Abstract
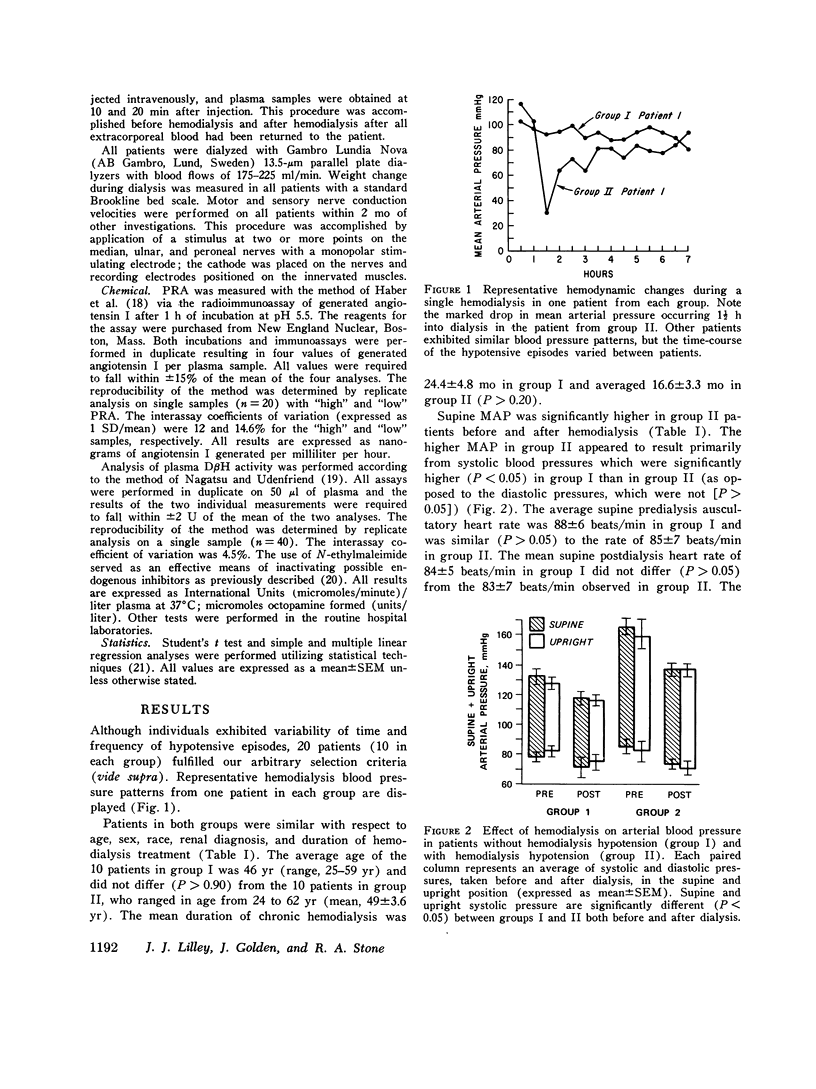
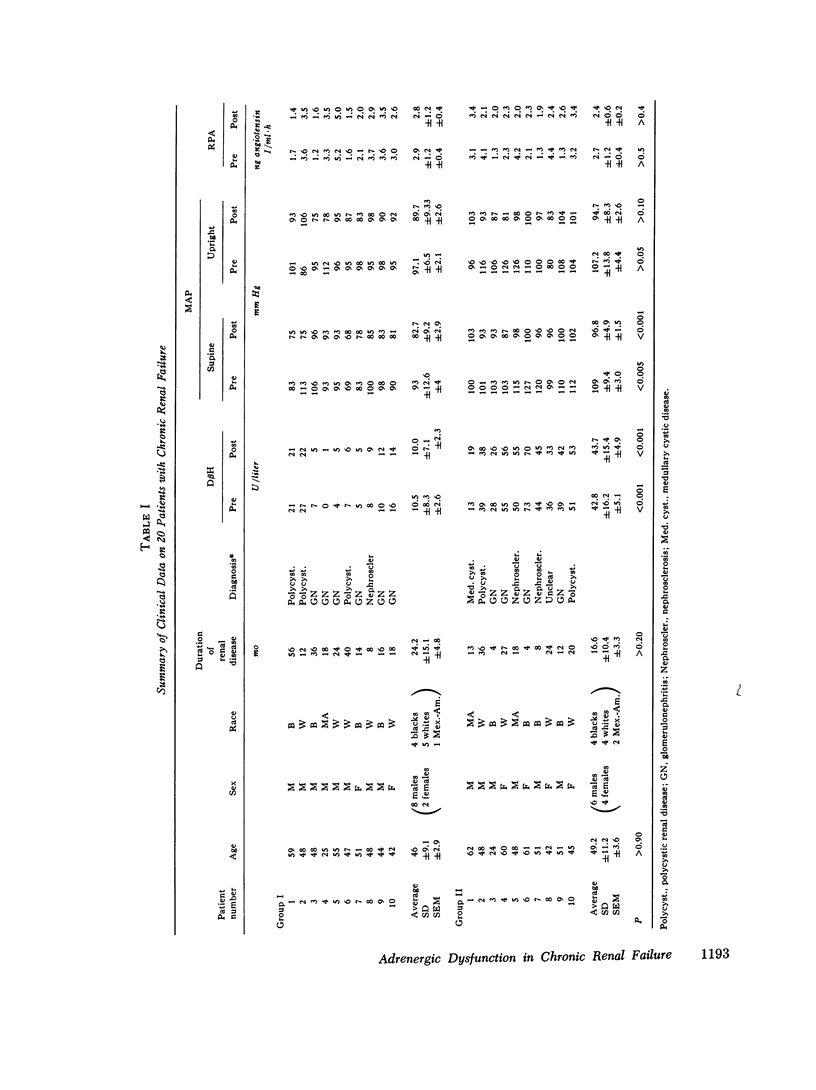
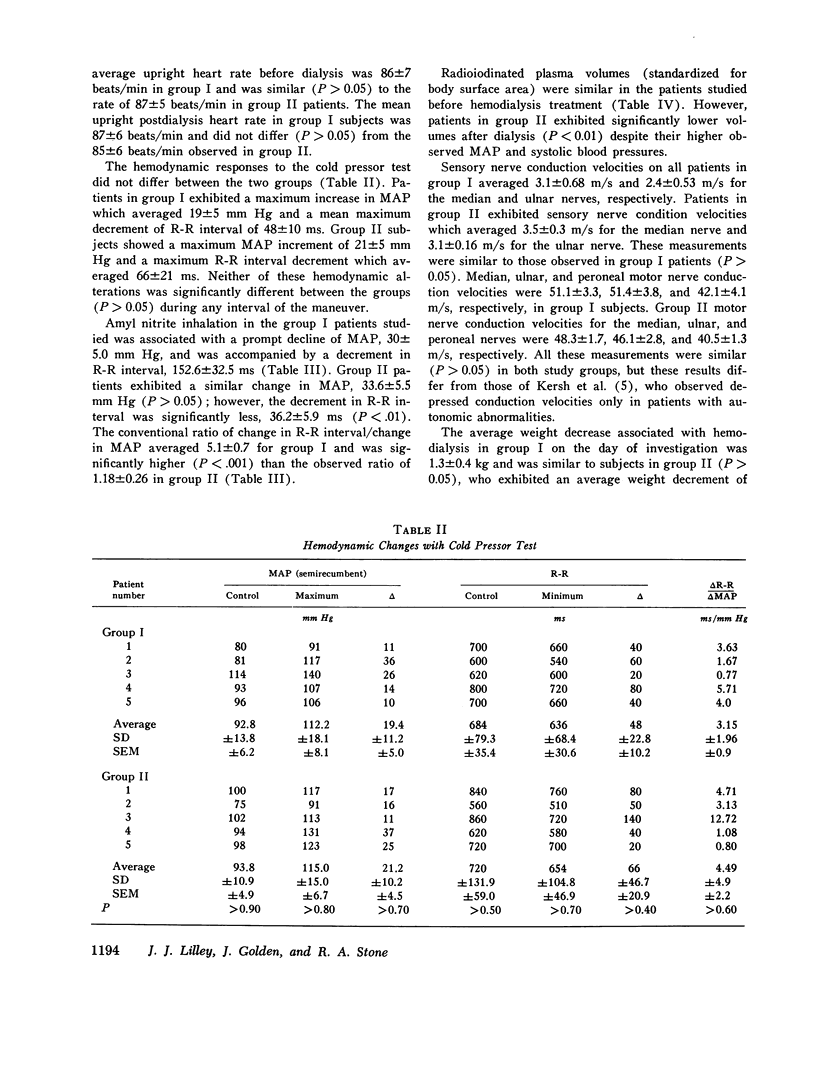
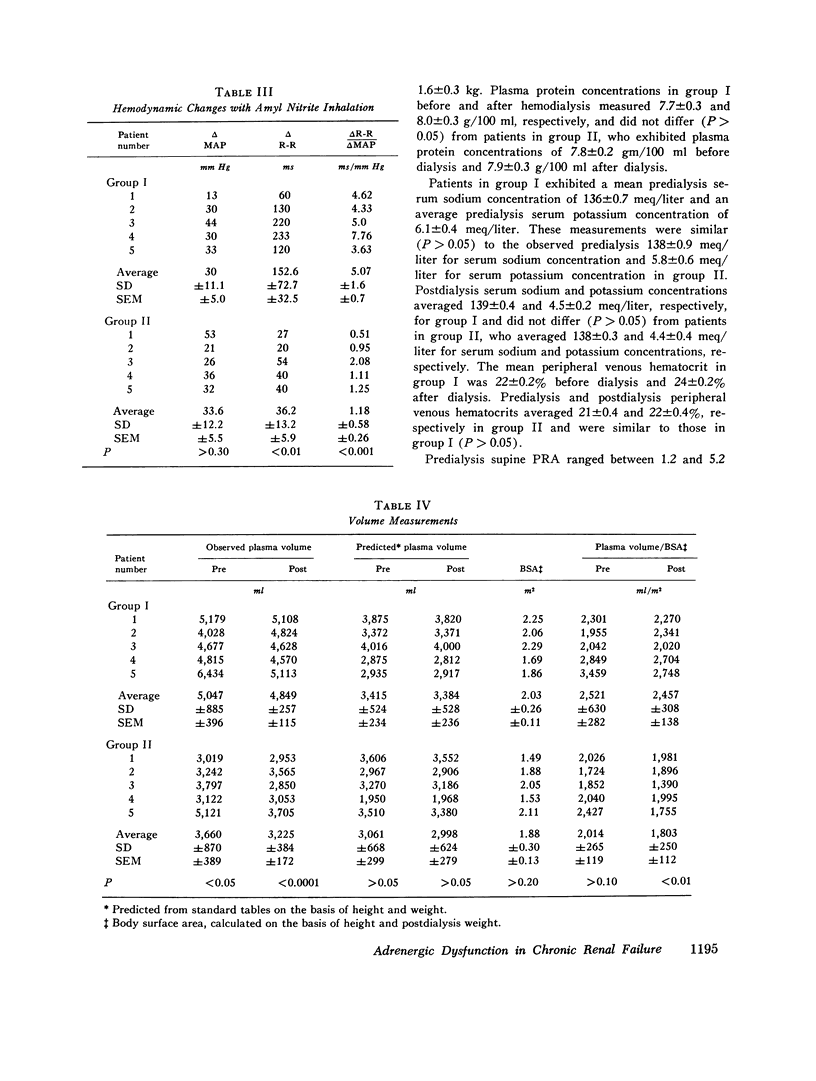
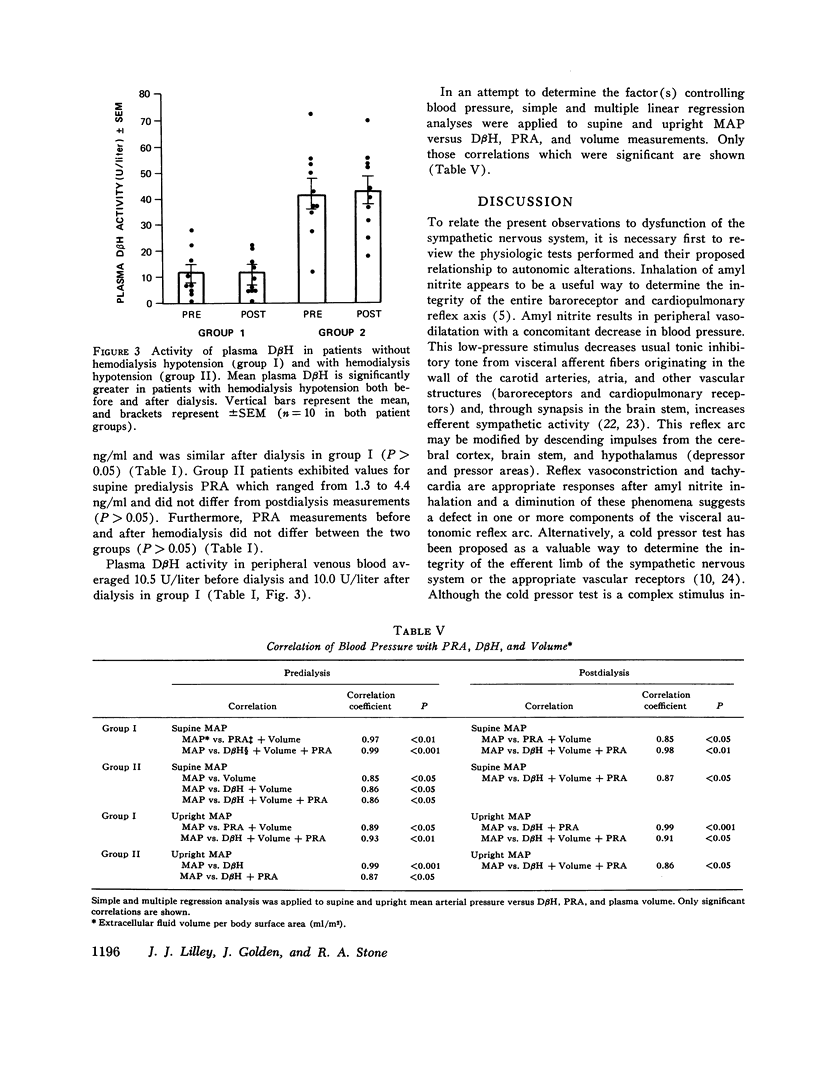
Previous investigations have suggested that significant hypotension during hemodialysis may result from abnormalities of sympathetic nervous system activity. To further evaluate these phenomena, plasma dopamine beta-hydroxylase (D beta H) and cold pressor test (proposed indexes of efferent sympathetic nervous system activity) and amyl nitrite inhalation (an index of the entire baroreceptor reflex arc) were studied in two groups of patients: group I, patients exhibiting a mean arterial pressure decrease to less than 70 mm Hg during less than 10% of dialyses; group II (hemodialysis hypotension), patients with a mean arterial pressure decrease to less than 70 mm Hg during more than 90% of dialyses. The groups were similar with respect to plasma renin activity, renin response to ultrafiltration, age, duration of dialysis, nerve conduction velocity, plasma protein concentration, hematocrit, dialysis weight change, resting heart rate, sex, race, blood pressure and heart rate response to cold pressor test, and 125I-albumin plasma volume. Supine mean arterial pressure was higher in patients with hemodialysis hypotension than in patients without hemodialysis hypotension (group I) both before and after dialysis. Plasma D beta H activity was significantly higher in patients with hemodialysis hypotension (group II) than in group I both before and after dialysis. Amyl nitrite inhalation, expressed as change in delta R-R interval/mean arterial pressure decrease, was less in hemodialysis hypotension patients. These results suggest that hemodialysis hypotension may result from a lesion in the baroreceptors, cardiopulmonary receptors, or visceral afferent nerves. Furthermore, elevated mean arterial pressure in patients with hemodialysis hypotension may be neurogenic in origin, as reflected by plasma D beta H activity, and appears similar to the hypertension that follows baroreceptor deafferentation of experimental animals.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi G., Ponticelli C., Bardi U., Redaelli B., Campolo L., De Ponti C., Graziani G. Role of the kidney in 'salt and water dependent hypertension' of end-stage disease. Clin Sci. 1972 Jan;42(1):47–55. doi: 10.1042/cs0420047. [DOI] [PubMed] [Google Scholar]
- Bristow J. D., Brown E. B., Jr, Cunningham D. J., Goode R. C., Howson M. G., Sleight P. The effects of hypercapnia, hypoxia and ventilation on the baroreflex regulation of the pulse interval. J Physiol. 1971 Jul;216(2):281–302. doi: 10.1113/jphysiol.1971.sp009525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. J., Curtis J. R., Lever A. F., Robertson J. I., De Wardener H. E., Wing A. J. Plasma renin concentration and the control of blood pressure in patients on maintenance haemodialysis. Nephron. 1969;6(3):329–349. doi: 10.1159/000179737. [DOI] [PubMed] [Google Scholar]
- Chalmers J. P. Brain amines and models of experimental hypertension. Circ Res. 1975 Apr;36(4):469–480. doi: 10.1161/01.res.36.4.469. [DOI] [PubMed] [Google Scholar]
- DeQuattro V., Miura Y. Neurogenic factors in human hypertension: mechanism or myth? Am J Med. 1973 Sep;55(3):362–378. doi: 10.1016/0002-9343(73)90136-8. [DOI] [PubMed] [Google Scholar]
- DeQuattro V., Nagatsu T., Maronde R., Alexander N. Catecholamine synthesis in rabbits with neurogenic hypertension. Circ Res. 1969 Apr;24(4):545–555. doi: 10.1161/01.res.24.4.545. [DOI] [PubMed] [Google Scholar]
- Dustan H. P., Tarazi R. C., Bravo E. L. Physiologic characteristics of hypertension. Am J Med. 1972 May;52(5):610–622. doi: 10.1016/0002-9343(72)90052-6. [DOI] [PubMed] [Google Scholar]
- Engelman K., Portnoy B., Sjoerdsma A. Catecholamines-cyclic amp-angiotensin receptors. Plasma catecholamine concentrations in patients with hypertension. Circ Res. 1970 Jul;27(1 Suppl 1):141–146. [PubMed] [Google Scholar]
- Geffen L. Serum dopamine beta-hydroxylase as an index of sympathetic function. Life Sci. 1974 May 1;14(9):1593–1604. doi: 10.1016/0024-3205(74)90262-8. [DOI] [PubMed] [Google Scholar]
- Gewirtz G. P., Kopin I. J. Release of dopamine-beta-hydroxylase with norepinephrine during cat splenic nerve stimulation. Nature. 1970 Jul 25;227(5256):406–407. doi: 10.1038/227406a0. [DOI] [PubMed] [Google Scholar]
- Gutkin M., Levinson G. E., King A. S., Lasker N. Plasma renin activity in end-stage kidney disease. Circulation. 1969 Oct;40(4):563–574. doi: 10.1161/01.cir.40.4.563. [DOI] [PubMed] [Google Scholar]
- Haber E., Koerner T., Page L. B., Kliman B., Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969 Oct;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- Kersh E. S., Kronfield S. J., Unger A., Popper R. W., Cantor S., Cohn K. Autonomic insufficiency in uremia as a cause of hemodialysis-induced hypotension. N Engl J Med. 1974 Mar 21;290(12):650–653. doi: 10.1056/NEJM197403212901203. [DOI] [PubMed] [Google Scholar]
- Kim K. E., Neff M., Cohen B., Somerstein M., Chinitz J., Onesti G., Swartz C. Blood volume changes and hypotension during hemodialysis. Trans Am Soc Artif Intern Organs. 1970;16:508–514. [PubMed] [Google Scholar]
- Lamprecht F., Williams R. B., Kopin I. J. Serum dopamine-beta-hydroxylase during development of immobilization-induced hypertension. Endocrinology. 1973 Mar;92(3):953–956. doi: 10.1210/endo-92-3-953. [DOI] [PubMed] [Google Scholar]
- Lazarus J. M., Hampers C. L., Lowrie E. G., Merrill J. P. Baroreceptor activity in normotensive and hypertensive uremic patients. Circulation. 1973 May;47(5):1015–1021. doi: 10.1161/01.cir.47.5.1015. [DOI] [PubMed] [Google Scholar]
- Liedtke A. J., Urschel C. W., Kirk E. S. Total systemic autoregulation in the dog and its inhibition by baroreceptor reflexes. Circ Res. 1973 Jun;32(6):673–677. doi: 10.1161/01.res.32.6.673. [DOI] [PubMed] [Google Scholar]
- Louis W. J., Doyle A. E., Anavekar S. Plasma norepinephrine levels in essential hyoertension. N Engl J Med. 1973 Mar 22;288(12):599–601. doi: 10.1056/NEJM197303222881203. [DOI] [PubMed] [Google Scholar]
- Mancia G., Donald D. E. Demonstration that the atria, ventricles, and lungs each are responsible for a tonic inhibition of the vasomotorcenter in the dog. Circ Res. 1975 Feb;36(2):310–318. doi: 10.1161/01.res.36.2.310. [DOI] [PubMed] [Google Scholar]
- Molinoff P. B., Brimijoin S., Weinshilboum R., Axelrod J. Neurally mediated increase in dopamine-beta-hydroxylase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):453–458. doi: 10.1073/pnas.66.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftchi N. E., Wooten G. F., Lowman E. W., Axelrod J. Relationship between serum dopamine-beta-hydroxylase activity, catecholamine metabolism, and hemodynamic changes during paroxysmal hypertension in quadriplegia. Circ Res. 1974 Dec;35(6):850–861. doi: 10.1161/01.res.35.6.850. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Udenfriend S. Photometric assay of dopamine- -hydroxylase activity in human blood. Clin Chem. 1972 Sep;18(9):980–983. [PubMed] [Google Scholar]
- Pickering T. G., Gribbin B., Oliver D. O. Baroreflex sensitivity in patients on long-term haemodialysis. Clin Sci. 1972 Nov;43(5):645–657. doi: 10.1042/cs0430645. [DOI] [PubMed] [Google Scholar]
- Reid J. L., Lewis P. J., Dollery C. T. Central and peripheral mechanisms in the maintenance of experimental hypertension in the rabbit. Clin Sci Mol Med. 1973 Nov;45(5):701–709. doi: 10.1042/cs0450701. [DOI] [PubMed] [Google Scholar]
- Rockson S., Stone R., Van der Weyden M., Kelley W. N. Lesch-Nyhan syndrome: evidence for abnormal adrenergic function. Science. 1974 Dec 6;186(4167):934–935. doi: 10.1126/science.186.4167.934. [DOI] [PubMed] [Google Scholar]
- Rush R. A., Geffen L. B. Radioimmunoassay and clearance of circulating dopamine- -hydroxylase. Circ Res. 1972 Sep;31(3):444–452. doi: 10.1161/01.res.31.3.444. [DOI] [PubMed] [Google Scholar]
- Rush R. A., Thomas P. E., Udenfriend S. Measurement of human dopamine-beta-hydroxylase in serum by homologous radioimmunoassay. Proc Natl Acad Sci U S A. 1975 Feb;72(2):750–752. doi: 10.1073/pnas.72.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalekamp M. A., Beevers D. G., Briggs J. D., Brown J. J., Davies D. L., Fraser R., Lebel M., Lever A. F., Medina A., Morton J. J. Hypertension in chronic renal failure. An abnormal relation between sodium and the renin-angiotensin system. Am J Med. 1973 Sep;55(3):379–390. doi: 10.1016/0002-9343(73)90137-x. [DOI] [PubMed] [Google Scholar]
- Shoukas A. A., Sagawa K. Control of total systemic vascular capacity by the carotid sinus baroreceptor reflex. Circ Res. 1973 Jul;33(1):22–33. doi: 10.1161/01.res.33.1.22. [DOI] [PubMed] [Google Scholar]
- Stone R. A., Kirshner N., Gunnells J. C., Robinson R. R. Changes of plasma dopamine-beta-hydroxylase activity and other plasma constituents during the cold pressor test. Life Sci. 1974 May 1;14(9):1797–1805. doi: 10.1016/0024-3205(74)90281-1. [DOI] [PubMed] [Google Scholar]
- Takeshita A., Tanaka S., Kuroiwa A., Nakamura M. Reduced baroreceptor sensitivity in borderline hypertension. Circulation. 1975 Apr;51(4):738–742. doi: 10.1161/01.cir.51.4.738. [DOI] [PubMed] [Google Scholar]
- Thomson P. D., Melmon K. L. Clinical assessment of autonomic function. Anesthesiology. 1968 Jul-Aug;29(4):724–731. doi: 10.1097/00000542-196807000-00019. [DOI] [PubMed] [Google Scholar]
- Wallin B. G., Delius W., Hagbarth K. E. Comparison of sympathetic nerve activity in normotensive and hypertensive subjects. Circ Res. 1973 Jul;33(1):9–21. doi: 10.1161/01.res.33.1.9. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R., Axelrod J. Serum dopamine-beta-hydroxylase activity. Circ Res. 1971 Mar;28(3):307–315. doi: 10.1161/01.res.28.3.307. [DOI] [PubMed] [Google Scholar]
- Wooten G. F., Cardon P. V. Plasma dopamine- -hydroxylase activity. Elevation in man during cold pressor test and exercise. Arch Neurol. 1973 Feb;28(2):103–106. doi: 10.1001/archneur.1973.00490200051006. [DOI] [PubMed] [Google Scholar]
- de Potter W. P., de Schaepdryver A. F., Moerman E. J., Smith A. D. Evidence for the release of vesicle-proteins together with noradrenaline upon stimulation of the splenic nerve. J Physiol. 1969 Oct;204(2):102P+–102P+. [PubMed] [Google Scholar]


