Abstract
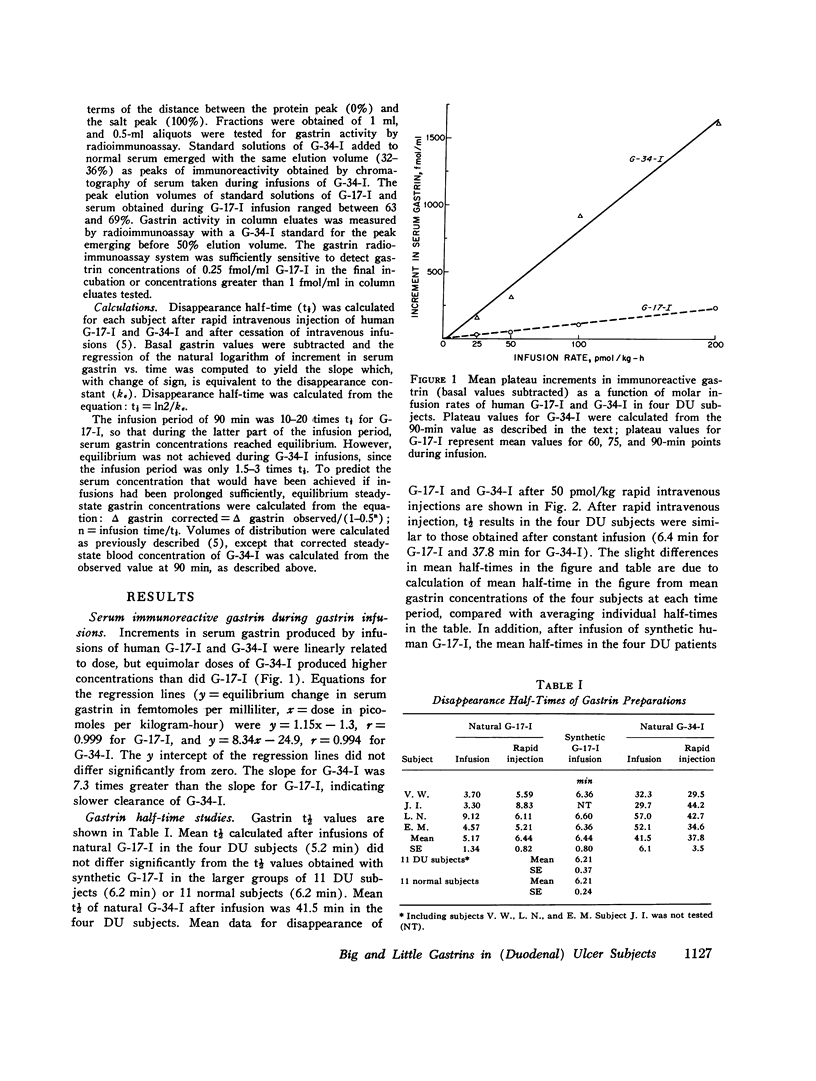
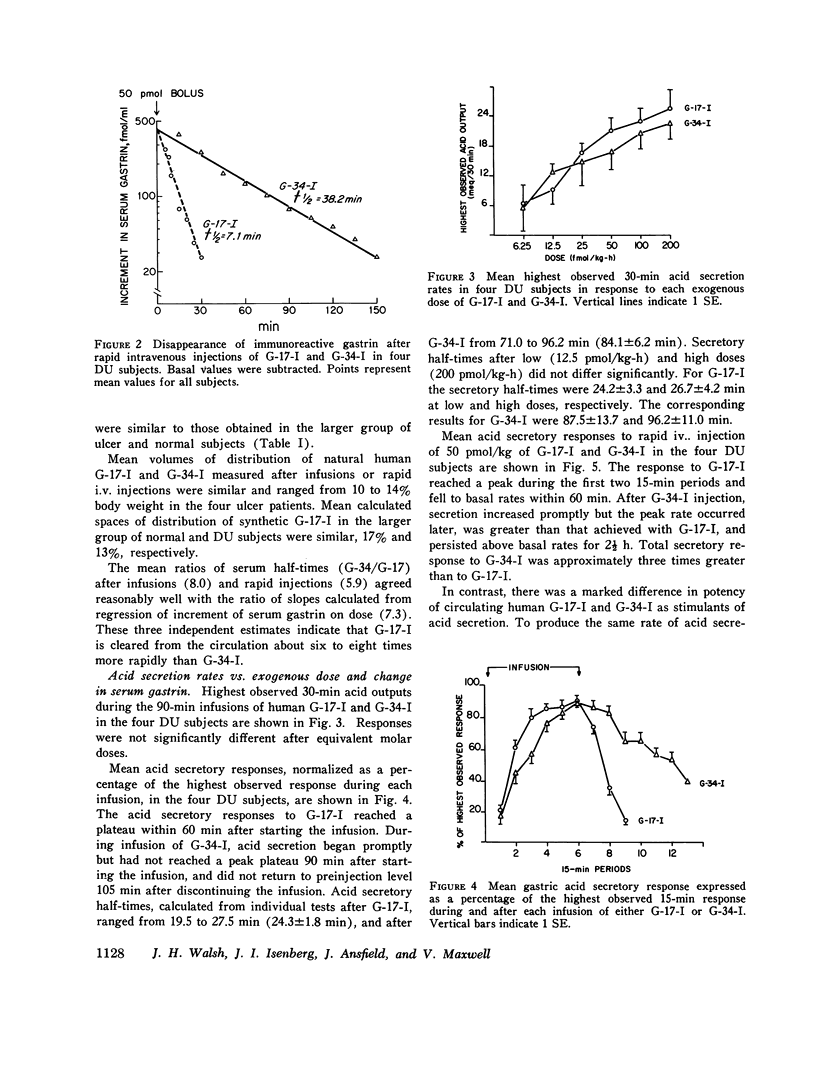
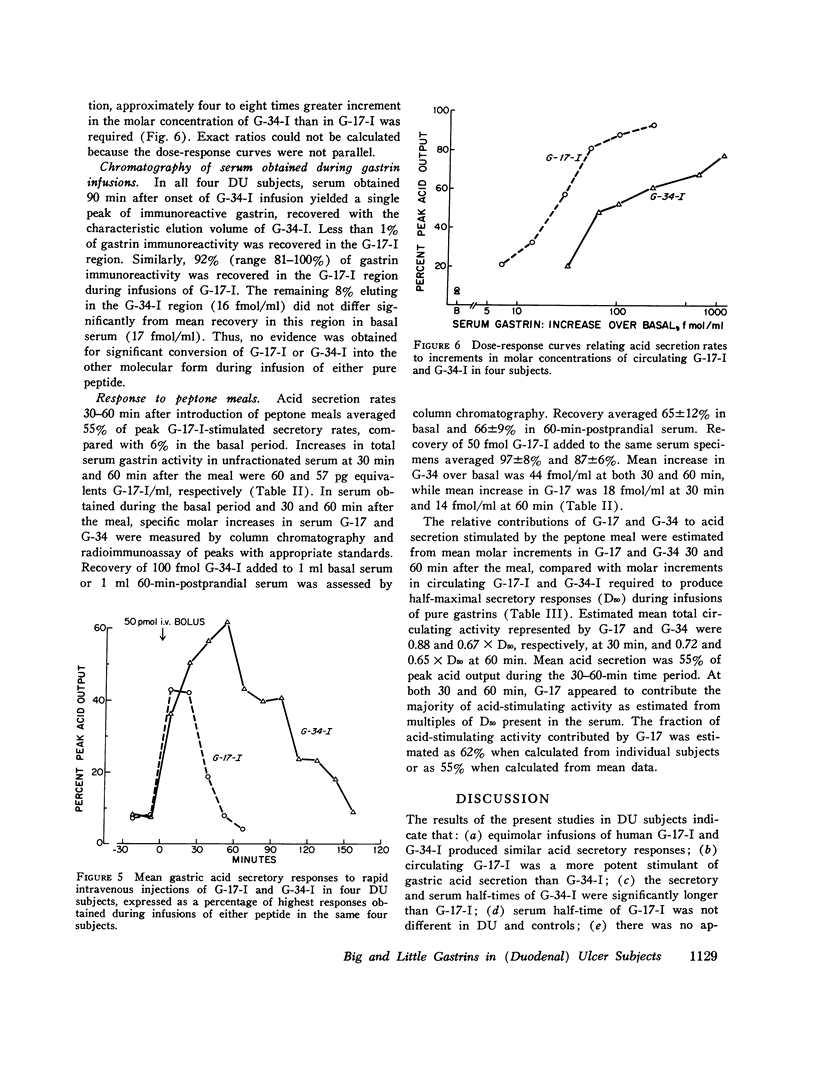
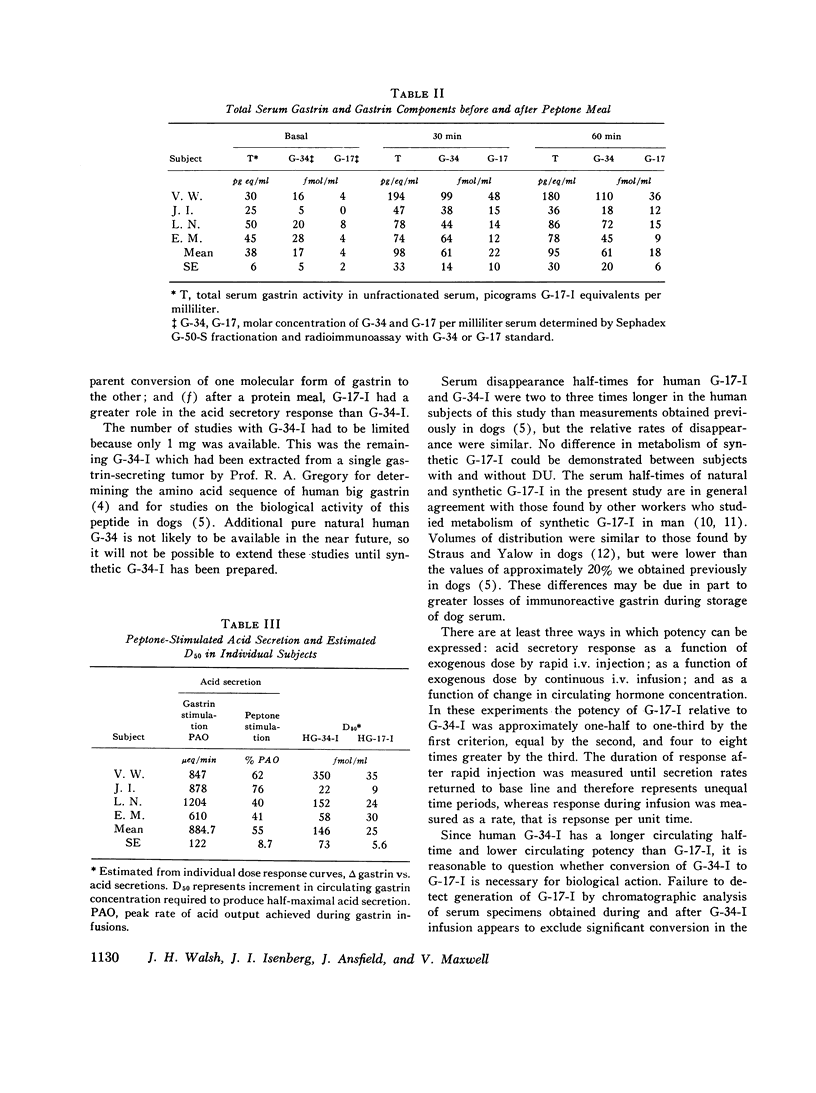
Acid-stimulating action and clearance of pure natural human big gastriin (HG-34-I) and little gastrin (HG-17-I) were assessed in four male subjects with inactive duodenal ulcer (DU) disease. Disappearance half-times for HG-17-I after intravenous infusion (5.2 min) or rapid intravenous injection (6.4 min) were six to eight times shorter than those for HG-34-I (41.5 and 37.8 min, respectively). Studies of clearance of synthetic human little gastrin (HG-17-I) were performed in three of these same four DU subjects, eight additional male DU subjects, and eleven normal male subjects. The disappearance halftime of synthetic HG-17-I averaged 6.2 min in both the DU subjects and the normal subjects. These data suggest that clearance of exogenous gastrin is not altered in patients with DU. Acid secretion in response to rapid intravenous injection of HG-34-I reached a higher peak and lasted longer than in response to an equimolar dose of HG-17-I; the total response to HG-34-I was about three times that to HG-17-I. During constant intravenous infusion, acid responses to equimolar exogenous doses of the two peptides were similar but the increment in molar concentration of circulating gastrin was six to eight times greater with HG-34-I than with HG-17-I. Chromatography of serum obtained during infusions of HG-34-I revealed no evidence of conversion to HG-17-I, nor was there any increase in circulating G-34 activity during infusions of HG-17-I. The increment in serum gastrin concentration required to produce half-maximal stimulation of gastric acid secretion (D50) was estimated in each subject for each gastrin from curves relating acid secretion to change in serum gastrin concentration produced by infusion of these peptides. After instilling peptone solution into the stomach, acid secretion was measured by intragastric titration, and increases in circulating G-17 and G-34 were determined by chromatography and radioimmunoassay of serum. Increases in circulating G-17 and G-34 in response to the peptone meal, taken together, were equivalent to 1.5 times the D50 determined from infusions of G-34 and G-17. Acid secretion during the same time period averaged 55% of maximal rates. Although G-34 comprised approximately three-fourths of the total molar concentration of circulating gastrin after stimulation, it was estimated to contribute less than half of the acid-stimulating activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dockray G. J., Walsh J. H. Amino terminal gastrin fragment in serum of Zollinger-Ellison syndrome patients. Gastroenterology. 1975 Feb;68(2):222–230. [PubMed] [Google Scholar]
- Fordtran J. S., Walsh J. H. Gastric acid secretion rate and buffer content of the stomach after eating. Results in normal subjects and in patients with duodenal ulcer. J Clin Invest. 1973 Mar;52(3):645–657. doi: 10.1172/JCI107226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli P. C., Elder J. B., Smith I. S., Hunter W. M., Gillespie I. E. The half life (T and one-half) of synthetic human gastrin I in man. Br J Surg. 1970 Nov;57(11):848–848. [PubMed] [Google Scholar]
- Gregory R. A. The Bayliss-Starling lecture 1973. The gastrointestinal hormones: a review of recent advances. J Physiol. 1974 Aug;241(1):1–32. doi: 10.1113/jphysiol.1974.sp010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg J. I., Grossman M. I., Maxwell V., Walsh J. H. Increased sensitivity to stimulation of acid secretion by pentagastrin in duodenal ulcer. J Clin Invest. 1975 Feb;55(2):330–337. doi: 10.1172/JCI107936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardi M., Maxwell V., Sturdevant R. A., Isenberg J. I. Metiamide, an H2-receptor blocker, as inhibitor of basal and meal-stimulated gastric acid secretion in patients with duodenal ulcer. N Engl J Med. 1974 Aug 22;291(8):373–376. doi: 10.1056/NEJM197408222910801. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F., Vikelsoe J. Immunoreactive gastrin components in human serum. Gut. 1974 Feb;15(2):102–111. doi: 10.1136/gut.15.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrumpf E., Semb L. S., Vold H. Metabolic clearance and disappearance rates of synthetic human gastrin in man. Scand J Gastroenterol. 1973;8(8):731–734. [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Studies on the distribution and degradation of heptadecapeptide, big, and big big gastrin. Gastroenterology. 1974 May;66(5):936–943. [PubMed] [Google Scholar]
- Walsh J. H., Debas H. T., Grossman M. I. Pure human big gastrin. Immunochemical properties, disappearance half time, and acid-stimulating action in dogs. J Clin Invest. 1974 Aug;54(2):477–485. doi: 10.1172/JCI107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Further studies on the nature of immunoreactive gastrin in human plasma. Gastroenterology. 1971 Feb;60(2):203–214. [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size and charge distinctions between endogenous human plasma gastrin in peripheral blood and heptadecapeptide gastrins. Gastroenterology. 1970 May;58(5):609–615. [PubMed] [Google Scholar]
- Yalow R. S., Wu N. Additional studies on the nature of big big gastrin. Gastroenterology. 1973 Jul;65(1):19–27. [PubMed] [Google Scholar]



