Abstract
Epidermal growth factor receptor (EGFR) mutations occur in 17% of non-small-cell lung cancer (NSCLC) patients with notable response to single agent therapy but with low complete remission rate and, eventually, disease progression. Priming BIM, a pro-apoptotic signaling BH3-only protein, induces sensitivity to erlotinib in EGFR-mutant cell lines. Synthetic lethal approaches and preemptive therapies based on the initial expression of BIM may significantly improve the treatment outcome. EGFR mutations result in transient pro-death imbalance of survival and apoptotic signaling in response to EGFR inhibition. SHP2 is essential to the balance between ERK and the phosphoinositide-3-kinase (PI3K)/AKT and signal transducer activator of transcription (STAT) activity, while mTOR can be an additional marker for patients with high BIM expression. Furthermore, stromal hepatocyte growth factor (HGF) confers EGFR tyrosine kinase inhibitor (TKI) resistance and induces interreceptor crosstalk with integrin-b4, Eph2, CUB domain-containing protein-1 (CDCP1), AXL and JAK1. Only by understanding better, and in more depth, complex cancer molecular biology will we have the information that will help us to design strategies to augment efficacy of EGFR TKIs and offer our patients the best, most correct therapeutic option.
Keywords: Lung cancer, epidermal growth factor receptor (EGFR) mutations, biomarkers, signaling pathways
Introduction
A recent meta-analysis of patients with non-small-cell lung cancer (NSCLC) and epidermal growth factor receptor (EGFR) activating mutations showed that first-generation EGFR tyrosine kinase inhibitors (TKIs) significantly delayed disease progression but had no effect on overall survival (1). Erlotinib, gefitinib and the second-generation, irreversible EGFR TKI afatinib have offered patients with metastatic EGFR positive lung cancer a therapeutic alternative that has proven its superiority over standard platinum-based chemotherapy (2-4). However, primary or acquired resistance limits the therapeutic success of these targeted agents (2). The expression levels of the proapoptotic protein BIM have been found to predict responsiveness to kinase inhibitors in treatment-naïve cancer patients, confirming that this molecule is implicated in modulation of cancer cell dependence on EGFR and other oncogenic models (5,6). The levels of all three major splicing isoforms, BIM extra-long (BIM-EL), BIM long and BIM short, are induced after erlotinib treatment in drug-sensitive PC-9 cells, but not in drug-resistant H1650 [that lacks expression of the phosphatase and tensin homolog (PTEN) protein] and in H1975 cells (that harbor the ‘gatekeeper’ mutation T790M-EGFR). EGFR signaling influences BIM expression and phosphorylation status mainly via the ERK pathway, and erlotinib appears to induce significant dephosphorylation of BIM-EL which results in an increase in its proapoptotic function (7,8). However, pretreatment BIM expression levels may not be enough to predict outcome to EGFR TKIs. The two primary signaling pathways activated by EGFR are the mitogen-activated protein kinase (MAPK) and the phosphoinositide-3-kinase (PI3K) axes. Src tyrosine kinases, activation of the signal transducer activator of transcription 3 (STAT3) pathway and downstream signaling have also been well documented (9). EGFR phosphorylation leads to recruitment of multiple effector proteins through recognition and binding of Src-homology 2 domain-containing phosphatase 2 (SHP2) to phosphotyrosine motifs on the receptor (9). SHP2 (encoded by PTPN11), is a ubiquitously expressed SH2 domain-containing protein tyrosine phosphatase (PTP). Despite its direct function in protein dephosphorylation, SHP2 plays an overall positive role in transducing signals initiated from growth factors/cytokines and extracellular matrix proteins and in initiating various downstream signaling cascades, including PI3K, MAPK and STAT3 (9,10).
In this short review we will try to demonstrate, by reviewing the current literature, that “first-line EGFR TKIs monotherapy for patients with mutant EGFR NSCLC is incomplete” and EGFR inhibitors, reversible or irreversible, are unlikely to provide cures in the majority of patients.
BIM expression in treatment naïve cancers predicts responsiveness to EGFR TKIs, but almost 2/3 of patients have low BIM mRNA levels at baseline
We were able to examine BIM mRNA levels in pretreatment tumour samples from 83 patients included in the EURTAC trial (2,5). BIM expression was low or intermediate in 53 (63.96%) and high in 30 (36.14%) patients. PFS to erlotinib was 12.9 months for those with high, and 7.2 months for those with low/intermediate, BIM expression levels, while among chemotherapy-treated patients, it was 5.8 and 5.5 months, respectively (P=0.0003) (5). Overall survival was 28.6 months for patients with high BIM expression and 22.1 months for those with low/intermediate BIM expression (P=0.0364). Multivariate analyses showed that erlotinib was a marker of longer PFS [hazard ratio (HR) =0.35; P=0.0003], while high BIM expression was a marker of longer PFS (HR =0.49; P=0.0122) and overall survival (HR =0.53; P=0.0323) (5). SHP2 plays a fundamental role in NSCLC cells harboring EGFR mutations (11,12). SHP2 is required for the full activation of the MAPK/ERK pathway and its catalytic activity regulates the PI3K/AKT pathway resulting in the positive effect of SHP2 on cell survival (9,12-14). Cragg and colleagues have reported that concurrent treatment of H3255, HCC827, or H1650 cells with gefitinib and a MEK inhibitor does not result in substantially enhanced apoptosis (15). In contrast, SHP2 knockdown reduces ERK phosphorylation and increases cellular sensitivity to gefitinib in cells expressing EGFR mutants, but also in cells expressing wild-type EGFR (11). Activation of receptor tyrosine kinases, including EGFR, results in SHP2 phosphorylation at Y542, which is required for normal SHP2-mediated ERK activation in response to many growth factors (11). Surprisingly, the EGFR L858R mutation leads to decreased ability to activate ERK compared to wild-type EGFR, which correlates with decreased EGFR internalization and reduced phosphorylation of SHP2 and sensitivity to gefitinib (16). Lazzara and colleagues were able to demonstrate that SHP2 Y542 phosphorylation was induced in the EGFR wild type H1666 cells (that carry an uncommon BRAF mutation, G465V) in response to EGF, but not in the H3255 cells which harbor the missense L858R exon 21 mutation, suggesting that SHP2 activity may be less efficiently promoted by EGFR L858R (16). The reduced SHP2 phosphorylation and full ERK activation may partially correlate with decreased EGFR internalization, given that activating mutations of EGFR are endocytosis-impaired (16). However, the mutant-bearing (del19) PTEN-null cell line H1650 did exhibit inducible SHP2 Y542 phosphorylation (16). Therefore, further studies are needed to define the mechanism underlying differential SHP2 involvement beyond the apparent link to receptor internalization.
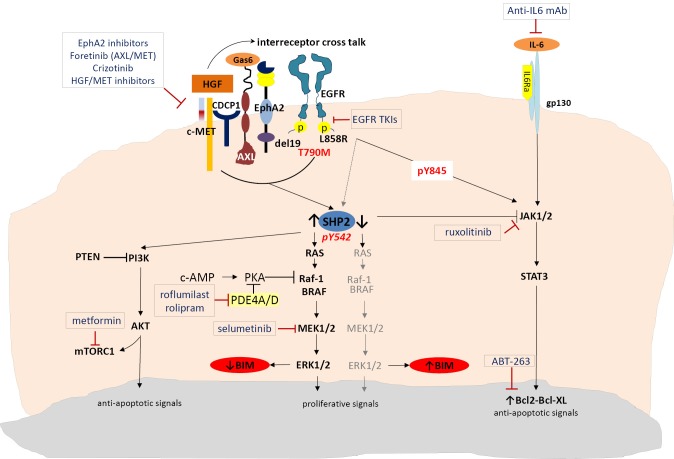
SHP2 is also required for sustained activation of ERK and epithelial morphogenesis downstream from the MET receptor tyrosine kinase (17,18). Several MET inhibitors have been tested so far that can be classified according to their mechanism of action in selective MET inhibitors (tivantinib, EMD 1204831, SGX523, INCB0280), unselective MET inhibitors (crizotinib, cabozantinib, foretinib, golvatinib, MGC D265 and MK-2461) and antibodies targeting MET (onartuzumab) or hepatocyte growth factor (HGF) (ficlatuzumab, rilotumumab or TAK-701) (19,20). Upon activation of MET by its ligand, HGF, which is provided by stromal cells, EGFR signaling is dramatically altered (21). HGF anticipates the mode of action in EGFR mutant tumours, as EGFR tyrosine kinase activity, as well as the classical downstream signaling, is no longer required for tumour growth (21). Specifically, HGF confers EGFR TKI resistance by inducing two novel cancer-promoting functions: first, it abolishes classical EGFR signaling, which makes cancer cells independent of these signaling mechanisms and neutralizes the point of action for EGFR TK-targeted drugs. Second, it enables the EGFR to interact with proteins, which are known to be markers of a highly metastatic phenotype like the CUB domain-containing protein-1 (CDCP1), EphA2 and AXL, interactions that cannot be affected by EGFR TKI treatment (Figure 1) (21). Thus, treatment with HGF/MET inhibitors together with EGFR-targeted therapies, as well as targeting HGF/MET-induced EGFR interactors may both be necessary for elimination of tumour growth (21). At the same time, Gas6/AXL-mediated stimulation of ERK is attributed, in part, to its ability to activate SHP2 (18). Foretinib is an oral multikinase inhibitor targeting MET, RON, AXL, and VEGFR, while YW327.6S2 is the first reported fully humanized AXL blocking antibody that blocks AXL functions by downregulating its expression as well as inhibiting the ligand Gas6 (22,23).
Figure 1.
Mechanisms for BIM regulation.
EphA2 is a member of the erythropoietin-producing hepatocellular (Eph) family of receptor tyrosine kinases. Unlike traditional oncogenes that often function only in tumour cells, EphA2 mediates cell-cell interactions both in tumour cells and in the tumour microenvironment, namely the tumor stroma and tumor vasculature. EphA2 is often overexpressed in a variety of malignant cancers, including breast, lung, prostate and colon (17). EphA2 phosphorylates Tyr542 and Tyr580 of SHP2 to enhance and prolong ERK activation downstream of receptor tyrosine kinases in cells stimulated with growth factors, such as EGF, HGF or Gas6 (17). Miura et al., were able to demonstrate that prolonged and enhanced ERK activation in cells stimulated with growth factors were reduced in cells depleted of EphA2 with simultaneous reduction of Tyr542/580 phosphorylation (17). The SHP2-dependent ERK activation signal pathway was hyperactivated promoting cancer cell proliferation in tumors with EphA2 overexpression, measured by mRNA or immunohistochemistry (IHC) (17). Very interestingly, the G391R EphA2 mutation has been identified in a squamous cell cancer cell line (H2170) but also in samples from patients exhibiting NSCLC with squamous histology (24). This mutation activates downstream effectors of EphA2 including mTOR, making this receptor a useful molecular therapeutic target even for squamous NSCLC (24). Until now, only a few small molecule inhibitors of EphA2 have been identified (25) but dasatinib has been reported to have potent inhibitory activity against this receptor (26).
Targeting the phospho-peptide binding site in SHP2 seems to be a feasible approach for developing SHP2- selective inhibitors but, despite the great need, little progress has been made. In a recent study, a small-molecule inhibitor (#220-324) was identified that selectively inhibits SHP2 and blocks SHP2-mediated signaling and cellular function (10). Until further studies are performed to optimize this compound and develop new SHP2 inhibitors with increased activity and selectivity suitable for preclinical and clinical studies, combining EGFR TKIs with MET, AXL or EphA2 inhibitors can be a rational and innovative synthetic lethality approach for EGFR mutant NSCLC patients with low baseline BIM expression and high SHP2 activity (Figure 1). It seems that IHC staining and mRNA expression of SHP2 are well correlated and can be used as a biomarker for response (27,28).
Additionally, the MAPK pathway can be cross-regulated by the cAMP pathway. This occurs through inhibition of the Raf-1 kinase by PKA, a main effector of cAMP (29). Upregulation of the tumor-promoting factors PDE4A and PDE4D in lung cancer (including the H1975 cell line) impairs cAMP generation through cAMP hydrolysis, activating the MAPK pathway and thus downregulating BIM (29). Whether drugs already approved for nononcologic indications, for example roflumilast which is used to treat asthma and chronic obstructive pulmonary disease, can be safely and effectively repurposed as PDE4 inhibitors in combination with EGFR TKIs, warrants further investigation (Figure 1). It is worth mentioning that cAMP is also involved in regulation of mammalian target of rapamycin (mTOR) transcription by diacylglycerol kinase α (DGKα) (30,31). mTOR mRNA levels have been shown to correlate strongly with DGKα mRNA levels in several tumours, and cells treated with PDE4 inhibitors show a significant decrease in mTOR transcription, indicating that DGKα regulates mTOR transcription, probably via modulation of cAMP levels (31).
High BIM at baseline is not enough to offer the maximum benefit to NSCLC EGFR mutant patients treated with EGFR TKIs
Even patients with high BIM levels at baseline (which means that the ERK pathway may not be very active) eventually develop resistance and disease relapse after a median PFS of 12.9 months (5). Binding of EGF to the EGFR induces dimerization, autophosphorylation and transactivation of the receptor’s tyrosine kinase activity, providing a variety of binding sites for a series of proteins, thereby initiating activation of downstream signaling pathways (32). For instance, Y845 (pY845) phosphorylation stabilizes the activation loop, maintains the enzyme in an active state and regulates STAT3/5 activity. Phospho-tyrosine 992 (pY992) within EGFR provides a binding motif for phospholipase C-γ (PLC-γ), initiating downstream signaling, including PKC and subsequent ERK activation. Phospho-tyrosine 1068 (pY1068) and 1086 (pY1086) provide a binding motif for Grb2/SH2 domain, which also leads to ERK and AKT activation (32). Phospho-tyrosine 1173 (pY1173) and 1148 (pY1148) represent a motif for PLC-γ and Shc, both of which can initiate activation of the ERK cascade. Interestingly, pY1068, pY1148, and pY1173 are essential for EGFR internalization and degradation, as well as for tyrosine kinase activity (32). In 2004, Sordella and colleagues were able to demonstrate the differential EGF-induced tyrosine phosphorylation pattern seen with wild-type and mutant EGFR receptors (32). EGF-induced phosphorylation of Y1045 and Y1173 is almost indistinguishable between wild-type and mutant EGFRs, whereas phosphorylation of Y992 and Y1068 is substantially increased in both mutants. Y845 is highly phosphorylated in the L858R missense mutant, but not in the wild-type or deletion mutant, and hence appears to be unique in distinguishing between the two types of EGFR mutations (32). Therefore, the effects of EGFR-activating mutations might be most appropriately characterized as “oncogene imbalance”, since the ERK pathway is altered in the opposite direction to AKT and STAT (16). In the EURTAC study, patients with deletion 19 had significantly better PFS to erlotinib compared to chemotherapy, in comparison with the smaller group of patients with the L858R missense exon 21 mutation, for whom PFS to erlotinib was not significantly different from the chemotherapy treated group (2). We have previously commented on the findings that the EGFR L858R mutation leads to decreased ability to activate ERK compared to wild-type EGFR which correlates with decreased EGFR internalization, reduced phosphorylation of SHP2 and reduced sensitivity to gefitinib (16). In addition, we can now speculate that these differences in outcome between the two classic mutations can be through full STAT3/5 activation by the missense exon 21 mutation. Inhibition of EGFR with EGFR TKIs has no effect on tyrosine phosphorylated STAT3 (33). Therefore combining EGFR TKIs (reversible or irreversible) with a JAK2 inhibitor, like ruxolitinib, can be more efficient in inducing apoptosis regardless of MAPK/ERK abrogation or high levels of BIM in EGFR mutant patients with the L858R mutation (Figure 1).
Activating mutations of EGFR may enhance IL-6 production and autocrine stimulation of STAT3 activity, but additional cellular factors are important in modulating this pathway and the response of cells to IL6. The PC9 (del19) cells that harbor activated EGFR have essentially absent STAT3 activity, measured either by immunoblot or DNA binding assay (33). While activating mutations of EGFR may enhance IL-6 production and autocrine stimulation of STAT3 activity, additional cellular factors are important in modulating this pathway and the response of cells to IL6. Indeed, STAT3 activity in lung cancer cells is regulated by IL-6 in conjunction with JAK1/2 activity. SHP2 is a positive regulator of cell growth and migration through stimulation of the MAPK/ERK pathway, but a negative regulator of interferon signaling and the JAK/STAT3 pathway. It has been demonstrated by You and colleagues that SHP2 is involved in protecting cells from the cytotoxic effect of IFNs and that it acts as a negative effector in mediating activation of STATs induced by IFN-α or IFN-γ (34). Therefore, in cells that SHP2 is silent, and BIM or BIM-EL levels remain elevated through the activity of EGFR TKIs, the JAK/STAT3 pathway can be hyperactive inducing anti-apoptotic signals and favoring tumour survival and progression. In these cases the combination of EGFR TKIs with a JAK2 inhibitor like ruxolitinib can abrogate tumor growth (33).
Does mTOR matter more than BIM?
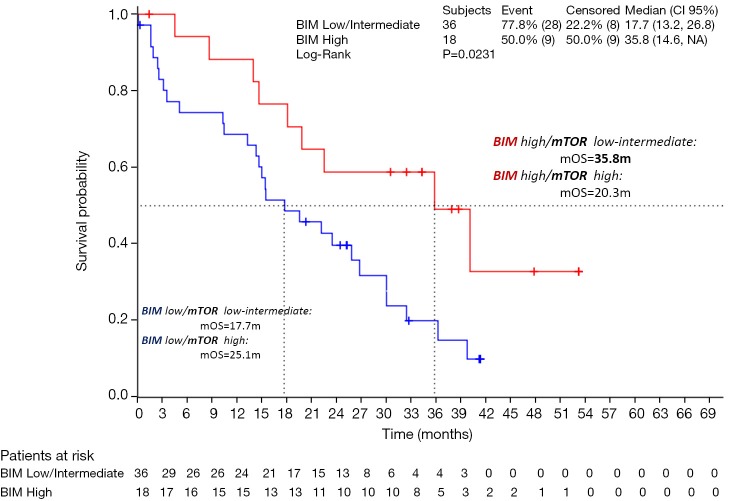
Regardless of BIM status, mTOR is a serine/threonine kinase that is often deregulated during cancer growth. It has been shown that mTOR is important for the oncogenic transformation induced specifically by PI3K and AKT. mTOR integrates cues from nutrients and growth factors, acting as a nexus point for cellular signals to control growth, metabolism, and longevity. Deregulation of either of mTOR’s two complexes, mTORC1 or mTORC2, leads to diseases of metabolism, including cancer and diabetes (35). We were able to examine mTOR mRNA levels in 48 tumor samples from the EURTAC study. Eighteen patients (37.5%) had high mRNA expression by terciles and 30 (62.5%) had low/intermediate mTOR mRNA levels. Also, we were able to correlate the mTOR levels with high levels of BIM. For instance, patients with high BIM and low-intermediate mTOR, had a median overall survival of 35.5 months, compared to 20.3 months for the group of patients with high BIM and high mTOR (Figure 2) (unpublished data).
Figure 2.
Effect of mTOR on the survival of patients with high BIM.
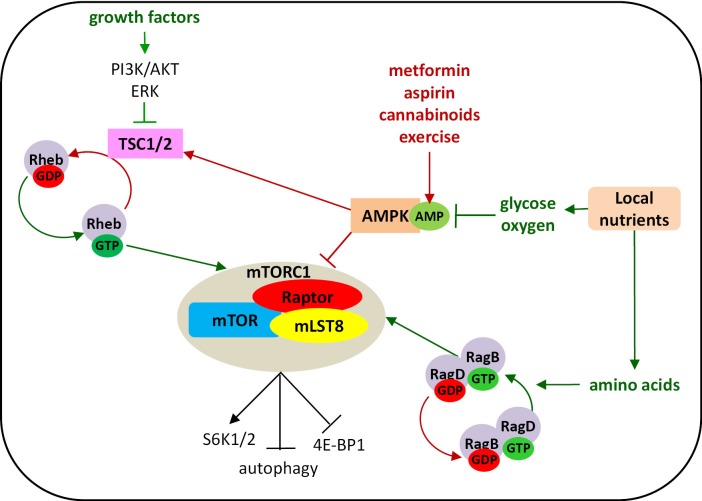
mTOR warrants further exploration to determine whether it is a stronger biomarker than BIM to predict outcome of patients treated with EGFR TKIs or chemotherapy. mTORC1 is essential to the decision process between anabolism and catabolism (36). This complex, which consists of mTOR, Raptor, and mLST8, is activated by amino acids, growth factors and cellular energy to drive nutrient uptake and, subsequently, proliferation (36). The molecular details of these nutrient-sensing processes are not yet fully elucidated. Amino acids activate the Rag GTPases to regulate mTORC1 localization to the lysosomes and growth factors signal through the PI3K-AKT or the ERK pathways to activate mTORC1 by releasing the Ras homolog enriched in brain (Rheb) GTPase from repression by the tumour suppressors tuberous sclerosis 1 and 2 (TSC1, TSC2). Finally, low-energy conditions inhibit mTORC1 by activating AMP-activated protein kinase (AMPK) (36). mTORC1 phosphorylates and activates the ribosomal S6 kinases (S6K1 and S6K2), which are required for translation of a group of mRNAs, and inactivates the binding protein of eukaryotic translation initiation factor 4E (4E/BP), thereby facilitating 4E-mediated translation (37). At the same time, mTORC1 is known to be a major negative regulator of autophagy. Altogether, these effects imply that mTORC1 increases protein synthesis and reduces protein degradation (37). AMPK serves as an energy sensor in all eukaryotic cells and also occupies a central role in linking metabolism and cancer development. It is activated in response to an increase in the AMP:ATP ratio during hypoxia, starvation, glucose deprivation or muscle contraction and regulates aerobic glycolysis (the Warburg effect) in cancer cells and suppresses tumour growth in vivo (38). Under starvation conditions, AMPK plays a critical role for cell survival by stimulating energy production and limiting use of energy by active biosynthetic pathways usually operating in proliferating cells (38). Many recent studies have shown that exercise or pharmacologic activators of AMPK, such as metformin, cannabinoids, and aspirin (a synthetic derivative of salicylate), cause AMPK activation and inhibit or delay the onset of tumours in different animal cancer models (38-40). Cannabinoid-mediated metabolism results in strong induction of autophagy and inhibition of cell growth in pancreatic cancer cells (40) (Figure 3).
Figure 3.
Regulation of mTORC1 activity.
The anticancer mechanism of action of metformin is ambiguous. Although it is an antidiabetic drug, activation of AMPK through phosphorylation of AMPKα at Thr-172 has been widely accepted as a possible mechanism (41). However, most studies, which evaluate the antitumor activity of metformin, use concentrations much higher than the recommended therapeutic doses for clinical use. When concentrations are decreased to the same as that found in plasma and tissues of individuals receiving therapeutic doses, inhibition of cell proliferation is not observed (42). A recent study by Gou and colleagues demonstrated that low concentrations of metformin were associated with reduction of ERK and mTOR phosphorylation independent of AKT and AMPK phosphorylation in pancreatic cancer cells (42). These low concentrations of metformin were effective on specific subpopulations of pancreatic cancer cells expressing CD133, a surface marker considered characteristic of cells with extensive proliferative and self-renewal characteristics (cancer stem cells). A similar selective inhibitory effect of metformin was observed on CD133 positive cancer glioblastoma cells (43). In NSCLC, IHC assessment of CD133 expression is correlated with pathological stage and is predictive of unfavorable prognosis for stages II-IV (44). These results provide a basis for combination of metformin with current therapies to improve prognosis of cancer patients and allocate a role to IHC evaluation of CD133 as a biomarker to predict response (43).
Conclusions
If we wish to radically change treatment of EGFR mutant NSCLC to the benefit of our patients, we should start thinking about a different approach based on information derived from additional biomarkers. Patients with low BIM levels at baseline may benefit from the combination of EGFR TKIs with compounds that downregulate or abrogate activity of SHP2, like MET, AXL or EphA2 inhibitors. It should be seriously considered whether, at time of progression, a JAK2 inhibitor should be added in order to overcome loss of the negative impact of SHP2 on the JAK/STAT pathway. Patients with high BIM levels at baseline may have a hyperactive JAK/STAT pathway through either the L858R mutation or loss of SHP2 activity. The combination of EGFR TKIs plus a JAK inhibitor should be seriously considered in these cases. In general, patients with high BIM expression benefit from erlotinib or similar EGFR TKIs, but analysis of mTOR could further improve outcome by selecting patients with high mTOR for combination therapy with EGFR TKIs and mTOR inhibitors. We propose this line of research at the levels of cell lines or xenograft models and at the level of biomarker discovery in tumour samples, in order to verify in the most accurate possible way our assumptions and contribute to the radical transformation of treatment of EGFR mutant lung cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [DOI] [PubMed] [Google Scholar]
- 4.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [DOI] [PubMed] [Google Scholar]
- 5.Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [DOI] [PubMed] [Google Scholar]
- 6.Wannesson L, Viteri S, Costa C, et al. Signaling pathways modulating dependence of lung cancer on mutant epidermal growth factor receptor and mechanisms of intrinsic and acquired resistance to tyrosine kinase inhibitors. Curr Pharm Des 2013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med 2007;4:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med 2007;4:1669-79; discussion 1680. [DOI] [PMC free article] [PubMed]
- 9.Dasari VR, Velpula KK, Alapati K, et al. Cord blood stem cells inhibit epidermal growth factor receptor translocation to mitochondria in glioblastoma. PLoS One 2012;7:e31884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Yu B, Liu W, Yu WM, et al. Targeting protein tyrosine phosphatase SHP2 for the treatment of PTPN11-associated malignancies. Mol Cancer Ther 2013;12:1738-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furcht CM, Muñoz Rojas AR, Nihalani D, et al. Diminished functional role and altered localization of SHP2 in non-small cell lung cancer cells with EGFR-activating mutations. Oncogene 2013;32:2346-55, 2355.e1-10. [DOI] [PMC free article] [PubMed]
- 12.Aceto N, Sausgruber N, Brinkhaus H, et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med 2012;18:529-37. [DOI] [PubMed] [Google Scholar]
- 13.Ivins Zito C, Kontaridis MI, Fornaro M, et al. SHP-2 regulates the phosphatidylinositide 3'-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J Cell Physiol 2004;199:227-36. [DOI] [PubMed] [Google Scholar]
- 14.Wu CJ, O’Rourke DM, Feng GS, et al. The tyrosine phosphatase SHP-2 is required for mediating phosphatidylinositol 3-kinase/Akt activation by growth factors. Oncogene 2001;20:6018-25. [DOI] [PubMed] [Google Scholar]
- 15.Cragg MS, Kuroda J, Puthalakath H, et al. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med 2007;4:1681-89; discussion 1690. [DOI] [PMC free article] [PubMed]
- 16.Lazzara MJ, Lane K, Chan R, et al. Impaired SHP2-mediated extracellular signal-regulated kinase activation contributes to gefitinib sensitivity of lung cancer cells with epidermal growth factor receptor-activating mutations. Cancer Res 2010;70:3843-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura K, Wakayama Y, Tanino M, et al. Involvement of EphA2-mediated tyrosine phosphorylation of Shp2 in Shp2-regulated activation of extracellular signal-regulated kinase. Oncogene 2013;32:5292-301. [DOI] [PubMed] [Google Scholar]
- 18.Maroun CR, Naujokas MA, Holgado-Madruga M, et al. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol 2000;20:8513-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menis J, Giaj Levra M, Novello S.MET inhibition in lung cancer. Trans Lung Cancer Res 2013;2:23-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res 2013;19:2310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gusenbauer S, Vlaicu P, Ullrich A.HGF induces novel EGFR functions involved in resistance formation to tyrosine kinase inhibitors. Oncogene 2013;32:3846-56. [DOI] [PubMed] [Google Scholar]
- 22.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res 2010;16:3507-16. [DOI] [PubMed] [Google Scholar]
- 23.Ye X, Li Y, Stawicki S, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 2010;29:5254-64. [DOI] [PubMed] [Google Scholar]
- 24.Faoro L, Singleton PA, Cervantes GM, et al. EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J Biol Chem 2010;285:18575-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noberini R, Koolpe M, Peddibhotla S, et al. Small molecules can selectively inhibit ephrin binding to the EphA4 and EphA2 receptors. J Biol Chem 2008;283:29461-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Q, Jorgensen C, Pawson T, et al. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer 2008;99:1074-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu R, Yu Y, Zheng S, et al. Overexpression of Shp2 tyrosine phosphatase is implicated in leukemogenesis in adult human leukemia. Blood 2005;106:3142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong S, Li FQ, Zhang Q, et al. Expression and clinical significance of SHP2 in gastric cancer. J Int Med Res 2012;40:2083-9. [DOI] [PubMed] [Google Scholar]
- 29.Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet 2013;382:720-31. [DOI] [PubMed] [Google Scholar]
- 30.Dominguez CL, Floyd DH, Xiao A, et al. Diacylglycerol kinase α is a critical signaling node and novel therapeutic target in glioblastoma and other cancers. Cancer Discov 2013;3:782-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosell R, Karachaliou N.Lung cancer: maintenance therapy and precision medicine in NSCLC. Nat Rev Clin Oncol 2013;10:549-50. [DOI] [PubMed] [Google Scholar]
- 32.Sordella R, Bell DW, Haber DA, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004;305:1163-7. [DOI] [PubMed] [Google Scholar]
- 33.Song L, Rawal B, Nemeth JA, et al. JAK1 activates STAT3 activity in non-small-cell lung cancer cells and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling. Mol Cancer Ther 2011;10:481-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You M, Yu DH, Feng GS. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol 1999;19:2416-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csibi A, Fendt SM, Li C, et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 2013;153:840-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab 2011;22:94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng L, Yang W, Wu F, et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res 2013;19:5372-80. [DOI] [PubMed] [Google Scholar]
- 39.Hawley SA, Fullerton MD, Ross FA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 2012;336:918-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dando I, Donadelli M, Costanzo C, et al. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis 2013;4:e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocha GZ, Dias MM, Ropelle ER, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res 2011;17:3993-4005. [DOI] [PubMed] [Google Scholar]
- 42.Gou S, Cui P, Li X, et al. Low concentrations of metformin selectively inhibit CD133+ cell proliferation in pancreatic cancer and have anticancer action. PLoS One 2013;8:e63969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Würth R, Pattarozzi A, Gatti M, et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: a role for metformin-induced inhibition of Akt. Cell Cycle 2013;12:145-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizugaki H, Sakakibara-Konishi J, Kikuchi J, et al. CD133 expression: a potential prognostic marker for non-small cell lung cancers. Int J Clin Oncol 2013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]