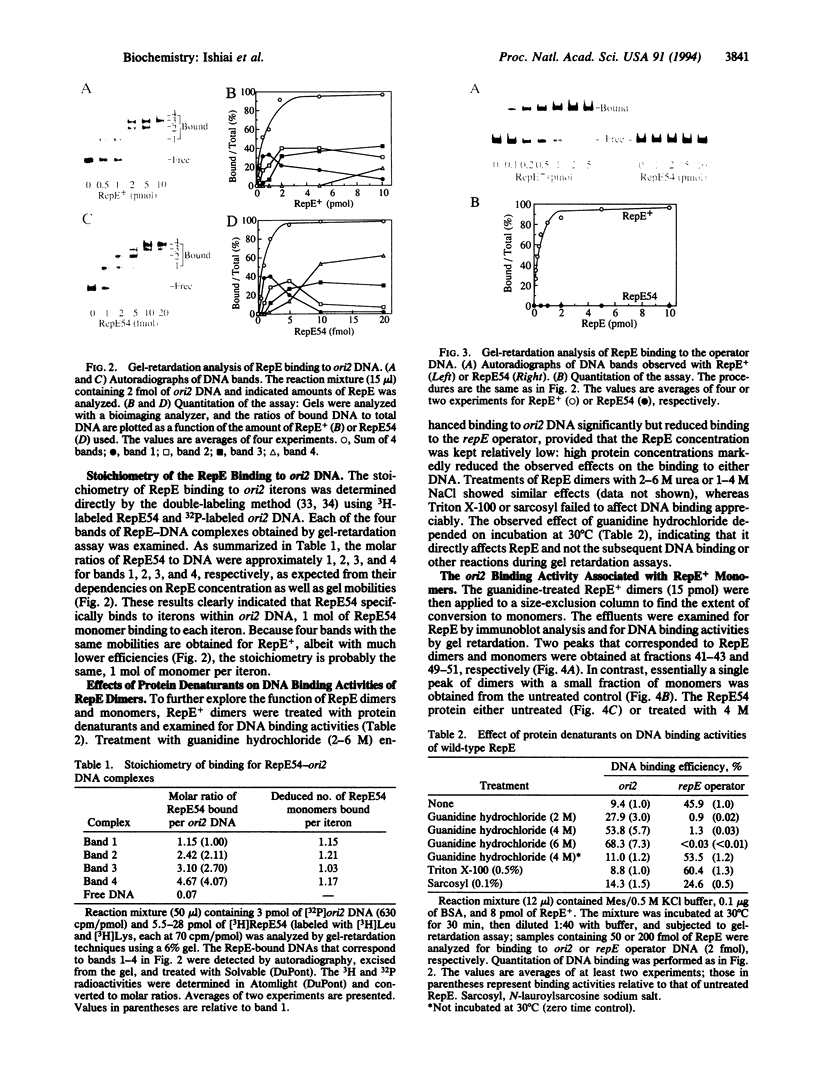
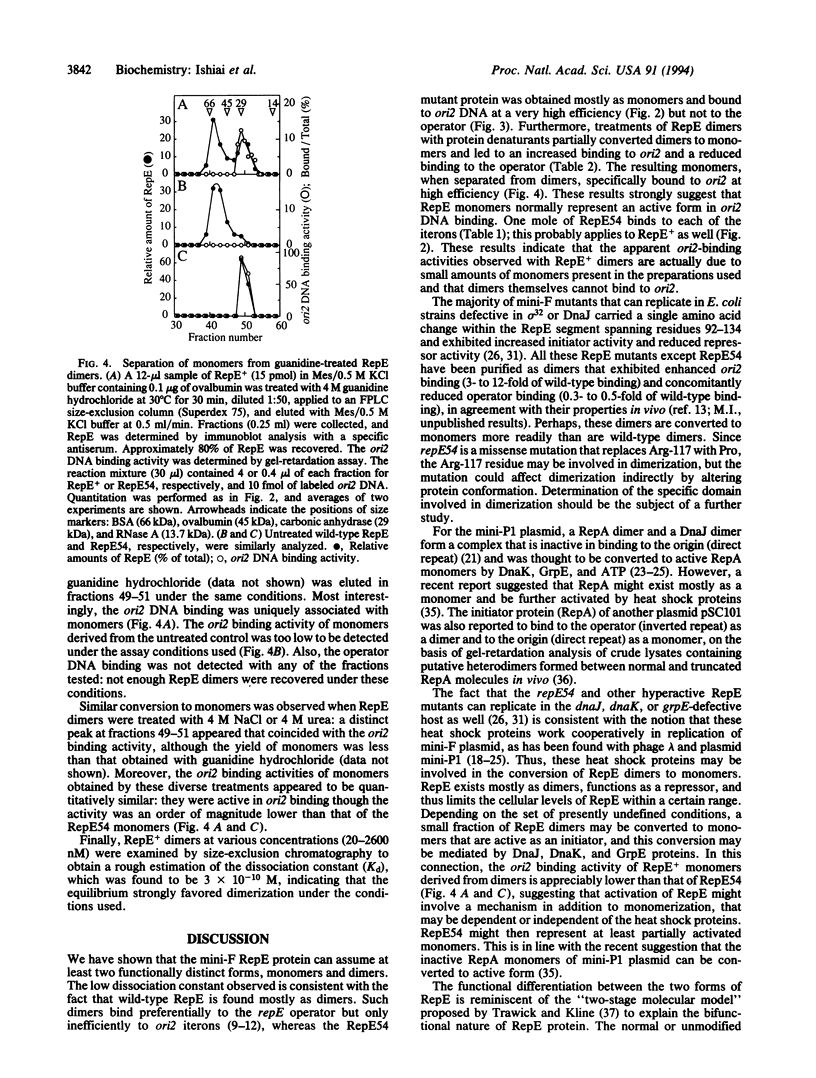
Abstract
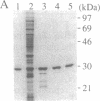
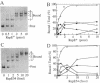
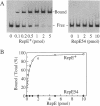
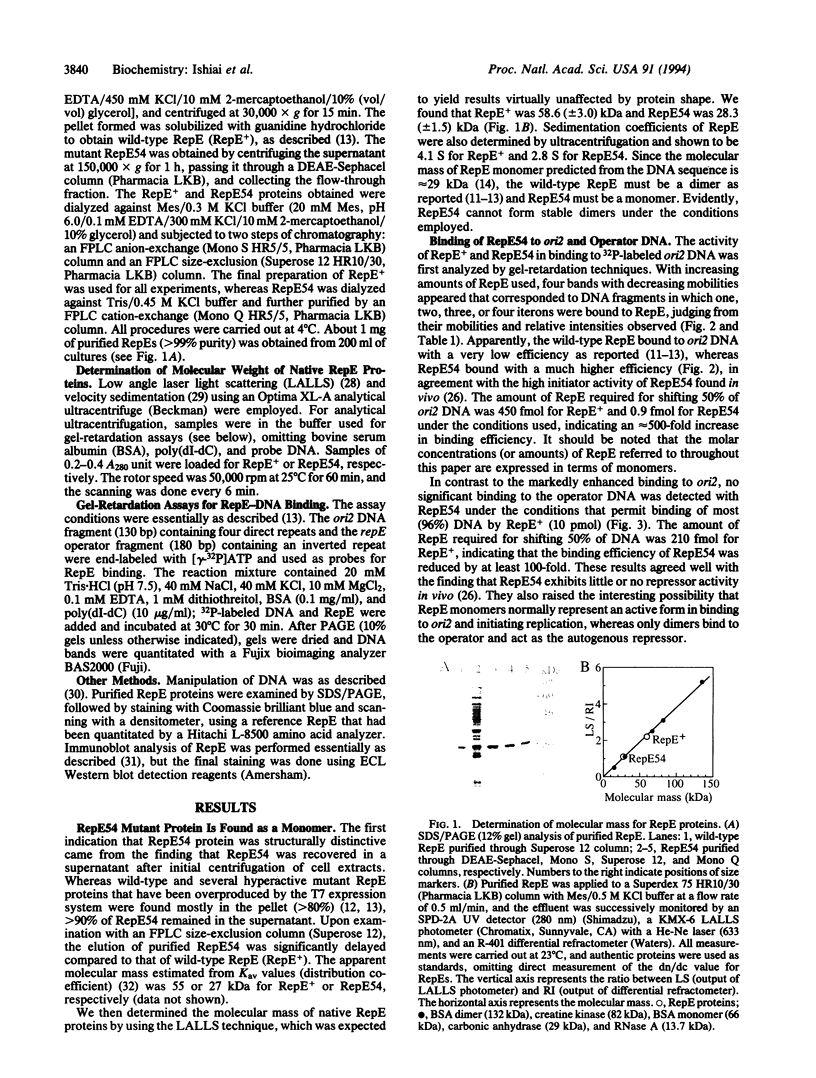
Replication of mini-F plasmid requires the plasmid-encoded RepE initiator protein and several host factors including DnaJ, DnaK, and GrpE, heat shock proteins of Escherichia coli. The RepE protein plays a crucial role in replication and exhibits two major functions: initiation of replication from the origin, ori2, and autogenous repression of repE transcription. One of the mini-F plasmid mutants that can replicate in the dnaJ-defective host produces an altered RepE (RepE54) with a markedly enhanced initiator activity but little or no repressor activity. RepE54 has been purified from cell extracts primarily in monomeric form, unlike the wild-type RepE that is recovered in dimeric form. Gel-retardation assays revealed that RepE54 monomers bind to ori2 (direct repeats) with a very high efficiency but hardly bind to the repE operator (inverted repeat), in accordance with the properties of RepE54 in vivo. Furthermore, the treatment of wild-type RepE dimers with protein denaturants enhanced their binding to ori2 but reduced binding to the operator: RepE dimers were partially converted to monomers, and the ori2 binding activity was uniquely associated with monomers. These results strongly suggest that RepE monomers represent an active form by binding to ori2 to initiate replication, whereas dimers act as an autogenous repressor by binding to the operator. We propose that RepE is structurally and functionally differentiated and that monomerization of RepE dimers, presumably mediated by heat shock protein(s), activates the initiator function and participates in regulation of mini-F DNA replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bukau B., Walker G. C. Delta dnaK52 mutants of Escherichia coli have defects in chromosome segregation and plasmid maintenance at normal growth temperatures. J Bacteriol. 1989 Nov;171(11):6030–6038. doi: 10.1128/jb.171.11.6030-6038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta S., Mukhopadhyay G., Papp P. P., Lewis M. S., Chattoraj D. K. Activation of DNA binding by the monomeric form of the P1 replication initiator RepA by heat shock proteins DnaJ and DnaK. J Mol Biol. 1993 Jul 5;232(1):23–34. doi: 10.1006/jmbi.1993.1367. [DOI] [PubMed] [Google Scholar]
- Ezaki B., Ogura T., Mori H., Niki H., Hiraga S. Involvement of DnaK protein in mini-F plasmid replication: temperature-sensitive seg mutations are located in the dnaK gene. Mol Gen Genet. 1989 Aug;218(2):183–189. doi: 10.1007/BF00331267. [DOI] [PubMed] [Google Scholar]
- Ishiai M., Wada C., Kawasaki Y., Yura T. Mini-F plasmid mutants able to replicate in Escherichia coli deficient in the DnaJ heat shock protein. J Bacteriol. 1992 Sep;174(17):5597–5603. doi: 10.1128/jb.174.17.5597-5603.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y., Wada C., Yura T. Binding of RepE initiator protein to mini-F DNA origin (ori2). Enhancing effects of repE mutations and DnaJ heat shock protein. J Biol Chem. 1992 Jun 5;267(16):11520–11524. [PubMed] [Google Scholar]
- Kawasaki Y., Wada C., Yura T. Mini-F plasmid mutants able to replicate in the absence of sigma 32: mutations in the repE coding region producing hyperactive initiator protein. J Bacteriol. 1991 Feb;173(3):1064–1072. doi: 10.1128/jb.173.3.1064-1072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y., Wada C., Yura T. Roles of Escherichia coli heat shock proteins DnaK, DnaJ and GrpE in mini-F plasmid replication. Mol Gen Genet. 1990 Jan;220(2):277–282. doi: 10.1007/BF00260494. [DOI] [PubMed] [Google Scholar]
- Keasling J. D., Palsson B. O., Cooper S. Cell-cycle-specific F plasmid replication: regulation by cell size control of initiation. J Bacteriol. 1991 Apr;173(8):2673–2680. doi: 10.1128/jb.173.8.2673-2680.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Sandhu G. S., Eckloff B. W., Aleff R. A. Site-specific proteolysis of mini-F plasmid replication protein RepE destroys initiator function and generates an incompatibility substance. J Bacteriol. 1992 May;174(9):3004–3010. doi: 10.1128/jb.174.9.3004-3010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L. J. Nonrandom F-plasmid replication in Escherichia coli K-12. J Bacteriol. 1992 Apr;174(7):2121–2123. doi: 10.1128/jb.174.7.2121-2123.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue T. M., Rhodes D. G. Determination of size, molecular weight, and presence of subunits. Methods Enzymol. 1990;182:566–587. doi: 10.1016/0076-6879(90)82045-4. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki S., Kuribayashi M., Miki T., Horiuchi T. An amber replication mutant of F plasmid mapped in the minimal replication region. Mol Gen Genet. 1983;191(2):231–237. doi: 10.1007/BF00334819. [DOI] [PubMed] [Google Scholar]
- Manen D., Upegui-Gonzalez L. C., Caro L. Monomers and dimers of the RepA protein in plasmid pSC101 replication: domains in RepA. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8923–8927. doi: 10.1073/pnas.89.19.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L., Ray D. S. Mechanism of autonomous control of the Escherichia coli F plasmid: different complexes of the initiator/repressor protein are bound to its operator and to an F plasmid replication origin. Nucleic Acids Res. 1986 Jul 25;14(14):5693–5711. doi: 10.1093/nar/14.14.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L., Ray D. S. Mechanism of autonomous control of the Escherichia coli F plasmid: purification and characterization of the repE gene product. Nucleic Acids Res. 1988 Jan 25;16(2):413–424. doi: 10.1093/nar/16.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraiso K., Tokino T., Murotsu T., Matsubara K. Replication of mini-F plasmid in vitro promoted by purified E protein. Mol Gen Genet. 1987 Mar;206(3):519–521. doi: 10.1007/BF00428895. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- Olsen P. H., Esmon N. L., Esmon C. T., Laue T. M. Ca2+ dependence of the interactions between protein C, thrombin, and the elastase fragment of thrombomodulin. Analysis by ultracentrifugation. Biochemistry. 1992 Jan 28;31(3):746–754. doi: 10.1021/bi00118a016. [DOI] [PubMed] [Google Scholar]
- Schneider G. J., Geiduschek E. P. Stoichiometry of DNA binding by the bacteriophage SP01-encoded type II DNA-binding protein TF1. J Biol Chem. 1990 Jun 25;265(18):10198–10200. [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Søgaard-Andersen L., Rokeach L. A., Molin S. Regulated expression of a gene important for replication of plasmid F in E. coli. EMBO J. 1984 Feb;3(2):257–262. doi: 10.1002/j.1460-2075.1984.tb01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T. Confirmation of molecular weight of Aspergillus oryzae alpha-amylase using the low angle laser light scattering technique in combination with high pressure silica gel chromatography. J Biochem. 1981 Feb;89(2):363–368. doi: 10.1093/oxfordjournals.jbchem.a133210. [DOI] [PubMed] [Google Scholar]
- Tilly K., Yarmolinsky M. Participation of Escherichia coli heat shock proteins DnaJ, DnaK, and GrpE in P1 plasmid replication. J Bacteriol. 1989 Nov;171(11):6025–6029. doi: 10.1128/jb.171.11.6025-6029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokino T., Murotsu T., Matsubara K. Purification and properties of the mini-F plasmid-encoded E protein needed for autonomous replication control of the plasmid. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4109–4113. doi: 10.1073/pnas.83.12.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Helinski D. R. Separation of the minimal replication region of the F plasmid into a replication origin segment and a trans-acting segment. Mol Gen Genet. 1982;186(3):372–377. doi: 10.1007/BF00729456. [DOI] [PubMed] [Google Scholar]
- Trawick J. D., Kline B. C. A two-stage molecular model for control of mini-F replication. Plasmid. 1985 Jan;13(1):59–69. doi: 10.1016/0147-619x(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Wada C., Akiyama Y., Ito K., Yura T. Inhibition of F plasmid replication in htpR mutants of Escherichia coli deficient in sigma 32 protein. Mol Gen Genet. 1986 May;203(2):208–213. doi: 10.1007/BF00333956. [DOI] [PubMed] [Google Scholar]
- Wada C., Imai M., Yura T. Host control of plasmid replication: requirement for the sigma factor sigma 32 in transcription of mini-F replication initiator gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8849–8853. doi: 10.1073/pnas.84.24.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L. A., Phua S. H., Bergquist P. L., Lane H. E. An Mr 29000 protein is essential for mini-F maintenance in E. coli. Gene. 1982 Sep;19(2):173–178. doi: 10.1016/0378-1119(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. Three Escherichia coli heat shock proteins are required for P1 plasmid DNA replication: formation of an active complex between E. coli DnaJ protein and the P1 initiator protein. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2690–2694. doi: 10.1073/pnas.87.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hoskins J., McKenney K. Function of DnaJ and DnaK as chaperones in origin-specific DNA binding by RepA. Nature. 1991 Mar 14;350(6314):165–167. doi: 10.1038/350165a0. [DOI] [PubMed] [Google Scholar]
- Wickner S., Hoskins J., McKenney K. Monomerization of RepA dimers by heat shock proteins activates binding to DNA replication origin. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7903–7907. doi: 10.1073/pnas.88.18.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Skowyra D., Hoskins J., McKenney K. DnaJ, DnaK, and GrpE heat shock proteins are required in oriP1 DNA replication solely at the RepA monomerization step. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10345–10349. doi: 10.1073/pnas.89.21.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. C., Nash H. A. The interaction of E. coli IHF protein with its specific binding sites. Cell. 1989 Jun 2;57(5):869–880. doi: 10.1016/0092-8674(89)90801-5. [DOI] [PubMed] [Google Scholar]