Abstract
The 7th edition of TNM for Lung Cancer represented a major advance from previous editions, in the process of revision, the size and breadth of the data base used, its international character, the intensity of the analysis and the critical nature of the internal and external validation undertaken before its launch in January 2010. This all came about by the involvement of the International Association for the Study of Lung Cancer (IASLC), which assumed the role previously performed by Dr. Mountain, of developing data-driven revisions for the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC). In taking on this task the IASLC made the global lung cancer community aware of the limitations of previous revisions and now stand accountable and subject to the same scrutiny. In this article we describe the achievements of the IASLC TNM and Prognostic Factors Committee, but also the short-comings of the 7th edition, as an essential step towards rectifying deficiencies and further improving the classification in future revisions.
Key Words: Lung cancer, prognostic factors, staging
Introduction
To properly understand the achievements of the 7th edition of TNM for Lung Cancer it is necessary to give a brief history of the TNM Classification itself. A more detailed history of this topic is available elsewhere (1).
A system to describe the anatomical extent of a cancer using the “T”, “N” and “M” descriptors was developed by Dr. Pierre Denoix, a surgical oncologist at the Institut Gustave-Roussy in Paris, and evolved over a series of articles in the 1940s and early 50s (2). The first international classification of malignant disease based on TNM was published in 1968 by the Union Internationale Contre le Cancer (UICC), which now prefers to be known by the English version of its title, Union for International Cancer Control (3), lung cancer being included under the section for “other sites”. This initial attempt at classification was arrived at by discussion and consensus, there being no data available. The American Joint Committee for Cancer Staging and End Results Reporting, now the American Joint Committee on Cancer (AJCC), orchestrated the collection of data through its Task Force on Lung Cancer. The analysis of a data base of 2,155 lung cancer cases resulted in “A system for the clinical staging of lung cancer” reported by Drs. Mountain, Carr and Anderson in 1973 (4). This formed the basis of the 2nd edition of the UICC TNM Classification of Malignant Disease published in 1975 (5) and the 1st edition of the AJCC Manual for Cancer Staging published in 1977 (6). Thereafter Dr. Mountain developed his own data base which informed future revisions up to and including the 5th edition published in 1997 (7,8), by which time the data base had accumulated 5,319 cases. There were no changes in the lung cancer classification in the 6th edition (9,10).
At a workshop sponsored by the International Association for the Study of Lung Cancer (IASLC) and held at the Brompton Hospital in London in 1996 Dr. Mountain presented his revisions for the 5th edition of TNM which had been approved by the UICC and AJCC and were due to come into force within a few weeks. The deficiencies of the underlying data were discussed: a relatively small number of cases, accumulated over 20 years, predominantly cases referred for surgical consideration and mostly derived from a single institution. The workshop attendees recommended “the establishment by the IASLC of a staging committee” to “represent the IASLC in negotiations with UICC and AJCC with regard to future revisions of classification” (11).
Achievements
With this introduction the achievements of the IASLC Staging and Prognostic Factors Committee can be put into perspective and enumerated.
In 1998, using the membership of the IASLC and pump-priming funding from the IASLC, a committee was established with members from all specialities involved in the treatment of lung cancer and from across the globe. The commitment of these early members was such that they largely self-funded their involvement for the first 2 years of the project (12).
High level support from the officers and head office of the IASLC secured long-term funding from philanthropic partners in the pharmaceutical industry. This provided administrative support for the committee and allowed us to contract with a not-for-profit data centre in Seattle, Cancer Research And Biostatistics (CRAB) with expertise in oncology and the collection and analysis of data from multi-centre, international studies.
Members of the lung cancer community supported this ambitious project by donating over 100,000 cases of lung cancer collected between 1990 and 2000. This data originated from 46 centres in over 20 countries around the globe and included cases treated by all modalities of care, including bi-modality and multi-modality regimens. Such a large data base allowed intensive internal and external validation, unprecedented in any previous revision (12).
As the proposals of each sub-committee were developed the data, analysis and proposals were published in the official journal of the IASLC, the Journal of Thoracic Oncology (JTO). These discussion articles were made available without subscription so that members and non-members were aware of the proposals and to enable an informed debate within national TNM committees. Once approved by the UICC and AJCC the IASLC produced site-specific educational materials (13) which were available at the 13th World Conference on Lung Cancer in September 2009. These contained precise figures illustrating each T, N and M descriptor. Never before had the global lung cancer community been so well informed of the pending changes for a new edition of TNM in lung cancer or been better prepared for its introduction.
The 7th edition of TNM for lung cancer was delivered, fully developed, on time and on budget to the UICC and AJCC. It complied with the requirements of both organisations with regard to process and timelines and was adopted in its entirety and without change. It came in to force on the 1st of January 2010 (14,15). The new edition retained the previous size cut-point of 3 cm separating T1 from T2 tumors. New size cut-points were introduced; 2 cm to separate T1a from T1b, 5 cm to separate T2a from T2b and 7 cm to separate T2b from T3 tumors. Size therefore became a T3 descriptor for the first time. There were changes to other T (16) and M categories (17). Cancers associated with additional tumor nodules (metastases) in the same lobe as the primary became T3, whilst those in other ipsilateral lobes became T4. Cancers associated with malignant pleural or pericardial effusions or nodules were moved from the T to an M category, linked with cases in which there were metastases in the opposite lung, as M1a. Distant haematogenous and nodal metastases became M1b. The N categories remained unchanged but for the first time these had been validated in an international data base of cases treated by all modalities of care (18). Some changes were also made in the resultant stage groupings (19). All of these changes were data driven and validated (20), aligning stage with prognosis more accurately than ever before. The use of TNM was shown to have prognostic value in the 13,000 cases of small-cell lung cancer (SCLC) within the database, in cases clinically staged (21) and the smaller number of surgically treated cases of SCLC in which pathological stage was available (22). Clinicians and pathologists were shown to have been correct in using the TNM classification for carcinoid tumors, although this was never previously sanctioned, and bronchopulmonary carcinoid tumors were included within the 7th edition of the TNM classification for the first time (23).
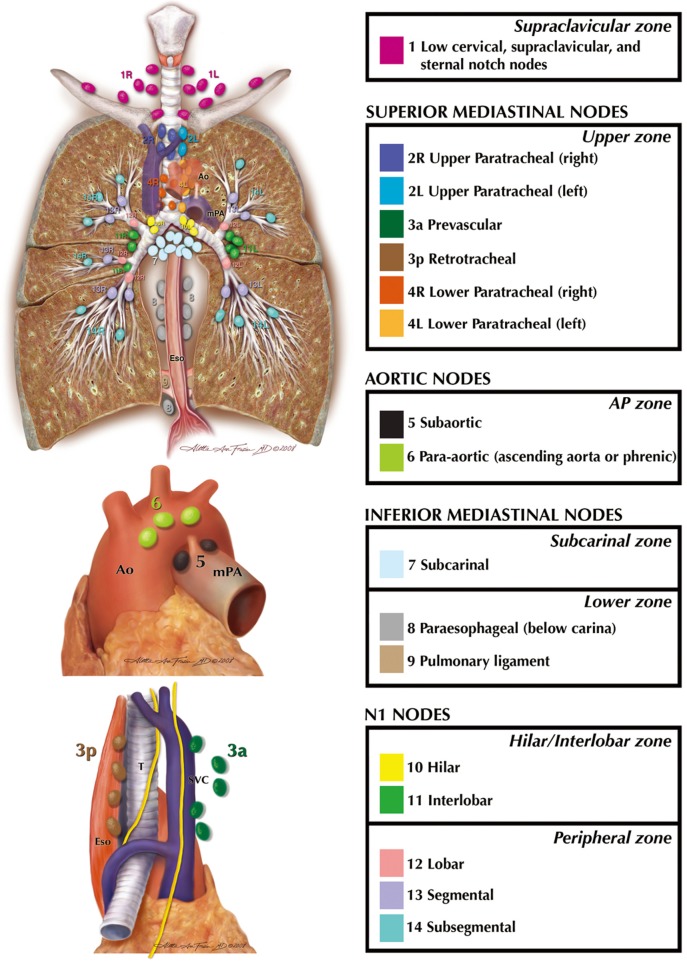
Additional issues, raised in discussions within the committee and in the literature review undertaken by the UICC, were addressed by consensus and, where available, by study of the available literature. The previous features used to distinguish pulmonary metastases from synchronous primary tumors were thought to have lagged behind developments in morphology, immunohistochemistry and molecular studies. The definition was therefore expanded and the role of the pathologist and these technological improvements were emphasised. An internationally agreed definition of “visceral pleural invasion” (VPI) was developed (24) adapting a system long in use by the Japan Lung Cancer Society (25) and also espoused by Hammar (26). The inconsistencies between the nodal map developed by Naruke and the Japan Lung Cancer Society (27) and that of Mountain and Dresler (28) were reconciled by international agreement and defined by precise anatomical boundaries (29). This led to the UICC and AJCC recognising the IASLC nodal map and its accompanying table of definitions as the recommended means to describe regional lymph node spread for lung cancer. It then became possible to re-introduce minimum requirements for lymph node evaluation at surgery and subsequent pathological examination as part of the expanded definition of a complete, R0, resection (30). The concept of nodal “zones” was developed, covering larger anatomical areas than individual “stations” in the hope that this would assist oncologists, treating patients with bulky nodal disease which could encompass more than one station, and widen the relevance of nodal mapping beyond mere surgeons. A version of the IASLC nodal map is shown in Figure 1 and the table of definitions in Table 1.
Figure 1.
The IASLC Nodal Map, reconciling the discrepancies between the Mountain/Dressler and Naruke maps. Each nodal station is colour-coded and listed. Those within a distinct nodal “zone” are grouped within the box. Reprinted courtesy of the International Association for the Study of Lung Cancer and with permission of Aletta Frazier, MD. Copyright ©2009, 2010 Aletta Ann Frazier, MD.
Table 1. The table of definitions for the nodal stations in the IASLC nodal map.
| Nodal station | Description | Definition |
|---|---|---|
| #1 (left/right) | Low cervical, supraclavicular and sternal notch nodes | Upper border: lower margin of cricoid cartilage |
| Lower border: clavicles bilaterally and, in the midline, the upper border of the manubrium, 1R designates right-sided nodes, 1L, left-sided nodes in this region | ||
| #L1 and #R1 limited by the midline of the trachea | ||
| #2 (left/right) | Upper paratracheal nodes | 2R: Upper border, apex of the right lung and pleural space and, in the midline, the upper border of the manubrium; Lower border, intersection of caudal margin of innominate vein with the trachea |
| 2L: Upper border, apex of the left lung and pleural space and, in the midline, the upper border of the manubrium; Lower border, superior border of the aortic arch | ||
| As for #4, in #2 the oncologic midline is along the left lateral border of the trachea | ||
| #3 | Pre-vascular and retrotracheal nodes | 3a: prevascular |
| On the right | ||
| Upper border: apex of chest | ||
| Lower border: level of carina | ||
| Anterior border: posterior aspect of sternum | ||
| Posterior border: anterior border of superior vena cava | ||
| On the left | ||
| Upper border: apex of chest | ||
| Lower border: level of carina | ||
| Anterior border: posterior aspect of sternum | ||
| Posterior border: left carotid artery | ||
| 3p: retrotracheal | ||
| Upper border: apex of chest | ||
| Lower border: carina | ||
| #4 (left/right) | Lower paratracheal nodes | 4R: includes right paratracheal nodes, and pretracheal nodes extending to the left lateral border of trachea |
| Upper border: intersection of caudal margin of innominate vein with the trachea | ||
| Lower border: lower border of azygos vein | ||
| 4L: includes nodes to the left of the left lateral border of the trachea, medial to the ligamentum arteriosum | ||
| Upper border: upper margin of the aortic arch | ||
| Lower border: upper rim of the left main pulmonary artery | ||
| #5 | Subaortic (aorto-pulmonary window) | Subaortic lymph nodes lateral to the ligamentum arteriosum |
| Upper border: the lower border of the aortic arch | ||
| Lower border: upper rim of the left main pulmonary artery | ||
| #6 | Para-aortic nodes (ascending aorta or phrenic) | Lymph nodes anterior and lateral to the ascending aorta and aortic arch |
| Upper border: a line tangential to the upper border of the aortic arch | ||
| Lower border: the lower border of the aortic arch | ||
| #7 | Subcarinal nodes | Upper border: the carina of the trachea |
| Lower border: the upper border of the lower lobe bronchus on the left; the lower border of the bronchus intermedius on the right | ||
| #8 (left/right) | Para-esophageal nodes (below carina) | Nodes lying adjacent to the wall of the esophagus and to the right or left of the midline, excluding subcarinal nodes |
| Upper border: the upper border of the lower lobe bronchus on the left; the lower border of the bronchus intermedius on the right | ||
| Lower border: the diaphragm | ||
| #9 (left/right) | Pulmonary ligament nodes | Nodes lying within the pulmonary ligament |
| Upper border: the inferior pulmonary vein | ||
| Lower border: the diaphragm | ||
| #10 (left/right) | Hilar nodes | Includes nodes immediately adjacent to the mainstem bronchus and hilar vessels including the proximal portions of the pulmonary veins and main pulmonary artery |
| Upper border: the lower rim of the azygos vein on the right; upper rim of the pulmonary artery on the left | ||
| Lower border: interlobar region bilaterally | ||
| #11 | Interlobar nodes | Between the origin of the lobar bronchi |
| #11s: between the upper lobe bronchus and bronchus intermedius on the right | ||
| #11i: between the middle and lower lobe bronchi on the right | ||
| Optional sub-categories | ||
| #12 | Lobar nodes | Adjacent to the lobar bronchi |
| #13 | Segmental nodes | Adjacent to the segmental bronchi |
| #14 | Sub-segmental nodes | Adjacent to the subsegmental bronchi |
Reprinted with permission of the International Association for the Study of Lung Cancer. Copyright ©2009, 2010 IASLC
So much for the achievements. What “hurdles” were encountered and which are left for the next phase of the IASLC TNM and Prognostic Factors Committee?
Hurdles
During the evolution of the IASLC proposals for the revision of the 6th edition we were aware of some limitations of our database and had to make difficult decisions. The solutions we settled upon and the retrospective nature of the data base have created some issues for those now using the 7th edition of the TNM Classification for Lung Cancer.
A dilemma was encountered as we sought to accommodate sub-groups of T or M cases that had been identified to have a prognosis that differed significantly from the rest of the cases within that category. If we kept the group within the original category and identified it by new alphabetical subscripts retrospective compatibility would be feasible and cases within existing data bases could be translated from the TNM version by which they were originally classified to the new edition of TNM. This had been managed with all previous revisions. Unfortunately it soon became apparent that the number of sub-categories required to accommodate all of the changes would exceed 20 and the number of resultant stage groupings would be in the region of 180 (19). This was clearly impractical with the technologies available globally at that time. For this reason it was reluctantly decided to move these sub-groups to other T and M categories which shared a similar prognosis, keeping the numbers of categories manageable but sacrificing backward compatibility for existing data bases, including our data donors!
A major limitation of our data was its retrospective nature. We chose to accept the short-coming of such data as the only way we could collect sufficient cases to inform revisions of the classification within the timelines dictated by the UICC and AJCC. However, the limitations of retrospective data collection brought with it several frustrations. Whilst in all cases we knew which category of T, N and M formed the basis of the clinical or pathological stage grouping, in only a minority did we know the descriptor which resulted in the case being assigned to that category and in few did we know that all other descriptors in that category were absent. For example, we would know that the case was assigned to T2, but only in a minority would we be told that this was because the tumor was 3 cm or larger. If this was so, we were rarely given information about the presence or absence of VPI or the proximity of the tumor to established anatomical levels on bronchoscopy. In addition we were not always told how was the size was measured, whether on chest radiography or CT, and in which dimension, a single reading or more, and in which axis? Similarly with no international guidance as to a definition of VPI we were unsure how this was assessed by individual pathologists at that time, and whether an elastic stain had been utilized to clarify this involvement? We had to accept that such cases were recorded in the data base as “VPI present”. In accepting these limitations we should at least recognize that the same issues almost certainly applied to the data that informed all previous revisions. Although the 7th edition placed added emphasis on size, by including additional size cut-points and making size >7 cm a T3 descriptor, the problem of how best to measure size was just as pertinent for the 3 cm cut-point which had divided T1 and T2 tumors since the mid 1970s (4). In the prospective data set established by the IASLC Staging and Prognostic Factors Committee (31) to help inform future revisions of TNM we are collecting data on the largest dimension and the imaging modality used to measure size. In addition we ask for the status of all descriptors within each T and M category. Such data will allow us to investigate issues such as the interaction of VPI and size on prognosis.
A new category, T1a, has been created for very small tumors, those no larger than 2 cm. The prognosis of these cases when associated with N0 disease is statistically more favorable than tumors greater than 2 cm but no larger than 3 cm, T1b. Does this have implications for structured observation in Low-Dose CT (LDCT) screening programmes? Does it suggest that sub-lobar resection may be sufficient for such cases? There are many shortcomings in such assumptions. The proportion of such cases in our data base which were screen-detected is unknown, but this will be clarified within the prospective data base. The issue regarding sub-lobar resection has become more topical with the increasing use of LDCT screening. Many of the cancers discovered with such screening are adenocarcinomas often with a proportion of ground-glass opacity (GGO). The classification of such lesions has been clarified in the new IASLC/ATS/ERS Classification for adenocarcinoma (32). The present role of sub-lobar resection has been summarized in a consensus report from the IASLC Strategic Screening Advisory Committee (33), which clearly favors anatomical segmentectomy over wedge resection and sets out the specific situation in which this is appropriate as an alternative to lobectomy, with carefully crafted caveats.
“It is recommended that anatomical segmentectomy be reserved for the CT screening detected pure GGO lesions or part-solid lesions below 2 cm located in the peripheral third of the lung, after frozen section of N1 and N2 lymph nodes have confirmed the T1aN0M0 status. In addition frozen section or cytological evaluation of resection margins is recommended.”
There are 2 ongoing trials assessing the role of sub-lobar resection in small peripheral cancers, one in the United States, CALGB 140503, and another in Japan, JCOG 0802. These should provide definitive advice when the results become available (34).
In some situations we have moved descriptors between T and M categories which may result in a case being assigned to a different stage grouping from that of earlier editions of TNM. One such area concerned the classification of cancers associated with “additional tumor nodules” in the lobe of the primary, moved from T4 to T3 in the 7th edition, and in other ipsilateral lobes, moved from M1 to T4. Could we be sure that such changes were appropriate for all such cases? The answer is almost certainly not, but does it only apply to cases with a single additional nodule, or those with several or many? Does it only apply to so called “satellite” nodules adjacent to the primary tumor? Can we be sure that none of these cases in our data base were actually synchronous primary tumors? Clarification of these questions will have to await an analysis of from prospective data bases such as the one the IASLC has established. However we have now clarified that “additional tumor nodules” are “pulmonary metastases”, and improved the definition of “synchronous primary tumors”. If the management of a cases hinges on the distinction between additional nodules being metastases or synchronous primary tumors then biopsy of more than just the main lesion may be necessary.
Stage does not dictate treatment, it is only one factor in this decision, acting to “aid the clinician in the planning of treatment” (14). Inevitably however when stage grouping change there is an understandable question as to whether this should influence treatment algorithms. Several such changes occurred in the 7th edition:
(I) Tumors larger than 5 cm have been re-classified as T2b, and those >7 cm as T3. These cases, when associated with the N0 category were previously stage IB but are now upstaged to IIA and IIB respectively. Clinical trials have now established that adjuvant chemotherapy is beneficial after complete resection of stage II cases (35). Should these “new” stage II cases, large tumors which are N0, be treated along these lines? We should remind ourselves that these trials were conducted on stage II cases associated with N1 disease and must await the results of appropriate trials addressing the issue of adjuvant chemotherapy in large, node negative tumors, stratifying by size using the 7th edition cut-points (36);
(II) The classification of T4 tumors associated with invasion of adjacent structures has not changed but the stage grouping assigned to such cases when T4 is associated with the N0 and N1 categories has been down-staged to stage IIIA. Should all such cases now be considered for multimodality regimens which include surgery? One has to be cautious about such sweeping statements. Most surgical series of “resectable” T4 cases have been small, with highly selected cases collected over decades. Many such cases did not go to theatre with a classification of T4 but were thought to be less extensive and only found to be “T4”, “resectable” and node negative at surgery. The pre-operative assessment of “resectability” is always difficult, especially after induction chemotherapy and even more so after induction chemo-radiotherapy. It is also a very personal decision and one which cannot easily be conveyed by objective criteria. Until more data is accrued one can only advise that such advanced cases be assessed at specialist centres with experience in making these difficult decisions;
(III) The new descriptors appropriate for the classification of cases with additional tumor nodules in the lobe of the primary, and other ipsilateral lobes have already been alluded to and the reservations concerning this assignment mentioned. However, these changes have also resulted in down-staging in some circumstances. Those cases with additional tumor nodules in the lobe of the primary, classified as T3, when associated with the N0 category have been down-staged to stage IIB. One would expect that these cases would indeed be treated by surgery in patients who are sufficiently fit to withstand lobectomy as the additional lesions do not extend the extent of resection and subsequent pathological classification may show one (or more) to be synchronous primaries. The role of adjuvant chemotherapy will arise but at present there is no data to inform this decision. Such T3 cases associated with N1 and N2 categories, and the T4 lesions due to additional tumor nodules in other ipsilateral lobes associated with N0 and N1 categories have been down-staged to stage IIIA. Once again this stage has traditionally been the middle ground where most discussion at multi-disciplinary meetings is concentrated. One can only suggest that these cases now be subjected to the same deliberations and that treatment options include a discussion of surgery as part of the multi-modality care in appropriate cases. It is unlikely that trials will prove feasible in these cases and once again data from prospectively collected data with comprehensive data sets may help these decisions in future.
Conclusions
The 7th edition of TNM for lung cancer was an enormous improvement when compared to earlier editions. The process of revision has been dramatically altered and colleagues with data from around the globe have been able to influence the classification we all use in everyday practice. The new edition is based upon international data, on patients treated by all modalities of care and accrued over a relatively short period, during which time treatment and investigative algorithms were relatively stable. Stage has been aligned with prognosis more closely than ever before. However, as outlined in this article, it is far from perfect. We responded to criticisms of earlier revisions and have taken a giant step forwards with the 7th edition. The IASLC is now committed, on behalf of its members and the global lung cancer community, to the long-term financial and scientific burden of improving future revisions and expanding our activities to cover other thoracic malignancies, including mesothelioma and thymic tumors. The 8th edition of the TNM Classification of Malignant Tumors is scheduled to be enacted in September 2015. The IASLC Staging and Prognostic Factors Committee is well advanced in its preparation and has accumulated an even larger data base than that previously used for the 7th edition. Once our proposals have been identified and validated they will again be released for scrutiny in discussion articles in JTO. Readers are encouraged to become members of the IASLC to assist in this endeavor, ensure they are kept abreast of impending changes and be in a position to obtain the educational materials the IASLC plans to launch at the 16th World Conference on Lung Cancer in Denver, September 2015.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- 1.Goldstraw P. History of TNM in lung cancer. In: Goldstraw P. eds. IASLC staging manual in thoracic oncology (1st Edition). Florida: Editorial Rx, 2009:16-29. [Google Scholar]
- 2.Denoix PF. The TNM staging system. Bull Inst Nat Hyg 1952;7:743. [Google Scholar]
- 3.UICC. TNM classification of malignant tumours 1st, Geneva, 1968. [Google Scholar]
- 4.Mountain CF, Carr DT, Anderson WA. A system for the clinical staging of lung cancer. Am J Roentgenol Radium Ther Nucl Med 1974;120:130-8. [DOI] [PubMed] [Google Scholar]
- 5.UICC. TNM classification of malignant tumours 2nd, Geneva, 1975. [Google Scholar]
- 6.American Joint Committee on cancer staging and end results reporting. AJCC cancer staging manual (1st Edition). Philadelphia: Lippincott-Raven, 1977. [Google Scholar]
- 7.UICC. eds. TNM classification of malignant tumours (5th Edition). Berlin: Springer Verlag, 1997. [Google Scholar]
- 8.American Joint Committee on Cancer. AJCC Cancer Staging Manual. In: Fleming ID, Cooper JS, Henson DE, et al. eds. 5th. Philadelphia: Lipincott Raven, 1997. [Google Scholar]
- 9.American Joint Committee on Cancer. eds. AJCC cancer staging manual (6th Edition). New York: Springer, 2002. [Google Scholar]
- 10.UICC International Union Against Cancer. eds. TNM classification of malignant tumours (6th Edition). New York: Wiley-Liss, 2002. [Google Scholar]
- 11.Goldstraw P.Report on the international workshop on intrathoracic staging. London, October 1996. Lung Cancer 1997;18:107-11. [Google Scholar]
- 12.Goldstraw P, Crowley JJ, IASLC International Staging Project The international association for the study of lung cancer international staging project on lung cancer. J Thorac Oncol 2006;1:281-6. [Google Scholar]
- 13.Goldstraw P. eds. IASLC staging handbook in thoracic oncology (1st Edition). Florida: Editorial Rx Press, 2009. [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C. eds. UICC TNM classification of malignant tumours (7th Edition). New York: Wiley-Liss, 2009. [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, et al. eds. AJCC cancer staging manual (7th Edition). New York: Springer, 2009. [Google Scholar]
- 16.Rami-Porta R, Ball D, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593-602. [DOI] [PubMed] [Google Scholar]
- 17.Postmus PE, Brambilla E, Chansky K, et al. The IASLC lung cancer staging project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol 2007;2:686-93. [DOI] [PubMed] [Google Scholar]
- 18.Rusch VW, Crowley J, Giroux DJ, et al. The IASLC lung cancer staging project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12. [DOI] [PubMed] [Google Scholar]
- 19.Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [DOI] [PubMed] [Google Scholar]
- 20.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC lung cancer staging project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694-705. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [DOI] [PubMed] [Google Scholar]
- 22.Vallières E, Shepherd FA, Crowley J, et al. The IASLC lung cancer staging project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [DOI] [PubMed] [Google Scholar]
- 23.Travis WD, Giroux DJ, Chansky K, et al. The IASLC lung cancer staging project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1213-23. [DOI] [PubMed] [Google Scholar]
- 24.Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1384-90. [DOI] [PubMed] [Google Scholar]
- 25.The Japan Lung Cancer Society. eds. Classification of lung cancer: first English edition. Chiba: Kanehara and Co, 2000. [Google Scholar]
- 26.Hammar SP. Common tumors. In: Dail DH, Hammar SP. eds. Pulmonary pathology (2nd Edition). New York: Springer-Verlag, 1994:1138. [Google Scholar]
- 27.Naruke T, Suemasu K, Ishikawa S.Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg 1978;76:832-9. [PubMed] [Google Scholar]
- 28.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [DOI] [PubMed] [Google Scholar]
- 29.Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77. [DOI] [PubMed] [Google Scholar]
- 30.Union for International Cancer Control (UICC). eds. TNM supplement: a commentary on uniform use (4th Edition). Oxford: Wiley Blackwell, 2012. [Google Scholar]
- 31.Giroux DJ, Rami-Porta R, Chansky K, et al. The IASLC lung cancer staging project: data elements for the prospective project. J Thorac Oncol 2009;4:679-83. [DOI] [PubMed] [Google Scholar]
- 32.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Field JK, Smith RA, Aberle DR, et al. International association for the study of lung cancer computed tomography screening workshop 2011 report. J Thorac Oncol 2012;7:10-9. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [DOI] [PubMed] [Google Scholar]
- 35.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [DOI] [PubMed] [Google Scholar]
- 36.Cuffe S, Bourredjem A, Graziano S, et al. A pooled exploratory analysis of the effect of tumor size and KRAS mutations on survival benefit from adjuvant platinum-based chemotherapy in node-negative non-small cell lung cancer. J Thorac Oncol 2012;7:963-72. [DOI] [PMC free article] [PubMed] [Google Scholar]