOI] [PubMed] [Google Scholar]
- 45.Hoang T, Dahlberg SE, Schiller JH, et al. Randomized phase III study of thoracic radiation in combination with paclitaxel and carboplatin with or without thalidomide in patients with stage III non-small-cell lung cancer: the ECOG 3598 study. J Clin Oncol 2012;30:616-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young RJ, Tin AW, Brown NJ, et al. Analysis of circulating angiogenic biomarkers from patients in two phase III trials in lung cancer of chemotherapy alone or chemotherapy and thalidomide. Br J Cancer 2012;106:1153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bass KK, Mastrangelo MJ. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol Immunother 1998;47:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 2007;56:641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawai M, Kitade H, Mathieu C, et al. Inhibitory and stimulatory effects of cyclosporine A on the development of regulatory T cells in vivo. Transplantation 2005;79:1073-7. [DOI] [PubMed] [Google Scholar]
- 50.Kruijtzer CM, Schellens JH, Mezger J, et al. Phase II and pharmacologic study of weekly oral paclitaxel plus cyclosporine in patients with advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4508-16. [DOI] [PubMed] [Google Scholar]
- 51.Litzinger MT, Fernando R, Curiel TJ, et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood 2007;110:3192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerena-Lewis M, Crawford J, Bonomi P, et al. A Phase II trial of Denileukin Diftitox in patients with previously treated advanced non-small cell lung cancer. Am J Clin Oncol 2009;32:269-73. [DOI] [PubMed] [Google Scholar]
- 53.Spadaro M, Caorsi C, Ceruti P, et al. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J 2008;22:2747-57. [DOI] [PubMed] [Google Scholar]
- 54.Hayes TG, Falchook GF, Varadhachary GR, et al. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Invest New Drugs 2006;24:233-40. [DOI] [PubMed] [Google Scholar]
- 55.Digumarti R, Wang Y, Raman G, et al. A randomized, double-blind, placebo-controlled, phase II study of oral talactoferrin in combination with carboplatin and paclitaxel in previously untreated locally advanced or metastatic non-small cell lung cancer. J Thorac Oncol 2011;6:1098-103. [DOI] [PubMed] [Google Scholar]
- 56.Kelly RJ, Giaccone G. The role of talactoferrin alpha in the treatment of non-small cell lung cancer. Expert Opin Biol Ther 2010;10:1379-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parikh PM, Vaid A, Advani SH, et al. Randomized, double-blind, placebo-controlled phase II study of single-agent oral talactoferrin in patients with locally advanced or metastatic non-small-cell lung cancer that progressed after chemotherapy. J Clin Oncol 2011;29:4129-36. [DOI] [PubMed] [Google Scholar]
- 58.Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013;23:249-62. [DOI] [PubMed] [Google Scholar]
- 59.Sessa C, Cresta S, Noberasco C, et al. Phase I clinical and pharmacokinetic study of trabectedin and cisplatin in solid tumours. Eur J Cancer 2009;45:2116-22. [DOI] [PubMed] [Google Scholar]
- 60.Massuti B, Cobo M, Camps C, et al. Trabectedin in patients with advanced non-small-cell lung cancer (NSCLC) with XPG and/or ERCC1 overexpression and BRCA1 underexpression and pretreated with platinum. Lung Cancer 2012;76:354-61. [DOI] [PubMed] [Google Scholar]
- 61.Iclozan C, Antonia S, Chiappori A, et al. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 2013;62:909-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanna N, Lilenbaum R, Ansari R, et al. Phase II trial of cetuximab in patients with previously treated non-small-cell lung cancer. J Clin Oncol 2006;24:5253-8. [DOI] [PubMed] [Google Scholar]
- 63.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [DOI] [PubMed] [Google Scholar]
- 64.Pirker R.EGFR-directed monoclonal antibodies in non-small cell lung cancer. Target Oncol 2013;8:47-53. [DOI] [PubMed] [Google Scholar]
- 65.Clamon G, Herndon J, Kern J, et al. Lack of trastuzumab activity in nonsmall cell lung carcinoma with overexpression of erb-B2: 39810: a phase II trial of Cancer and Leukemia Group B. Cancer 2005;103:1670-5. [DOI] [PubMed] [Google Scholar]
- 66.Lara PN, Jr, Laptalo L, Longmate J, et al. Trastuzumab plus docetaxel in HER2/neu-positive non-small-cell lung cancer: a California Cancer Consortium screening and phase II trial. Clin Lung Cancer 2004;5:231-6. [DOI] [PubMed] [Google Scholar]
- 67.Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [DOI] [PubMed] [Google Scholar]
- 68.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007;13:5144-9. [DOI] [PubMed] [Google Scholar]
- 69.Hassan R, Cohen SJ, Phillips M, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 2010;16:6132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barlesi F, Scherpereel A, Rittmeyer A, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol 2013;31:3004-11. [DOI] [PubMed] [Google Scholar]
- 71.Cui J, Cai X, Zhu M, et al. The efficacy of bevacizumab compared with other targeted drugs for patients with advanced NSCLC: a meta-analysis from 30 randomized controlled clinical trials. PLoS One 2013;8:e62038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [DOI] [PubMed] [Google Scholar]
- 73.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [DOI] [PubMed] [Google Scholar]
- 74.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nemunaitis J, Sterman D, Jablons D, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst 2004;96:326-31. [DOI] [PubMed] [Google Scholar]
- 77.Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther 2006;13:555-62. [DOI] [PubMed] [Google Scholar]
- 78.Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 2006;24:4721-30. [DOI] [PubMed] [Google Scholar]
- 79.Nemunaitis J, Nemunaitis M, Senzer N, et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther 2009;16:620-4. [DOI] [PubMed] [Google Scholar]
- 80.Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013;31:2396-403. [DOI] [PubMed] [Google Scholar]
- 81.Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 2005;23:6674-81. [DOI] [PubMed] [Google Scholar]
- 82.Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 2011;137:1337-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol 2008;3:735-44. [DOI] [PubMed] [Google Scholar]
- 84.Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol 2011;12:1125-33. [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez PC, Neninger E, García B, et al. Safety, immunogenicity and preliminary efficacy of multiple-site vaccination with an Epidermal Growth Factor (EGF) based cancer vaccine in advanced non small cell lung cancer (NSCLC) patients. J Immune Based Ther Vaccines 2011;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krug LM, Dao T, Brown AB, et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol Immunother 2010;59:1467-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hassan R, Ho M.Mesothelin targeted cancer immunotherapy. Eur J Cancer 2008;44:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 2012;18:858-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brezicka T, Bergman B, Olling S, et al. Reactivity of monoclonal antibodies with ganglioside antigens in human small cell lung cancer tissues. Lung Cancer 2000;28:29-36. [DOI] [PubMed] [Google Scholar]
- 90.Grant SC, Kris MG, Houghton AN, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guérin. Clin Cancer Res 1999;5:1319-23. [PubMed] [Google Scholar]
- 91.Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [DOI] [PubMed] [Google Scholar]
- 92.Brunsvig PF, Kyte JA, Kersten C, et al. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res 2011;17:6847-57. [DOI] [PubMed] [Google Scholar]
- 93.Fernandez LE, Gabri MR, Guthmann MD, et al. NGcGM3 ganglioside: a privileged target for cancer vaccines. Clin Dev Immunol 2010;2010:814397. [DOI] [PMC free article] [PubMed]
- 94.Vázquez AM, Hernández AM, Macías A, et al. Racotumomab: an anti-idiotype vaccine related to N-glycolyl-containing gangliosides - preclinical and clinical data. Front Oncol 2012;2:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hegmans JP, Veltman JD, Lambers ME, et al. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med 2010;181:1383-90. [DOI] [PubMed] [Google Scholar]
- 96.Hirschowitz EA, Foody T, Kryscio R, et al. Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol 2004;22:2808-15. [DOI] [PubMed] [Google Scholar]
- 97.Hirschowitz EA, Foody T, Hidalgo GE, et al. Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer 2007;57:365-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Um SJ, Choi YJ, Shin HJ, et al. Phase I study of autologous dendritic cell tumor vaccine in patients with non-small cell lung cancer. Lung Cancer 2010;70:188-94. [DOI] [PubMed] [Google Scholar]
- 99.Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985;313:1485-92. [DOI] [PubMed] [Google Scholar]
- 100.Kimura H, Yamaguchi Y.Adjuvant immunotherapy with interleukin 2 and lymphokine-activated killer cells after noncurative resection of primary lung cancer. Lung Cancer 1995;13:31-44. [DOI] [PubMed] [Google Scholar]
- 101.Kimura H, Yamaguchi Y.A phase III randomized study of interleukin-2 lymphokine-activated killer cell immunotherapy combined with chemotherapy or radiotherapy after curative or noncurative resection of primary lung carcinoma. Cancer 1997;80:42-9. [PubMed] [Google Scholar]
- 102.Ma Y, Zhang Z, Tang L, et al. Cytokine-induced killer cells in the treatment of patients with solid carcinomas: a systematic review and pooled analysis. Cytotherapy 2012;14:483-93. [DOI] [PubMed] [Google Scholar]
- 103.Li R, Wang C, Liu L, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother 2012;61:2125-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iwai K, Soejima K, Kudoh S, et al. Extended survival observed in adoptive activated T lymphocyte immunotherapy for advanced lung cancer: results of a multicenter historical cohort study. Cancer Immunol Immunother 2012;61:1781-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakamoto M, Nakajima J, Murakawa T, et al. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded Tcells: a phase I clinical study. J Immunother 2011;34:202-11. [DOI] [PubMed] [Google Scholar]
- 106.Terme M, Ullrich E, Delahaye NF, et al. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol 2008;9:486-94. [DOI] [PubMed] [Google Scholar]
- 107.Iliopoulou EG, Kountourakis P, Karamouzis MV, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother 2010;59:1781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suzuki K, Kachala SS, Kadota K, et al. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res 2011;17:5247-56. [DOI] [PubMed] [Google Scholar]
- 109.Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 2011;6:824-33. [DOI] [PubMed] [Google Scholar]
- 110.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer 2006;94:275-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCoy MJ, Lake RA, van der Most RG, et al. Post-chemotherapy T-cell recovery is a marker of improved survival in patients with advanced thoracic malignancies. Br J Cancer 2012;107:1107-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCoy MJ, Nowak AK, van der Most RG, et al. Peripheral CD8(+) T cell proliferation is prognostic for patients with advanced thoracic malignancies. Cancer Immunol Immunother 2013;62:529-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wakabayashi O, Yamazaki K, Oizumi S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci 2003;94:1003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heuvers ME, Aerts JG, Cornelissen R, et al. Patient-tailored modulation of the immune system may revolutionize future lung cancer treatment. BMC Cancer 2012;12:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol 2009;27:591-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev 2003;192:161-80. [DOI] [PubMed] [Google Scholar]
- 117.Hegmans JP, Hemmes A, Aerts JG, et al. Immunotherapy of murine malignant mesothelioma using tumor lysate-pulsed dendritic cells. Am J Respir Crit Care Med 2005;171:1168-77. [DOI] [PubMed] [Google Scholar]
- 118.Heuvers ME, Muskens F, Bezemer K, et al. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer 2013;81:468-74. [DOI] [PubMed] [Google Scholar]
- 119.Hegmans JP, Hemmes A, Hammad H, et al. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur Respir J 2006;27:1086-95. [DOI] [PubMed] [Google Scholar]
- 120.Aerts JG, Smit EF, Groen HJ, et al. Dingemans, The number of myeloid derived suppressor cells (MDSC) is increased in patients with advanced disease non-squamous lungcancer (aNSQ) compared to healthy controls and early stage of non-squamous lungcancer (eNSQ). Subanalysis of the NVALT12 study. J Thorac Oncol 2013;8:764S. [Google Scholar]
- 121.Lievense LA, Bezemer K, Aerts JG, et al. Tumor-associated macrophages in thoracic malignancies. Lung Cancer 2013;80:256-62. [DOI] [PubMed] [Google Scholar]
- 122.Veltman JD, Lambers ME, van Nimwegen M, et al. Low-dose cyclophosphamide synergizes with dendritic cell-based immunotherapy in antitumor activity. J Biomed Biotechnol 2010;2010:798467. [DOI] [PMC free article] [PubMed]
- 123.Veltman JD, Lambers ME, van Nimwegen M, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 2010;10:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Veltman JD, Lambers ME, van Nimwegen M, et al. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br J Cancer 2010;103:629-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madan RA, Gulley JL, Fojo T, et al. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 2010;15:969-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cornelissen R, Heuvers ME, Maat AP, et al. New roads open up for implementing immunotherapy in mesothelioma. Clin Dev Immunol 2012;2012:927240. [DOI] [PMC free article] [PubMed]
- 127.Hoos A.Evolution of end points for cancer immunotherapy trials. Ann Oncol 2012;23Suppl 8:viii47-52. [DOI] [PubMed] [Google Scholar]